Aerobic Bacteria Isolated from Diabetic Foot Ulcers of Egyptian Patients: Types, Antibiotic Susceptibility Pattern and Risk Factors Associated with Multidrug-Resistant Organisms
Abstract
Introduction
Methods
Study design
Sample collection
Isolation of aerobic bacteria
Antibiotic susceptibility testing
Detection of extended-spectrum beta-lactamase (ESBL) production
Statistical analysis
Ethical approval
Results
Demographic characteristics of the studied patients
Distribution of organisms isolated from DFUs
Antibiotic susceptibility profiles
Distribution of MDR organisms and risk factor analysis
Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Sivanmaliappan, T.S.; Sevanan, M. Antimicrobial susceptibility patterns of Pseudomonas aeruginosa from diabetes patients with foot ulcers. Int J Microbiol. 2011, 2011, 605195. [Google Scholar] [CrossRef]
- Hadadi, A.; Omdeh Ghiasi, H.; Hajiabdolbaghi, M.; Zandekarimi, M.; Hamidian, R. Diabetic foot: Infections and outcomes in Iranian admitted patients. Jundishapur J Microbiol. 2014, 7, e11680. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas. IDF Diabetes Atlas. 9th edition. IDF; 2019. Available online: https://www.diabetesatlas.org/upload/resources/materi al/20200302_133351_IDFATLAS9e-fial-web.pdf (accessed on 25 December 2019).
- Lavery, L.A.; Armstrong, D.G.; Murdoch, D.P.; Peters, E.J.; Lipsky, B.A. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis 2007, 44, 562–565. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann Med 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Assaad-Khalil, S.H.; Zaki, A.; Abdel Rehim, A.; et al. Prevalence of diabetic foot disorders and related risk factors among Egyptian subjects with diabetes. Prim Care Diabetes. 2015, 9, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020, 36 (Suppl 1), e3280. [Google Scholar] [CrossRef] [PubMed]
- Gentry, L.O. Diagnosis and management of diabetic foot ulcer. J Antimicrob Chemother. 1993, 32 (Suppl A), 77–89. [Google Scholar] [CrossRef] [PubMed]
- Banashankari, G.S.; Rudresh, H.K.; Harsha, A.H. Prevalence of gram negative bacteria in diabetic foot—A clinico-microbiological study. Al Ameen J Med Sci 2012, 5, 224–232. [Google Scholar]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Shahi, S.K.; Kumar, A. Isolation and genetic analysis of multidrug resistant bacteria from diabetic foot ulcers. Front Microbiol. 2016, 6, 1464. [Google Scholar] [CrossRef]
- Kandemir, Ö.; Akbay, E.; Şahin, E.; Milcan, A.; Gen, R. Risk factors for infection of the diabetic foot with multiantibiotic resistant microorganisms. J Infect. 2007, 54, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Kumar, A.; Kumar, S.; Singh, S.K.; Gupta, S.K.; Singh, T.B. Prevalence of diabetic foot ulcer and associated risk factors in diabetic patients from north India. J Diab Foot Compl 2012, 4, 83–91. [Google Scholar]
- Dos Santos, V.P.; da Silveira, D.R.; Caffaro, R.A. Risk factors for primary major amputation in diabetic patients. Sao Paulo Med J. 2006, 124, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, M.; Papanas, N.; Panopoulou, M.; Papatheodorou, K.; Bounovas, A.; Maltezos, E. Tissue and swab culture in diabetic foot infections: Neuropathic versus neuroischemic ulcers. Int J Low Extrem Wounds. 2013, 12, 87–93. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards of Antibiotic Susceptibility Testing, 27th ed; Supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012, 18, 268–81. [Google Scholar] [CrossRef]
- Roschanski, N.; Fischer, J.; Guerra, B.; Roesler, U. Development of a multiplex real-time PCR for the rapid detection of the predominant beta-lactamase genes CTX-M, SHV, TEM and CIT-Type AmpCs in Enterobacteriaceae. PLoS ONE. 2014, 9, e100956. [Google Scholar] [CrossRef]
- Anvarinejad, M.; Pouladfar, G.; Japoni, A.; et al. Isolation and antibiotic susceptibility of the microorganisms isolated from diabetic foot infections in Nemazee Hospital, Southern Iran. J Pathog. 2015, 328796. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Pecoraro, R.E.; Ahroni, J.H. Foot ulceration and infections in elderly diabetics. Clin Geriatr Med. 1990, 6, 747–769. [Google Scholar] [CrossRef]
- Markakis, K.; Bowling, F.L.; Boulton, A.J. The diabetic foot in 2015: An overview. Diabetes Metab Res Rev. 2016, 32 (Suppl 1), 169–178. [Google Scholar] [CrossRef]
- Papini, M.; Cicoletti, M.; Fabrizi, V.; Landucci, P. Skin and nail mycoses in patients with diabetic foot. G Ital Dermatol Venereol. 2013, 148, 603–608. [Google Scholar]
- Perim, M.C.; Borges Jda, C.; Celeste, S.R.; et al. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev Soc Bras Med Trop. 2015, 48, 546–554. [Google Scholar] [CrossRef]
- Shankar, E.M.; Mohan, V.; Premalatha, G.; Srinivasan, R.S.; Usha, A.R. Bacterial etiology of diabetic foot infections in South India. Eur J Int Med. 2005, 16, 567–570. [Google Scholar] [CrossRef]
- Omar, N.S., El-Nahas; Gray, J. Novel antibiotics for the management of diabetic foot infections. Int J Antimicrob Agents. 2008, 31, 411–419. [Google Scholar] [CrossRef]
- Dwedar, R.; Ismail, D.K.; Abdulbaky, A. Diabetic foot infection: Microbiological causes with special reference to their antibiotic resistance pattern. Egypt J Med Microbiol. 2015, 24, 95–102. [Google Scholar] [CrossRef]
- Gadepalli, R.; Dhawan, B.; Kapil, A. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006, 29, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Bansal, E.; Garg, A.; Bhatia, S.; Attri, A.; Chander, J. Spectrum of microbial flora in diabetic foot ulcers. Indian J Pathol Microbiol. 2008, 51, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.; Abouelwafa, M.; Eltayeb, W.; Aboshanab, K. Antibiotic resistance pattern of aerobic bacteria isolated from patients with diabetic foot ulcers in Egypt. Afr J Microbiol Res. 2014, 8, 2947–2954. [Google Scholar] [CrossRef]
- Hefni, A.A.; Ibrahim, A.R.; Attia, K.M.; et al. Bacteriological study of diabetic foot infection in Egypt. J Arab Soc Med Res. 2013, 8, 26–32. [Google Scholar]
- Zubair, M. Prevalence and interrelationships of foot ulcer, risk factors and antibiotic resistance in foot ulcers in diabetic populations: A systematic review and meta-analysis. World J Diabetes 2020, 11, 78–89. [Google Scholar] [CrossRef]
- Shakil, S.; Khan, A.U. Infected foot ulcers in male and female diabetic patients: A clinico-bioinformative study. Ann Clin Microbiol Antimicrob. 2010, 9, 2. [Google Scholar] [CrossRef]
- Xie, X.; Bao, Y.; Ni, L.; et al. Bacterial profile and antibiotic resistance in patients with diabetic foot ulcer in Guangzhou, Southern China: Focus on the differences among different Wagner’s grades, IDSA/IWGDF grades, and ulcer types. Int J Endocrinol. 2017, 2017, 8694903. [Google Scholar] [CrossRef]
- Spichler, A.; Hurwitz, B.L.; Armstrong, D.G.; Lipsky, B.A. Microbiology of diabetic foot infections: From Louis Pasteur to ‘crime scene investigation’. BMC Med. 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.; Ramani, R.; Shivananda, P.G.; Kundaje, G.N. Bacteriology of diabetic foot ulcers. Indian J Pathol Microbiol. 1991, 34, 81–87. [Google Scholar] [PubMed]
- Hamid, M.H.; Arbab, A.H.; Yousef, B.A. Bacteriological profile and antibiotic susceptibility of diabetic foot infections at Ribat University hospital; a retrospective study from Sudan. J Diabetes Metab Disord. 2020, 19, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Chincholikar, D.A.; Pal, R.B. Study of fungal and bacterial infections of the diabetic foot. Indian J Pathol Microbiol. 2002, 45, 15–22. [Google Scholar]
- Dezfulian, A.; Salehian, M.T.; Amini, V.; et al. Bacteriological study of diabetic foot infections in an Iranian hospital. Iran Red Crescent Med J. 2011, 13, 590–591. [Google Scholar]
- Amini, M.; Davat, A.; Piri, M. Determination of the resistance pattern of prevalent aerobic bacterial infections of diabetic foot ulcer. Iranian J Pathol. 2013, 8, 21–26. [Google Scholar]
- Saseedharan, S.; Sahu, M.; Chaddha, R.; et al. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz J Microbiol. 2018, 49, 401–406. [Google Scholar] [CrossRef]
- Murali, T.S.; Kavitha, S.; Spoorthi, J.; et al. Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J Med Microbiol. 2014, 63 Pt 10, 1377–1385. [Google Scholar] [CrossRef]
- Al Benwan, K.; Al Mulla, A.; Rotimi, V.O. A study of the microbiology of diabetic foot infections in a teaching hospital in Kuwait. J Infect Public Health. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Motta, R.N.; Oliveira, M.M.; Magalhães, P.S.; et al. Plasmid-mediated extended-spectrum beta lactamase-producing strains of Enterobacteriaceae isolated from diabetes foot infections in a Brazilian diabetic center. Braz J Infect Dis. 2003, 7, 129–134. [Google Scholar] [CrossRef]
- Richard, J.L.; Sott, A.; Jourdan, N.; et al. Risk factors and healing impact of multidrug-resistant bacteria in diabetic foot ulcers. Diabetes Metab. 2008, 34 Pt 1, 363–369. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Fiayyaz, F.; Sabir, S.; Khurshid, M. Diabetes-associated infections: Development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020, 202, 953–965. [Google Scholar] [CrossRef]
- Adeyemo, A.T.; Kolawole, B.; Rotimi, V.O.; Aboderin, A.O. Multicentre study of the burden of multidrug-resistant bacteria in the aetiology of infected diabetic foot ulcers. Afr J Lab Med. 2021, 10, 1261. [Google Scholar] [CrossRef]
- Noor, S.; Borse, A.G.; Ozair, M.; Raghav, A.; Parwez, I.; Ahmad, J. Inflammatory markers as risk factors for infection with multidrug-resistant microbes in diabetic foot subjects. Foot (Edinb). 2017, 32, 44–48. [Google Scholar] [CrossRef]
- Hartemann-Heurtir, A.; Robert, J.; Jacqueminet, S.; et al. Diabetic foot ulcer and multirug-resistant organisms: Risk factors and impact. Diabet Med. 2004, 21, 710–715. [Google Scholar] [CrossRef]
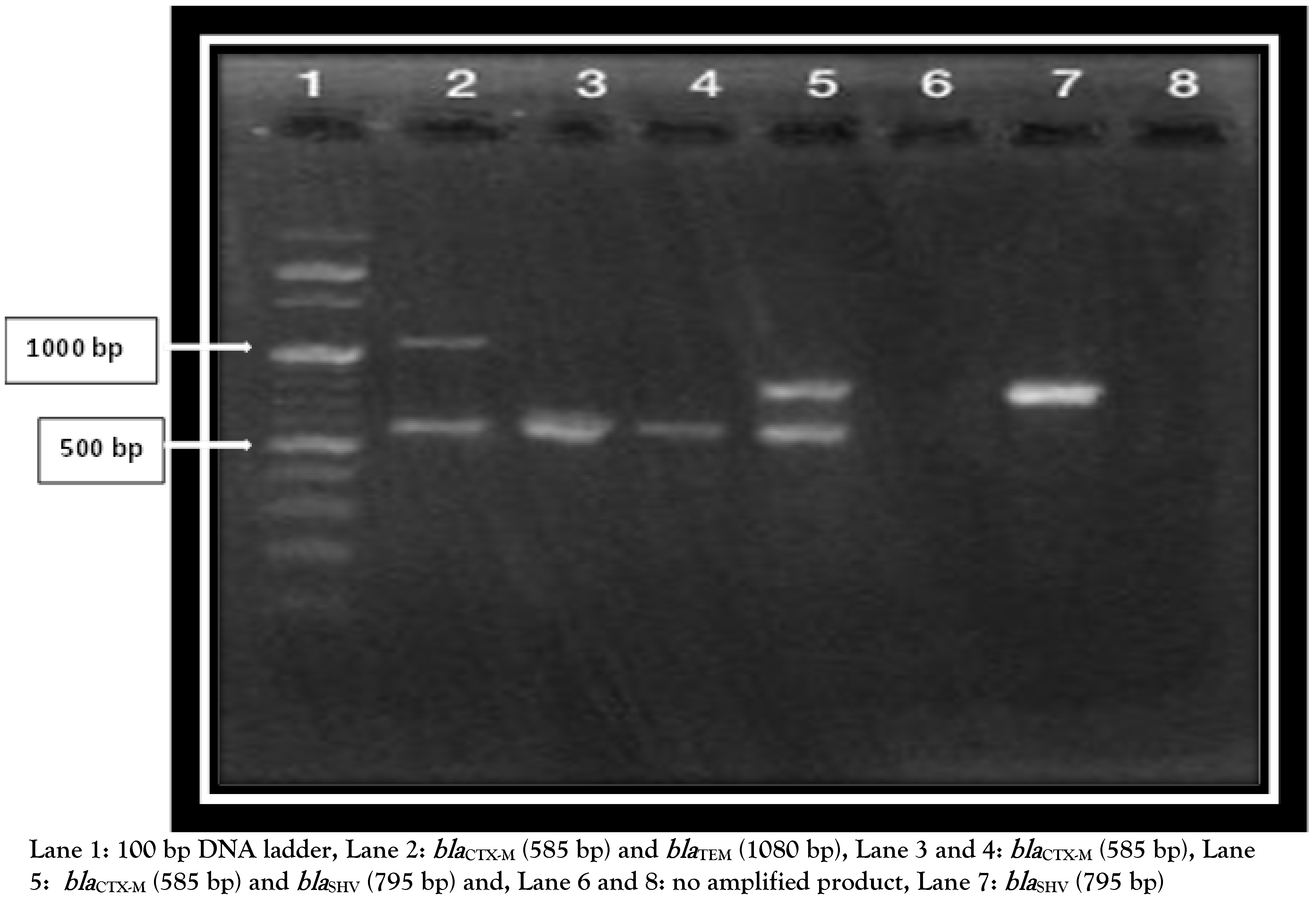
| Gene | Primer | Sequence | Amplicon size (bp) |
|---|---|---|---|
| blaCTX-M | CTX-M-F CTX-M-R | 5′CGATGTGCAGTACCAGTAA3′ 5′TTAGTGACCAGAATCAGCGG3′ | 585 |
| blaTEM | TEM-F TEM-R | 5′GCGGAACCCCTATTTG3′ 5′ACCAATGCTTAATCAGTGAG3′ | 1080 |
| blaSHV | SHV-F SHV-R | 5′TTATCTCCCTGTTAGCCACC3′ 5′GATTTGCTGATTTCGCTCGG3′ | 795 |
| Variables | DFUs No (%) | Univariate analysis Crude OR (95% CI) | Multivariate analysis Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Infected (No = 82) | Non-infected (No = 22) | |||
| Age | ||||
| <50 years | 34 (42.5) | 7 (31.8) | 0.659 (0.243-1.789) | |
| ≥50 years | 48 (58.5) | 15 (68.2) | ||
| Sex | ||||
| Male | 51 (62.2) | 12 (54.5) | 1.371 (0.530-3.547) | |
| Female | 31 (37.8) | 10 (45.5) | ||
| Duration of DM | ||||
| <10 years ≥10 years | 32 (39) 50 (61) | 15 (68.2) 7 (31.8) | 3.348 (1.231-9.109)* | 6.718 (1.3-34.3)* |
| Duration of DFU | ||||
| <3 months | 74 (90.2) | 19 (86.4) | 0.685 (0.166-2.831) | |
| ≥3 months | 8 (9.8) | 3 (13.6) | ||
| Severity of DFU | ||||
| Severe | 54 (65.9) | 6 (27.3) | 5.143 (1.812-14.6)* | 1.845 (0.43-7.8) |
| Non-severe | 28 (34.1) | 16 (72.7) | ||
| Size of DFU | ||||
| <4 cm | 55 (67.1) | 20 (90.9) | 4.90 (1.07-22.55)* | 4.26 (0.67-27.23) |
| ≥4 cm | 27 (32.9) | 2 | ||
| Site of ulcer | ||||
| Dorsal Plantar | 12 (14.6) 70 (85.4) | 6 (27.3) 16 (72.7) | 2.188 (0.713-6.707) | |
| Previous hospitalization | ||||
| Yes No | 47 (57.3) 35 (42.7) | 2 (9.1) 20 (10090.9) | 13.4 (2.9-61.27)* | 12.2 (1.97-75.61)* |
| Previous antibiotics intake | ||||
| Yes No | 50 (61) 32 (39) | 5 (22.7) 17 (77.3) | 5.31 (1.78-15.82)* | 1.56 (0.336-7.186) |
| Peripheral neuropathy | ||||
| Yes No | 52 (63.4) 30 (36.6) | 3 (13.6) 19 (86.4) | 10.978 (2.998-40.197)* | 9.5 (1.9-48.14)* |
| Gram stain (No, %) | Organism | Number | Frequency (%) |
|---|---|---|---|
| S. aureus | 18 | 22 | |
| Gram-positive cocci (36, 43.9%) | S. epidermidis | 7 | 8.5 |
| S. haemolyticus | 5 | 6.1 | |
| S. hominis | 4 | 4.8 | |
| S. simulans | 2 | 2.4 | |
| K. pneumoniae | 22 | 26.8 | |
| Gram-negative bacilli (46, 56.1%) | P. mirabilis | 14 | 17.1 |
| P. aeruginosa | 6 | 7.3 | |
| E. coli | 2 | 2.4 | |
| R. ornithinolytica | 2 | 2.4 | |
| Total | 82 | 100 |
| Antibiotic | Gram-positive cocci, n (%) | ||
|---|---|---|---|
| S. aureus (n=18) | CoNS (n=18) | Total (n=36) | |
| Ampicillin | 18 (100) | 18 (100) | 36 (100) |
| Amoxicillin/clavulanic acid | 18 (100) | 17 (94.4) | 35 (97.2) |
| Cefoxitin | 18 (100) | 18 (100) | 36 (100) |
| Erythromycin | 16 (88.9) | 16 (88.9) | 32 (88.9) |
| Clindamycin | 8 (44.4) | 7 (39) | 15 (41.7) |
| Doxycycline | 15 (83.3) | 10 (55.6) | 25 (69.4) |
| Levofloxacin | 14 (77.8) | 14 (77.8) | 28 (77.8) |
| Rifampicin | 16 (88.9) | 18 (100) | 34 (94.4) |
| Gentamicin | 8 (44.4) | 6 (33.3) | 14 (38.9) |
| Trimethoprim/sulfamethoxazole | 14 (77.7) | 18 (100) | 32 (88.9) |
| Vancomycin | 3 (16.7) | 1 (5.6) | 4 (11.1) |
| Linezolid | 2 (11.1) | 1 (5.6) | 3 (8.3) |
| Antibiotic | Gram-negative bacilli, n (%) | |||||
|---|---|---|---|---|---|---|
| K. pneumoniae (n=22) | P. mirabilis (n=14) | P. aeruginosa (n=6) | E. coli (n=2) | R. ornithinolytica (n=2) | Total (n=46) | |
| Piperacillin/tazobactam | 2 (9.1) | 0 | 0 | 0 | 0 | 2 (4.3) |
| Cefaclor | 22 (100) | 13 (92.9) | 6 (100) | 2 (100) | 2 (100) | 45 (97.8) |
| Cefotaxime | 22 (100) | 13 (92.9) | 6 (100) | 1 (50) | 1 (50) | 43 (93.5) |
| Ceftazidime | 18 (81.8) | 12 (85.7) | 5 (83.3) | 1 (50) | 2 (100) | 38 (82.6) |
| Cefepime | 22 (100) | 7 (50) | 3 (50) | 1 (50) | 1 (50) | 34 (73.9) |
| Levofloxacin | 20 (90.9) | 6 (42.9) | 6 (100) | 2 (100) | 1 (50) | 35 (76.1) |
| Trimethoprim/sulfamethoxazole | 22 (100) | 14 (100) | 5 (83.3) | 0 | 1 (50) | 42 (91.3) |
| Amikacin | 0 | 0 | 0 | 0 | 2 (100) | 2 (4.3) |
| Meropenem | 4 (18.2) | 0 | 0 | 0 | 1 (50) | 5 (10.9) |
| Aztreonam | 22 (100) | 10 (71.4) | 4 (66.7) | 2 (100) | 1 (50) | 39 (84.8) |
| Tigecycline | 2 (9.1) | 0 | 0 | 0 | 0 | 2 (4.3) |
| ESBL gene | Type of Enterobacteriaceae, n (%) | P value | |||
|---|---|---|---|---|---|
| K. pneumoniae | P. mirabilis | E. coli | Total | ||
| blaCTX-M | 4 (33.3) | 3 (42.9) | 0 | 7 (35%) | |
| blaTEM | 1 (8.3) | 1 (14.3) | 0 | 2 (10) | |
| blaCTX-M,blaTEM | 3 (25) | 1 (14.3) | 0 | 4 (20) | 0.8 |
| blaCTX-M,blaTEM,blaSHV | 4 (33.3) | 2 (28.6) | 1 (100) | 7 (35%) | |
| Total | 12 | 7 | 1 | 20 | |
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | P value | Crude OR | 95% CI | P value | Adjusted OR | 95% CI |
| Age | 0.281 | 0.375 | 0.063-2.23 | |||
| Sex | 0.804 | 1.182 | 0.316-4.424 | |||
| Duration of DM | 0.911 | 1.125 | 0.142-8.937 | |||
| Duration of DFU | 0.049 | 0.105 | 0.011-0.986 | 0.538 | 0.442 | 0.033-5.93 |
| Severity of DFU | 0.105 | 3.606 | 0.765-17.00 | |||
| Size of DFU | 0.054 | 4.533 | 0.972-21.14 | |||
| Site of DFU | 0.112 | 0.343 | 0.092-1.28 | |||
| Previous hospitalization | 0.005 | 9.286 | 1.98-43.44 | 0.756 | 1.426 | 0.152-13.427 |
| Previous antibiotic intake | 0.025 | 7.2 | 1.27-40.67 | 0.207 | 5.003 | 0.410-61.09 |
| Peripheral neuropathy | <0.001 | 20 | 3.8-104.6 | 0.007 | 15.5 | 2.104-114.28 |
© 2021 by the authors.
Share and Cite
Mashaly, M.; El kheir, M.A.; Ibrahim, M.; Khafagy, W. Aerobic Bacteria Isolated from Diabetic Foot Ulcers of Egyptian Patients: Types, Antibiotic Susceptibility Pattern and Risk Factors Associated with Multidrug-Resistant Organisms. GERMS 2021, 11, 570-582. https://doi.org/10.18683/germs.2021.1292
Mashaly M, El kheir MA, Ibrahim M, Khafagy W. Aerobic Bacteria Isolated from Diabetic Foot Ulcers of Egyptian Patients: Types, Antibiotic Susceptibility Pattern and Risk Factors Associated with Multidrug-Resistant Organisms. GERMS. 2021; 11(4):570-582. https://doi.org/10.18683/germs.2021.1292
Chicago/Turabian StyleMashaly, Mervat, Mohamed Abo El kheir, Mohamed Ibrahim, and Wael Khafagy. 2021. "Aerobic Bacteria Isolated from Diabetic Foot Ulcers of Egyptian Patients: Types, Antibiotic Susceptibility Pattern and Risk Factors Associated with Multidrug-Resistant Organisms" GERMS 11, no. 4: 570-582. https://doi.org/10.18683/germs.2021.1292
APA StyleMashaly, M., El kheir, M. A., Ibrahim, M., & Khafagy, W. (2021). Aerobic Bacteria Isolated from Diabetic Foot Ulcers of Egyptian Patients: Types, Antibiotic Susceptibility Pattern and Risk Factors Associated with Multidrug-Resistant Organisms. GERMS, 11(4), 570-582. https://doi.org/10.18683/germs.2021.1292




