Prevalence of Antimicrobial Resistance in Gram-Negative Clinical Isolates from a Major Secondary Hospital in Kuwait: A Retrospective Descriptive Study
Abstract
Introduction
Methods
Ethical considerations
Statistical analysis
Results
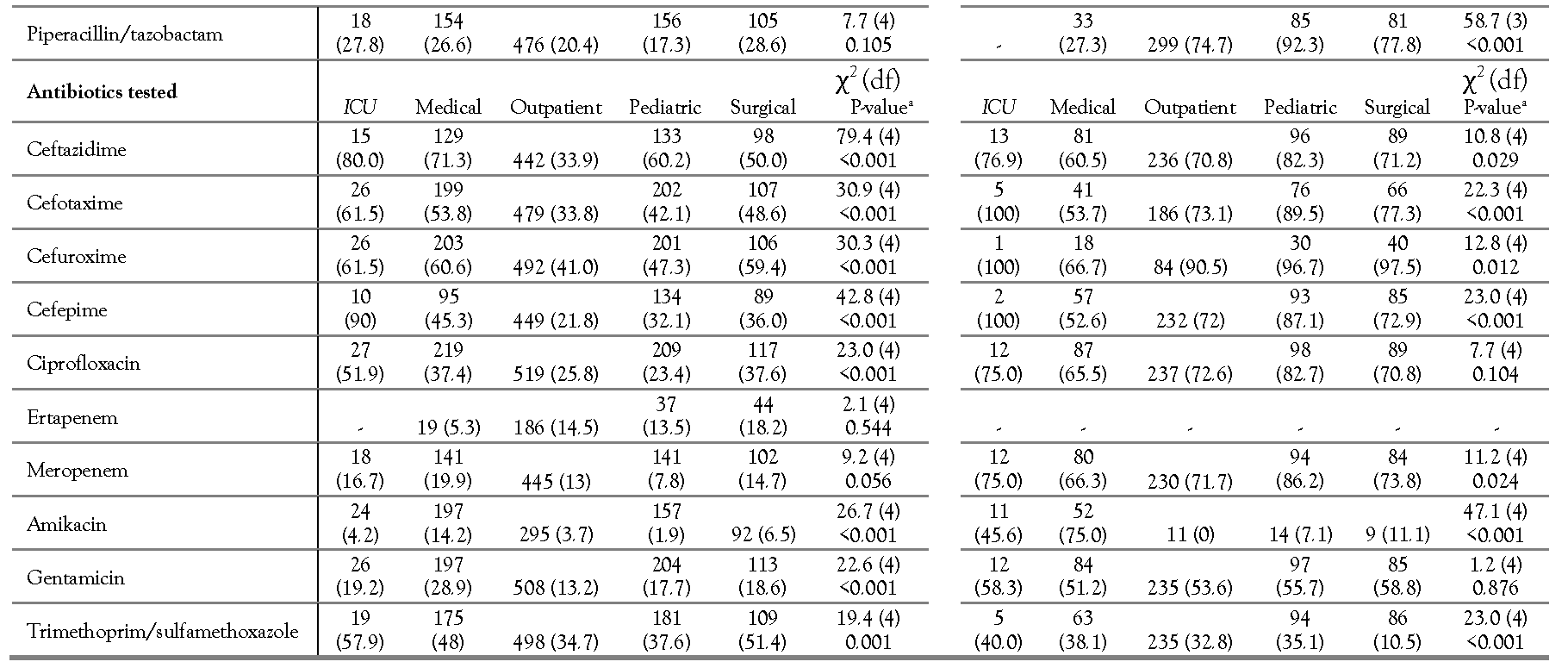
Individual antibiotic resistance
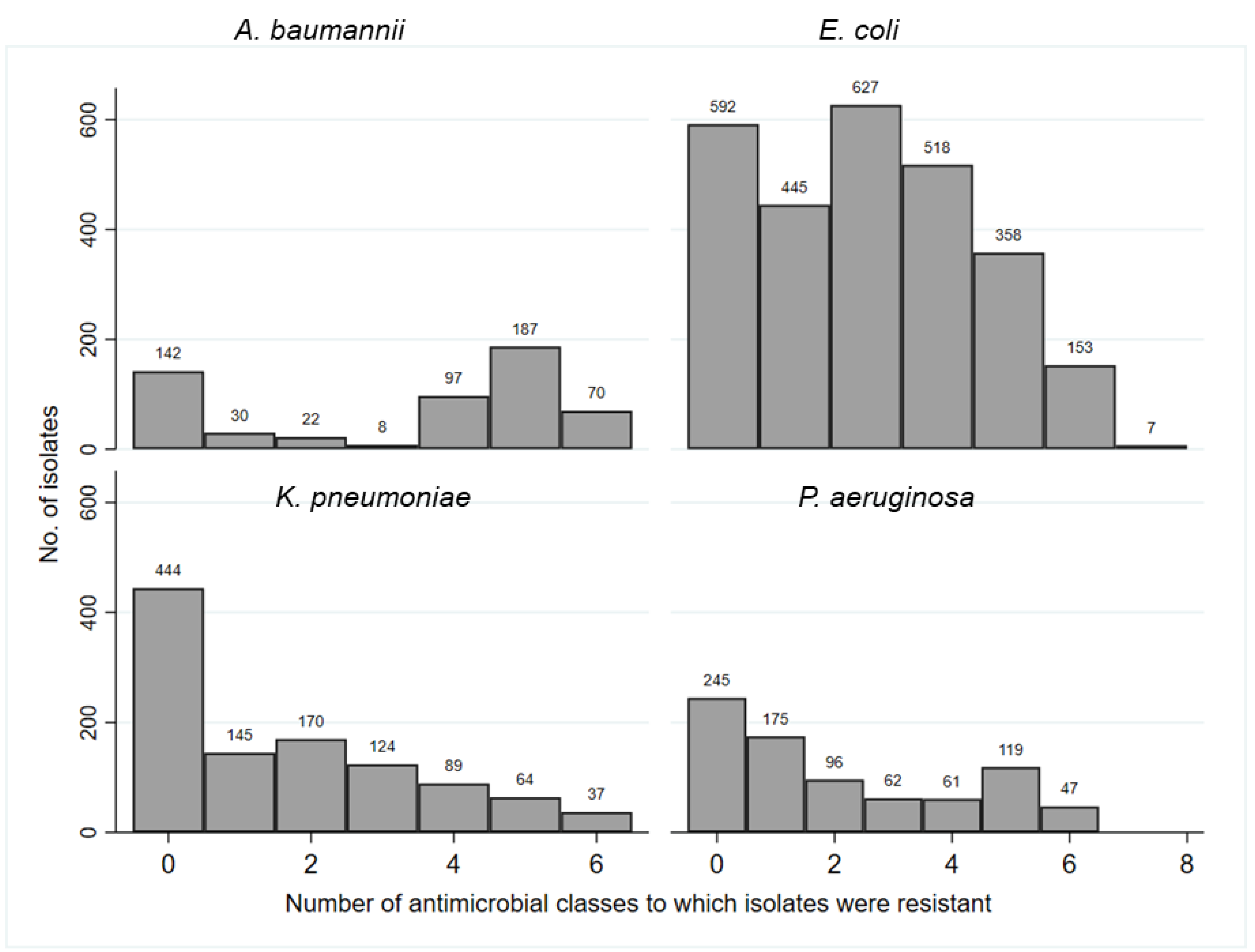
Multidrug resistance
Discussion
Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health, Organization. Global action plan on antimicrobial resistance. Geneva, Switzerland. 2015. Available online: http://apps.who.int/iris/bitstream/10665/193736/1/9 789241509763eng.pdf?ua=1 (accessed on 14 July 2019).
- Al Johani, S.M.; Akhter, J.; Balkhy, H.; El-Saed, A.; Younan, M.; Memish, Z. Prevalence of antimicrobial resistance among gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 2010, 30, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Forde, B.M.; Alfaresi, M.; et al. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci Rep. 2015, 5, 15082. [Google Scholar] [CrossRef] [PubMed]
- Yezli, S.; Shibl, A.M.; Livermore, D.M.; Memish, Z.A. Prevalence and antimicrobial resistance among Gram- negative pathogens in Saudi Arabia. J Chemother. 2014, 26, 257–272. [Google Scholar] [CrossRef] [PubMed]
- El-Saed, A.; Balkhy, H.H.; Al-Dorzi, H.M.; Khan, R.; Rishu, A.H.; Arabi, Y.M. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int J Infect Dis. 2013, 17, e696–701. [Google Scholar] [CrossRef] [PubMed]
- Al-Sweih, N.A.; Al-Hubail, M.A.; Rotimi, V.O. Emergence of tigecycline and colistin resistance in Acinetobacter species isolated from patients in Kuwait hospitals. J Chemother. 2011, 23, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.; Shahin, M.; Rotimi, V.O. Surveillance and trends of antimicrobial resistance among clinical isolates of anaerobes in Kuwait hospitals from 2002 to 2007. Anaerobe. 2010, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vali, L.; Dashti, K.; Opazo-Capurro, A.F.; Dashti, A.A.; Al Obaid, K.; Evans, B.A. Diversity of multi-drug resistant Acinetobacter baumannii population in a major hospital in Kuwait. Front Microbiol. 2015, 6, 743. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, R.A.; Rotimi, V.O.; Al Hubail, M.; et al. Wide dissemination of GES-type carbapenemases in Acinetobacter baumannii isolates in Kuwait. Antimicrob Agents Chemother. 2013, 57, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabs current.pdf (accessed on 28 November 2020).
- Centers for Disease Control and Prevention. Pneumonia (ventilator-associated [VAP] and nonventilator-associated pneumonia [PNEU]) event. 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcur rent.pdf (accessed on 28 November 2020).
- Centers for Disease Control and Prevention. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) events. 2020. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccautic urrent.pdf (accessed on 28 November 2020).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, CLSI supplement M100, 27th Ed. ed; CLSI: Wayne, PA, 2017. [Google Scholar]
- Al Sweih, N.; Jamal, W.; Rotimi, V.O. Spectrum and antibiotic resistance of uropathogens isolated from hospital and community patients with urinary tract infections in two large hospitals in Kuwait. Med Princ Pract. 2005, 14, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Al Benwan, K.; Al Sweih, N.; Rotimi, V.O. Etiology and antibiotic susceptibility patterns of community- and hospital-acquired urinary tract infections in a general hospital in Kuwait. Med Princ Pract. 2010, 19, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Husam, S.K.; Khalid, M.B.; Abiola, C.S.; Giuseppe, A.B. Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiella pneumoniae: Trends in the hospital and community settings. J Infect Dev Ctries. 2009, 3, 295–299. [Google Scholar] [CrossRef]
- Saperston, K.N.; Shapiro, D.J.; Hersh, A.L.; Copp, H.L. A comparison of inpatient versus outpatient resistance patterns of pediatric urinary tract infection. J Urol. 2014, 191 Suppl. 5, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.Y.; El-Din, K.; Rotimi, V.O.; Chugh, T.D. An analysis of hospital-acquired bacteraemia in intensive care unit patients in a university hospital in Kuwait. J Hosp Infect. 1999, 43, 49–56. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; et al. Antimicrobial- resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Balkhy, H.H.; Walsh, T.R.; Paterson, D.L. β- lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin Microbiol Rev. 2013, 26, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Aggarwal, S.; Sharma, S.; Chhibber, S.; Harjai, K. Urinary tract infections caused by Pseudomonas aeruginosa: A minireview. J Infect Public Health. 2009, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.A.; Ravizzola, G.; Peroni, L.; Bonfanti, C.; Manca, N. Prevalence of multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa in an Italian hospital. J Infect Public Health. 2013, 6, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Babay, H.A. Antimicrobial resistance among clinical isolates of Pseudomonas aeruginosa from patients in a teaching hospital, Riyadh, Saudi Arabia, 2001-2005. Jpn J Infect Dis. 2007, 60, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Sartor, A.L.; Sidjabat, H.E.; et al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolates in the Gulf Cooperation Council States: Dominance of OXA-23-type producers. J Clin Microbiol. 2015, 53, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Troyano, A.; Sibila, O. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology. 2017, 22, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
  |
| Percentage of resistance per GNB and location | |||||||||||
| E. coli (χ2 = 110.2 (12), p<0.001)a | P. aeruginosa χ2 = 38.2 (12), p=0.001)a | ||||||||||
Location/MDR | Total no. of isolates | 0 | 1 | 2 | 3+b | Total no. of isolates | 0 | 1 | 2 | 3+b | |
| ICU | 52 | 13.7 | 15.4 | 17.3 | 53.9 | 19 | 47.4 | 21.1 | 5.3 | 26.3 | |
| Medical | 448 | 16.3 | 8.7 | 19.9 | 55.1 | 169 | 43.2 | 17,2 | 12.4 | 27.2 | |
| Outpatient | 1096 | 23.8 | 16.2 | 24.6 | 35.3 | 394 | 25.0 | 24.4 | 11.9 | 38.6 | |
| Pediatric | 1007 | 23.1 | 20.6 | 25.0 | 31.3 | 162 | 25.3 | 16.7 | 11.0 | 47.0 | |
| Surgical | 124 | 19.4 | 12.1 | 13.7 | 54.8 | 85 | 36.5 | 27.1 | 11.8 | 24.7 | |
| K. pneumoniae χ2 = 22.5 (12), p=0.033)a | A. baumannii (χ2 = 68.6 (12), p<0.001)a | ||||||||||
Location/MDR | Total no. of isolates | 0 | 1 | 2 | 3+b | Total no. of isolates | 0 | 1 | 2 | 3+b | |
| ICU | 29 | 38.5 | 6.9 | 17.2 | 41.4 | 36 | 69.4 | 2.8 | 2.8 | 25.0 | |
| Medical | 230 | 36.1 | 10.9 | 19.6 | 33.5 | 121 | 38.8 | 7.4 | 8.3 | 45.5 | |
| Outpatient | 525 | 45.3 | 14.5 | 15.6 | 24.7 | 244 | 20.9 | 3.7 | 4.1 | 71.3 | |
| Pediatric | 215 | 43.7 | 14.0 | 16.3 | 26.1 | 100 | 14.0 | 2.0 | 3.0 | 81.0 | |
| Surgical | 120 | 33.3 | 13.3 | 13.3 | 40.0 | 90 | 20.0 | 5.6 | 4.4 | 70.0 | |
| Percentage of resistance per microorganism and specimen typea | ||||||||
| Specimen type/MDR | Total no. of isolates | 0 | 1 | 2 | 3+d | Most frequent MDR phenotype (%)e | ||
| E. coli | Body fluids | 34 | 29.4 | 8.8 | 14.7 | 47.1 | PEN-CEP-FLO-SUL (16.7) | |
| Blood | 160 | 19.4 | 16.9 | 27.5 | 36.3 | PEN-CEP-FLO-SUL (10.2) | ||
| χ2=23.7 (12), | ||||||||
| Respiratoryb | 52 | 11.5 | 13.5 | 26.9 | 48.0 | PEN-CEP-SUL (17.4) | ||
| p=0.022 | ||||||||
| Wound, bone, or other tissuec | 128 | 21.1 | 7.0 | 24.2 | 47.7 | PEN-CEP-FLO-AMN-SUL (14.9) | ||
| Urine | 2326 | 22.2 | 17.2 | 22.9 | 37.7 | PEN-CEP-FLO-SUL (9.5) | ||
| Specimen type/MDR | Total no. of isolates | 0 | 1 | 2 | 3+ | Most frequent MDR phenotype (%) | ||
| K. pneumoniae | Body fluids | 13 | 69.2 | 15.4. | 7.7 | 7.7 | CEP-FLO-SUL (25.0) | |
| Blood | 126 | 42.9 | 11.9 | 9.5 | 35.7 | CEP-FLO-SUL (16.7) | ||
| χ2=20.4 (12), | ||||||||
| Respiratory | 140 | 37.7 | 12.1 | 12.9 | 37.1 | PEN-CEP-FLO-SUL-CAR (10.3) | ||
| p=0.060 | ||||||||
| Wound, bone, or other tissue | 101 | 42.6 | 11.9 | 13.9 | 31.7 | PEN-CEP-SUL-FLO-CAR (6.9) | ||
| Urine | 693 | 41.1 | 14.3 | 18.0 | 26.6 | PEN-CEP-SUL-FLO-CAR-AMN (5.9) | ||
| Specimen type/MDR | Total no. of isolates | 0 | 1 | 2 | 3+ | Most frequent MDR phenotype (%) | ||
| P. aeruginosa | Body fluids | 9 | 66.7 | 22.2 | 11.1 | 0 | None | |
| Blood | 84 | 26.2 | 31.0 | 10.7 | 32.1 | PEN-CEP-FLO-CAR-AMN (11.5) | ||
| χ2=32.1 (12), | ||||||||
| Respiratory | 275 | 30.2 | 16.7 | 10.2 | 42.9 | PEN-CEP-FLO-CAR-AMN (25.8) | ||
| p=0.001 | ||||||||
| Wound, bone, or other tissue | 187 | 38.0 | 19.3 | 11.2 | 31.6 | PEN-CEP-FLO-CAR-AMN (14.2) | ||
| Urine | 250 | 25.2 | 26.0 | 14.8 | 34.0 | PEN-CEP-FLO-CAR-AMN (11.8) | ||
| Specimen type/MDR | Total no. of isolates | 0 | 1 | 2 | 3+ | Most frequent MDR phenotype (%) | ||
| A. baumannii | Body fluids | 6 | 50 | 0 | 0 | 50.0 | PEN-CEP-FLO-CAR-AMN (100) | |
| Blood | 73 | 24.7 | 6.9 | 6.9 | 61.6 | PEN-CEP-FLO-CAR-AMN (35.2) | ||
| χ2=73.2 (12), | ||||||||
| Respiratory | 226 | 11.5 | 3.1 | 2.7 | 82.7 | PEN-CEP-FLO-CAR-AMN (39.6) | ||
| p<0.001 | ||||||||
| Wound, bone, or other tissue | 133 | 45.1 | 2.3 | 3.0 | 49.6 | PEN-CEP-FLO-CAR-AMN (38.4) | ||
| Urine | 118 | 27.1 | 7.6 | 10.2 | 55.1 | CEP-FLO-CAR-AMN (31.4) | ||
Share and Cite
Alali, W.Q.; AlFouzan, W.; Dhar, R. Prevalence of Antimicrobial Resistance in Gram-Negative Clinical Isolates from a Major Secondary Hospital in Kuwait: A Retrospective Descriptive Study. Germs 2021, 11, 498-511. https://doi.org/10.18683/germs.2021.1285
Alali WQ, AlFouzan W, Dhar R. Prevalence of Antimicrobial Resistance in Gram-Negative Clinical Isolates from a Major Secondary Hospital in Kuwait: A Retrospective Descriptive Study. Germs. 2021; 11(4):498-511. https://doi.org/10.18683/germs.2021.1285
Chicago/Turabian StyleAlali, Walid Q., Wadha AlFouzan, and Rita Dhar. 2021. "Prevalence of Antimicrobial Resistance in Gram-Negative Clinical Isolates from a Major Secondary Hospital in Kuwait: A Retrospective Descriptive Study" Germs 11, no. 4: 498-511. https://doi.org/10.18683/germs.2021.1285
APA StyleAlali, W. Q., AlFouzan, W., & Dhar, R. (2021). Prevalence of Antimicrobial Resistance in Gram-Negative Clinical Isolates from a Major Secondary Hospital in Kuwait: A Retrospective Descriptive Study. Germs, 11(4), 498-511. https://doi.org/10.18683/germs.2021.1285




