Introduction
Zoonotic diseases encompass a wide range of pathogens and modes of transmission. Pathogens that can cause zoonoses include viruses, bacteria and parasites. The transmission mainly occurs through food or by different vectors [
1]. It is estimated that around 60% of all human infections originate from animals [
1], and 75% of all new infections in humans occur due to interspecies transmission [
2]. Many zoonoses are endemic and characteristic for particular regions, usually where poverty, lack of access to clean water, and sewage systems are highly prevalent, and therefore the transmission of pathogens through contaminated food and water or direct contact with animals is facilitated [
3].
Roma people are the largest transnational minority group in Europe, with approximately 12 million living mainly in central and eastern European countries [
4]. The Roma settled in Europe in the 12
th century, initially migrating from north-west India [
5,
6], Throughout history, the European Roma have been disadvantaged, as they have been discriminated population in many European countries, and even victims of genocide during the Nazi rule [
7]. These disadvantages reflect even today in their social, economic, and political vulnerability [
8]. Many still live in poor socioeconomic conditions in isolated settlements [
9]. The settlements are often built on loose soil, with no access to clean drinking water and without proper sanitary facilities and sewage systems [
10]. Poverty is widespread among Roma due to a lack of formal education, illiteracy, segregation in the educational system, and unemployment [
11]. Roma people have significantly poorer health status, shorter life expectancy, and more often suffer from chronic and communicable diseases than their non-Roma counterparts [
8,
12], Communicable diseases are prevalent, and Roma children are particularly susceptible to parasitic diseases [
8]. Moreover, numerous unregistered animals, with no veterinary care, live in many Roma settlements and share already overcrowded living areas with people [
11,
13], As a consequence of poor living conditions and close contact with animals, zoonoses, such as cryptosporidiosis, microsporidiosis, toxoplasmosis, giardiasis, and infections with soil-transmitted helminths (STHs) are common [
11,
13,
14], Studies on parasitic zoonoses in European Roma are scarce and mainly limited to Central and Eastern European countries.
In this review, we aim to summarize the available data on prevalence and risk factors for parasitic zoonoses among Roma, and address the burning issues related to emerging zoonoses in European Roma population and possible measures that can be taken in order to minimize their spread.
Results
Study selection
Literature search provided a total of 588 relevant articles; 330 articles were excluded because the sample consisted of animals, instead of humans. Out of the remaining 258 studies, 7 were excluded because of the inadequate article type. Furthermore, 98 articles originated from European countries and were further processed. After the exclusion criteria were applied, the final sample consisted of 14 articles.
Characteristics of the studies
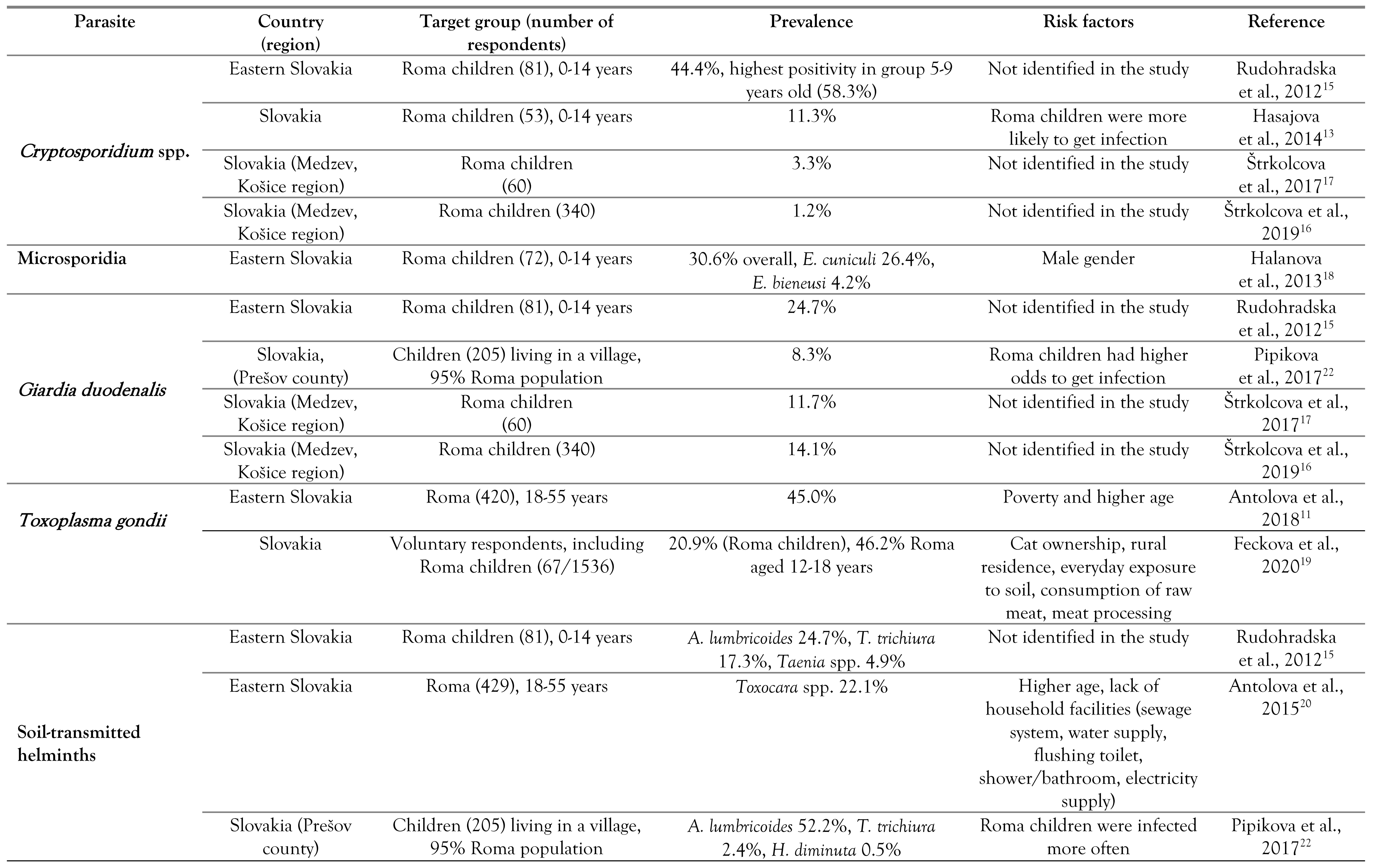
The 14 selected studies were published in the year range from 2011 to 2020. The majority of them (n=13) were conducted in Slovakia, with ten of them focusing on the area of east Slovakia, and one on North-eastern Slovakia. One study originated from Romania. Children were the population of interest in seven studies, whereas four studies included only adult participants and three studies examined samples consisting of adult respondents and children. The prevalence of
Cryptosporidium spp. was investigated in four studies, microsporidia in one,
Giardia duodenalis in four,
Toxoplasma gondii in two, soil-transmitted helminths in seven,
Trichinella spp. in two, and
Echinococcus spp. in two studies (
Table 1).
Findings on each of the parasites are presented in the sections below.
Prevalence of zoonotic parasitoses among Roma
Cryptosporidium
Cryptosporidium infection has a high prevalence among Roma children living in Eastern Slovakia settlements (44.4%) [
15]. On the other hand, studies conducted in 2016 and 2017 revealed a much lower prevalence of
Cryptosporidium infection among Roma children, 1.2%[
16] and 3.3%, respectively [
17]. A comparative study on the prevalence of
Cryptosporidium showed a 12-fold higher risk of infection in Slovakian Roma children versus non-Roma. The prevalence of infection among Roma (under five years) was 11.3%, while in children under one year old, it reached 22.7%. None of the non- Roma children were infected [
13].
Microsporidia
Enterocytozoon bieneusi was detected by real- time PCR in 4.2% and
Encephalitozoon cuniculi in 26.4% of stool samples from clinically healthy children (≤14 years old) of non-integrated Roma minority in Slovakia. Co-infections with
E. bieneusi and
E. cuniculi were detected only in one child (1.4%). Positive cases were observed in all age groups with the highest positivity (35.3%) in children aged six to nine years. The risk of
E. cuniculi infection was found to be nearly 1.7-fold higher in boys compared to girls and 4.3-fold higher for boys compared with girls in the case of
E. bieneusi infection [
18].
Toxoplasma gondii
A Slovakian study showed higher toxoplasma seropositivity in Roma (45.0%) compared to the majority population (24.1%). Poverty and higher age were identified as significant risk factors for the infection. Among Roma, the highest prevalence was detected in those older than 50 years, whereas in non-Roma group, highest seropositivity was in 30-39 years old participants. However, higher seropositivity in older participants can be explained by the accumulation of positive cases through years [
11].
Roma children are also at higher risk for infection compared to non-Roma children (20.9% vs. 7.1%). However, when results were categorized according to age, the highest prevalence of antibodies was found in Roma participants aged 12-18 (46.2%) [
19]. Cat ownership, rural residence, everyday exposure to soil, consumption of raw meat, and meat processing were found to increase the risk of infection [
19].
Soil-transmitted helminths (STHs)
Toxocara spp.
Toxocara infection has been more frequent in Roma than non-Roma adults (22.1% vs. 1%). Increasing age and worse sanitary conditions, such as lack of tap water, flushing toilet, bathroom and sewage system are associated with greater risk of infection [
20]. Moreover, another study showed that
Toxocara spp. infection is highly prevalent in Roma children (40.3%) and more frequent than in other high-risk groups, including hunters, veterinarians, and farmers. Female gender, living in rural areas, everyday contact with soil and frequent outdoor activities increased the risk of infection [
21].
Ascaris lumbricoides
In a study from 2017, among 81 of the healthy Roma children living in Eastern Slovakia, 24.7% tested positive for
Ascaris lumbricoides [
15]. In another Slovakian study that included Roma children, 73.3% of fecal samples were positive. Those children were living in settlements with poor communal hygiene, with streams in the village as a source of water [
17]. Several studies reported higher occurrence of this pathogen among Roma compared to non-Roma children. In a study from 2017, higher occurrence of parasitic diseases among Roma children in comparison to non-Roma children was observed (25.8% and 0.7%).
A. lumbricoides and
Trichuris trichiura were the most typical pathogens. A significant difference in the prevalence of parasites was observed between Roma children living in rural and urban areas (28.4% vs. 10.5%), which could be attributed to environmental contamination in rural areas. Boys and girls were equally affected. Additionally, Roma children living in Košice County showed a higher prevalence (31.8%), than those living in Prešov County (19.7%) [
10]. Another study confirmed higher occurrence of endoparasites among children living in village with predominantly Roma residents, compared to village mostly inhabited with general Slovakian population (53.2% vs. 0%).
Ascaris spp. was the dominant parasite among children (52.2%) and dogs (40.9%) living in village with over 95% of Roma population [
22]. In 2016, a study on the prevalence of intestinal parasites was conducted among Slovakian children from the Košice region aged <18 years. The prevalence of
A. lumbricoides in a group of Roma children was 49.4%, while in the non-Roma group it was 0.8% [
16].
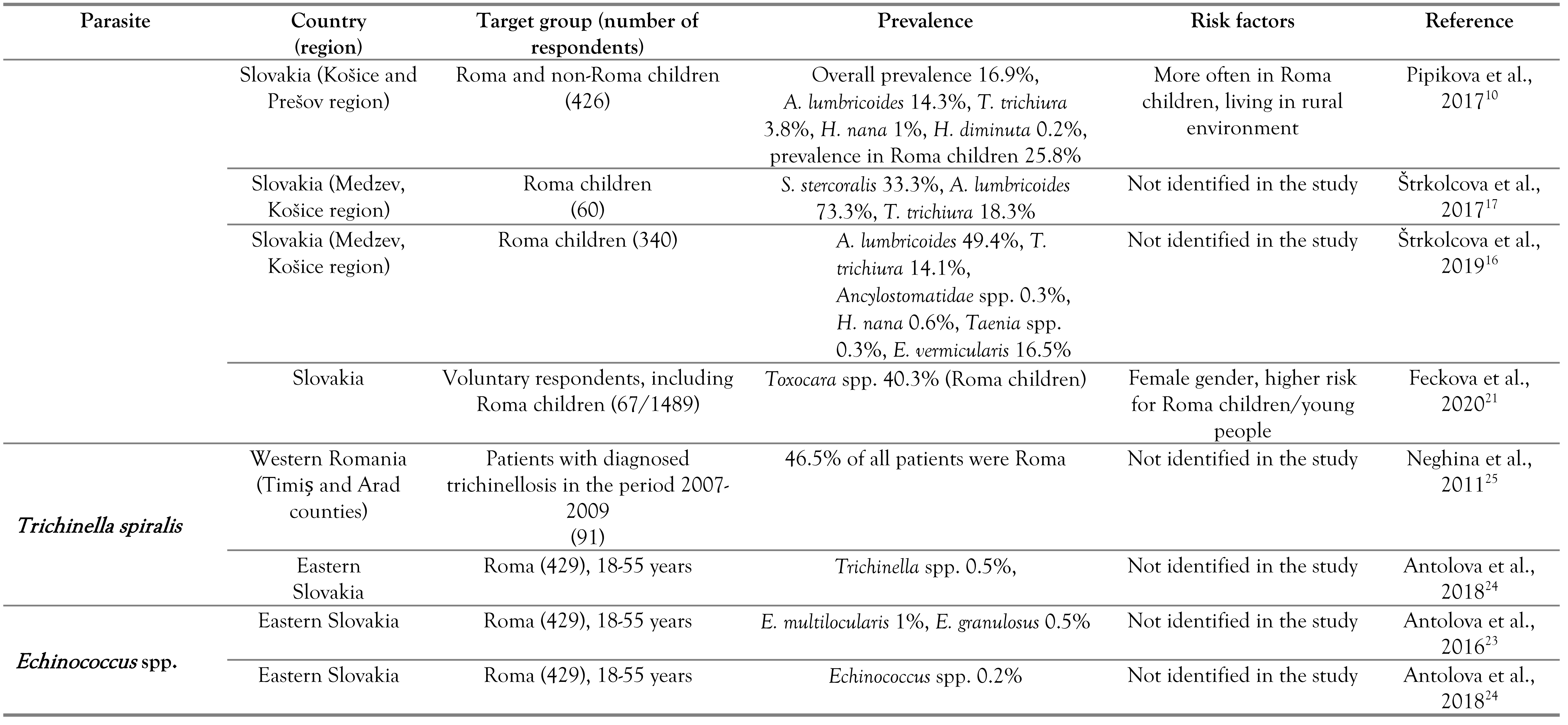
Trichuris trichiura
T. trichiura was detected in 17.3% of stool samples taken from healthy Roma children living in Eastern Slovakia. Prevalence was higher in children aged six to nine years [
15]. Furthermore, this parasite was found in 18.3% of Roma children living in Medzev, in poor sanitary conditions, with improper source of water (local streams) [
17]. In a study that compared the prevalence of parasites among two villages, the prevalence of
T. trichiura among children living in village with predominantly Roma residents was 2.4%, while in a village with predominantly Slovakian residents no cases of infection were detected [
22]. In a study from 2016, the prevalence among Roma children living in Košice region was 14.1%, while no positive samples were found in the non-Roma group [
16].
Strongyloides stercoralis
The Slovakian comparative study (2013- 2015) investigated the prevalence of STHs among Roma and non-Roma school children living in Medzev. Roma children were coming from a settlement with poor communal hygiene and improper water sources, that is, a stream in the village served as a source of water supply. Out of 60 examined Roma children, 33.3% had detectable IgG antibodies against
S. stercoralis, with the highest seroprevalence among children one to five years (38.1%), compared to 23.8% in non-Roma children. A high prevalence of soil contamination was observed; 13/14 soil samples taken in Roma settlement were positive for some of the endoparasites. When the prevalence of
S. stercoralis was compared between dogs from the settlements and dogs from the shelter, no significant difference was found [
17].
Echinococcus spp.
Two published studies from Slovakia analysed the seroprevalence of
Echinococcus spp. No significant difference was found, as seropositivity in Roma and non-Roma population was 0.5% vs. 0.8% for
E. granulosus, and 1% vs. 0.3% for
E. multilocularis in one study [
23], and 0.2% vs. 0% for
Echinococcus spp. in another study [
24].
Other STHs
In a study that included two villages with different population structure, the one with over 95% of Roma population had a higher prevalence of different endoparasites,
Hymenolepis diminuta was found in 0.5%, and
Enterobius vermicularis in 1% of the samples, while in a village inhabited mostly with Slovakian general population, these parasites weren’t detected [
22].
Among Roma children living in Košice region,
E. vermicularis had a prevalence of 16.5%,
Hymenolepis nana 0.6%,
Taenia spp. 0.3%, and
Ancylostomatidae spp. 0.3%. In 64.4% of children a mono-infection caused by one endoparasite species was detected. Mixed infections were confirmed in 23.2% of the examined samples. The highest number of positive samples was observed in children aged six to nine years. In the non-Roma group, a similar prevalence of
E. vermicularis (14.7%) and
Taenia spp. (0.8%) was found, while no cases of
Ancylostomatidae and
H. nana were detected [
16]. In a study that included 81 healthy Roma children living in Eastern Slovakia, 56.8% of stool samples were positive for some of the intestinal parasites.
Taenia spp. was found in 4.9% of the samples. Children aged six to nine years were more often positive to some of the parasites, while no
Taenia infection cases were found in children under one year old [
15].
Giardia duodenalis
A Slovakian study that investigated the prevalence of different parasites among healthy Roma children detected a high occurrence of the protozoan parasite,
G. duodenalis, in 24.7% of the respondents [
15]. Furthermore, this parasite was found in 11.7% of Roma children in another Slovakian study [
17]. Compared to non-Roma children, Roma children living in settlements were more frequently infected (14.1% vs. 1.6%) [
16]. Additionally,
G. duodenalis was detected in 8.3% of stool samples taken from the children living in village with predominantly Roma residents, whereas in a village where most of the residents were of Slovak general population, no positive samples were detected [
22].
Trichinella spp.
Between 2007 and 2009, the Roma minority accounted for most of the cases of trichinellosis in Romania. Most of the infected adults were unemployed and had lower incomes, resulting in more frequent consumption of home-made pork products [
25]. However, a recent Slovakian study in the Košice region reported no significant difference in
Trichinella spp. seroprevalence among Roma compared to non-Roma. Seropositivity to
Trichinella was confirmed in only two out of 429 Roma participants (0.5%). No seropositive cases were found in the non-Roma group of participants [
24].
Discussion
Our study aimed to investigate the prevalence and risk factors for parasitic zoonoses among Roma population living in Europe. All except one study included in the review originated from Slovakia. This is understandable given the high number of Roma residents in that country. However, there is no data on this issue from other countries with large proportion of Roma population, such as Bulgaria, Slovakia, Spain, etc. [
26], which would be helpful in order to get a more detailed picture of the state of Roma’s health. Furthermore, Roma who participated in the studies were mostly living in segregated settlements, however, this is true for the majority of the European Roma population [
9].
The data suggest that parasitic zoonoses, including cryptosporidiosis, microsporidiosis, toxoplasmosis, infection with the majority of STHs and G. duodenalis, are very common in Roma population. Cryptosporidium, T. gondii, STHs and Giardia spp. are more common in Roma children compared to non-Roma children, whereas T. gondii and STHs are also more common in Roma adults compared to non-Roma adults. On the contrary, trichinellosis, echinococcosis, and taeniasis have not been shown to be more prevalent among Roma compared to the general population.
Several possible reasons could be behind the higher prevalence of zoonotic diseases in Roma living in segregated settlements. No access to clean water and no monitoring of water quality is a major problem that leads to higher prevalence of water-borne parasitoses [
15,
27,
28], Additionally, inadequate garbage disposal and low hygienic standards attract animals, such as rodents, which also often share housing with people in settlements. Exposure to animals increases the chances of acquiring zoonotic diseases [
13,
18], and numerous animals in settlements increase the chance of soil contamination [
15,
29], High rates of soil contamination and frequent infection among animals living in settlements have been detected in two studies, suggesting that Roma are more exposed to sources of infection [
17,
22], Living in rural areas [
30], and frequent consummation of undercooked meat and unwashed vegetables and fruits represent another risk factor [
31],
-[
33] and those practices are standard in Roma as a consequence of inadequate sanitary conditions [
15]. Also, use of the woods for heating, that are contaminated with soil, through which people come in contact with parasites, influences the high prevalence of some endoparasites [
20]. It appears that Roma with low incomes or unemployed more often consume home-made meat products and are therefore more prone to get
Trichinella infection [
20], although another study on its prevalence among Roma detected no significant difference compared to non-Roma [
24]. Interestingly, although echinococcosis is transmitted through the same routes as other soil-transmitted helminths that are highly prevalent among Roma, this parasite does not affect Roma more often than the general population [
23,
24],
Preventive healthcare measures should be implemented in order to minimalize the risk of zoonotic diseases among Roma. Most of the risk factors are associated with poor hygiene and low socioeconomic standards, that also influence more common contact with infected animals or contaminated soil. Therefore, measures should aim better hygiene, access to clean water, improvement of sewage systems and better veterinary care for animals that live in settlements. Education about risks for infection and how to avoid them should be included in prevention plans.