Abstract
Introduction: With the exception of breast cancer, gynecologic neoplasms constitute the most common cancers that complicate pregnancy. Pregnancy therefore presents a window of opportunity for all pregnant women who do not take part in routine free cervical cancer screening program to undergo a free voluntary cytological test and human papillomavirus (HPV) DNA testing. This study aimed to determine prevalent HPV genotypes among pregnant women using exfoliated cells from cervical swabs and determine risk factors responsible for the upsurge of cervical precancerous lesions. Methods: In a cross-sectional study conducted from October 2017 to March 2018, a total of 482 pregnant women were enrolled. Cervical swabs and samples for cytology were collected from each enrolled participant during their routine prenatal consultation. The Papanicolaou’s (Pap) staining technique was performed and all cervical swab samples were amplified through conventional PCR. HPV genotypes were identified using the Roche Linear Array Genotyping Assay. SAS 9.2 software (SAS Institute Inc., USA) was used for statistical analysis and p values <0.05 were considered significant. Results: Among the 482 participants, 354 (73.4%) had normal cytology and 128 (26.6%) had abnormal cytology. HPV DNA was identified in 62/464 (13.4%). The most prominent HPV types identified were HPV 16 (24%), HPV 18 (36.4%), HPV 45 (28%), HPV 53 (18.9%) and HPV 67 (24.3%). Early intercourse, number of sexual partners and age at first pregnancy were some of the risk factors that influenced the etiology of preinvasive cervical lesion. Conclusions: Prevalent HPV types identified in our study were HPV 16, 18, 45, 53 and 67. Organizing effective screening programs in prenatal care facilities is crucial in order to detect prevalent HR-HPV types and precursors for cervical lesions. The addition of HPV vaccination in the national immunization program with focus on the different epidemiological HPV genotypes circulating in the country is warranted.
Introduction
Cervical lesions are precursor lesions for cervical carcinoma, which begin with infection of the metaplastic epithelium of the transformation zone of the cervix with one or more of the high-risk (HR) human papillomaviruses (HPVs) [1,2]. At least 1% of the childbearing women screened annually for cervical cancer using Papanicolaou (Pap) staining method are diagnosed with cervical intraepithelial neoplasia (CIN) [1,3]. The major causative factor of the disease is understood to be HPV, a double stranded DNA virus. Infection with HR-HPV types is the critical etiological factor in the development of cervical cancer. The highest prevalence of HPV infection occurs in adolescents and young adults between the ages of 15–25 years, accounting for more than 75% of new HPV infections [1,4].
The squamocolumnar junction is usually readily accessible due to the natural eversion of the transformation zone under the influence of high estrogen levels during pregnancy [5,6].
Available data on the prevalence of cervical lesions and HPV in pregnancy in Cameroon and its neighboring countries are scarce, and documented studies done on the existence of these preinvasive lesions in pregnancy are limited [7]. Routine screening for cervical abnormalities in pregnancy is not practiced frequently thereby making it almost impossible to know the prevalence rate of the disease in pregnancy, and cervical cancer rates have continued to remain unacceptably high [8]. The fear of inducing heavy bleeding, infection, and even the risk of an iatrogenic miscarriage represents the greatest concern that has significantly reduced routine screening of pregnant women [1]. Given the increasing incidence of HPV infection and preinvasive cervical lesions in young women, the beginning of pregnancy may be a window of opportunity for all pregnant women who do not otherwise take part in routine free cervical screening program to undergo a free voluntary cytological test and HPV DNA testing.
In Cameroon, like most developing countries, guidelines for population-based screening programs for cervical cancer are established and are mainly cytology based and visual inspection tests [9]. Despite the introduction of these screening programs, screening coverage is still very low. Several reasons such as poverty, lack of healthcare infrastructures and trained practitioners, absence of sustained prevention programs, lack of laboratory supplies, religion and beliefs are responsible for the failure to implement an effective screening program in Cameroon [10,11]. It is estimated that less than 5% of women that are at risk of developing cervical cancer have ever been screened [10].
Precancerous lesions of the cervix are most commonly treated in Cameroon by cryotherapy [9]. Surgical excision of the affected tissue is done (using the loop electrosurgical excision procedure) and necessary when the lesion is large. Excision by cone biopsy is reserved for more advanced or recurrent cases, especially those involving disease in the endocervical canal [9]. Treatment options for invasive cervical cancer (including squamous cell carcinoma and cervical adenocarcinoma) include surgery, radiotherapy and chemotherapy; these may be used in combination.
This study aimed to determine the prevalent HR-HPV types among pregnant women in Cameroon as a pre-requisite for HPV vaccine introduction using exfoliated cells from cervical swabs and to determine risk factors responsible for the upsurge of cervical precancerous lesions.
Methods
Study design
Ethical clearance for this study was obtained from the Cameroon National Ethical Committee N0. 2017/06/919/CE/CNERSH/SP. Informed consent was obtained from all participants. The study was conducted according to ethical guidelines and principles of the International Declaration of Helsinki 2013.
Between October 2017 and March 2018, a cross-sectional study was carried out in two hospitals in Yaoundé, Biyem-Assi district hospital and Marie Reine hospital Etoudi. Cervical swab and cytology samples were collected from pregnant women aged ≥25–40 years by trained personnel using standard techniques. Verification was done on enrolled participants for their eligibility to take part in the study; informed consent was signed by all eligible participants. Demographic and clinical data were collected with the aid of a questionnaire. The risk factors for cervical preinvasive disease such as age, educational status, age at coitarche, number of sexual partners since coitarche, marital status, contraceptive use and type of family planning method, parity, and previous history of sexually transmitted diseases, human immunodeficiency virus (HIV) status, history and duration of smoking were also obtained.
Risk factors were chosen based on the ease with which they can be elicited and recorded in a clinical setting and previous literature describing their association with the presence of cervical lesions or a plausible etiological role. Women with any abnormal cytology were referred to the Gynecology Department of the cancer management centers in Yaoundé for possible colposcopic examination and, if appropriate, biopsy and treatment.
Sample collection and analysis site
Cervical and cytology samples were collected from each participant by trained personnel. Cervical swabs were collected with a cervical brush (or Dacron swab, if the patient was in the last trimester of pregnancy), by inserting the total area of the brush into the cervix (1.5–2.0 cm) and rotating 180 °C and later placed in PreservCyt (CytycCorporation, USA).
Swab samples for cytology were collected using conventional methods; using a wooden Ayer spatula of about 220 mm long, the concave end of the spatula was placed against the cervix and rotated in circular fashion so that the entire area around the cervical opening and “squamo-columnar junction” was sampled. The convex end of the spatula was used for scraping vaginal lesions or sampling the “vaginal pool”, the collection of vaginal secretions just below the cervix. The sample taken from the cervix was spread on a thin labelled (using a pencil on the frosted end) glass slide. The smear was made as thin as possible to make it easier for the pathologist to read. Two slides were made for each participant. The slides were immediately fixed with 95% ethanol solution (within 10–15 s to avoid drying) and stored at room temperature for the duration of patient enrolment. These slides were stored at the laboratory of the Centre for the Study and Control of Communicable Diseases (CSCCD) of the Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon, until analysis.
Papanicolaou’s (Pap) staining
Before staining, all the slides were verified, prepared and validated by a trained cytologist. He later stained all verified slides using the Pap staining method, and after staining and drying, he examined the slides under the microscope (Zeiss optical microscope, ZEISS Axio Scope A1, Germany) for any cell abnormalities.
Each slide was scored according to the Bethesda 2001 system as unsatisfactory, negative, atypical squamous cells (ASC), atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells, which cannot exclude a high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesions (LSILs), high-grade squamous intraepithelial lesions (HSILs). Cytology readings were conducted blinded of clinical characteristics, HPV type infection and visual inspection of the cervix of the participants. Two slides were stained and analyzed for each patient. The most severe result was considered the final result, in case of differences between both slides, and slides with lesions were double checked by a cytologist external to the study. Differences were resolved by consensus.
HPV DNA extraction
Collected samples were immediately placed on ice packs and transported to the laboratory at CSCCD. The tubes were vortexed for 30 s and DNA extraction was done using the AmpliLute Liquide Media Extraction Kit (Roche Diagnostics, USA), as described in previous studies [9,10]. Briefly, 200 µL of samples were added to 200 µL of lysis solution and 20 µL of proteinase K solution. DNA was eluted in 100 µL of elution buffer.
HPV DNA amplification and detection using the linear array HPV genotyping test kit
HPV genotyping was performed using the Roche linear array HPV genotyping test kit (Roche Diagnostics) on all samples. Double-stranded DNA concentration was specifically quantified on the Rotor Gene 6000 PCR machine (Applied Biosystems, USA, gold-plated 96-well) according to the manufacturer’s instructions.
DNA amplification
The master mix (MMX) was prepared by adding 125 µL of HPV Mg2+ to the entire vial of MMX. The necessary calculations were done to determine the number of MMX vials needed for the number of samples to be processed according to the manufacturer’s instructions. Fifty microliters (50 µL) of the MMX were added to the amplification tubes and 50 µL of each processed specimen and control added to the appropriate labelled amplification tubes containing working MMX. PCR conditions were respected according to the manufacturer’s instructions.
Detection of amplicons
Genotyping was performed by hybridization of the amplified DNA. Test strip results were read independently by two molecular scientists. Both were blinded as to the HIV and cytological status detected in any of the study subjects. Samples that were beta globulin negative were excluded from the analysis. The HPV subtypes detected by the Roche array were classified as HR-HPV or low risk (LR)-HPV. A study subject was considered as HPV positive if the specific HPV genotype was detected on a cervical sample.
Statistical analysis
P values were calculated and used to determine whether a certain HPV subtype had an influence on a particular histological type of cervical cancer. The HPV types detected by the Roche linear array HPV genotyping test kit were categorized as follows: LR-HPV: HPV 6, 11, 26, 40, 42, 54, 55, 61, 62, 64, 66, 70, 71, 72, 81; probably HR-HPV: 53, 56; and HR-HPV: HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 69, 73, 82. For statistical analysis, the SAS 9.2 software (SAS Institute Inc., USA) was used and P values <0.05 were considered significant.
Results
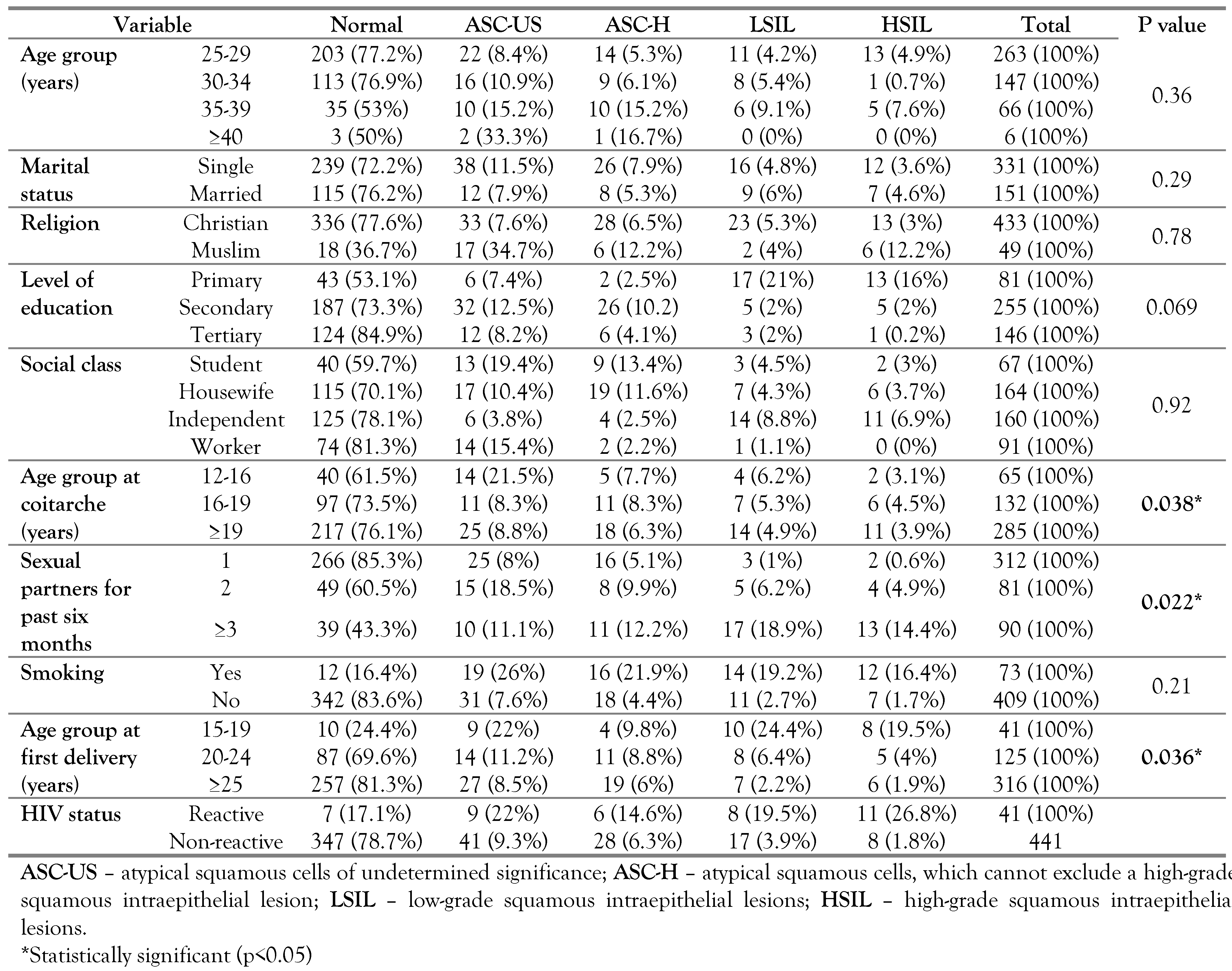
Among the 482 participants, 354 (73.4%) had normal cytology and 128 (26.6%) had abnormal cytology ranging from ASC-US to HSIL. The mean age was 27, and the most represented age range was 25–29 (51.7%) as presented in Table 1.

Table 1.
Sociodemographic characteristics, risk factors and cervical lesion.
Women with HSIL were referred to the Gynecology Department of the cancer management centers in Yaoundé, for possible colposcopic examination and, if appropriate, biopsy and treatment.
Early intercourse, number of sexual partners and age at first pregnancy were some of the risk factors that influenced the etiology of preinvasive cervical lesion as shown in Table 1. They were found to be statistically significant risk factors in the development of cervical lesion with p<0.05. Smoking, sexual frequency, use of contraceptives, parity and previous sexually transmitted diseases were all found not to be statistically significant risk factors.
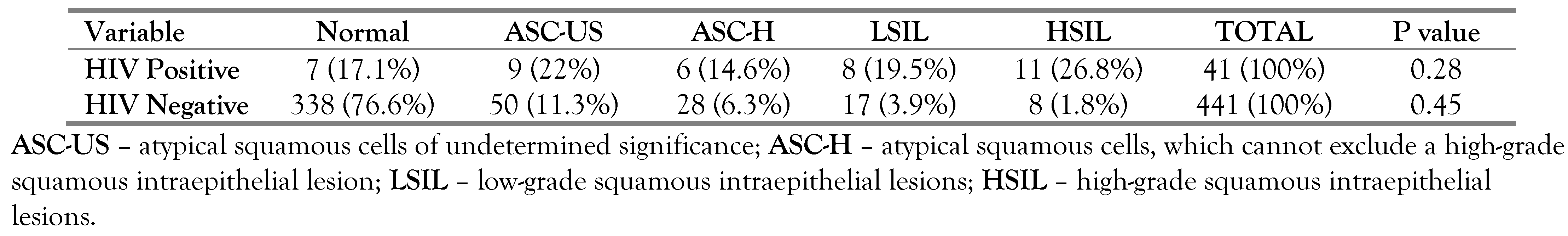
As presented in Table 1, most of the participants, n=347, (78.7%) were HIV negative. There was an association between cervical lesions and HIV status as shown in Table 2. HIV positive participants were more likely than HIV negative participants to have cervical abnormal lesions on cytology.

Table 2.
Correlation between cytology and HIV serology.
Polymerase chain reaction, HPV prevalence and types identified from cervical swabs
From the 482 eligible participants, 18 were eliminated either because of the quality of DNA or because of the absence of the beta globulin gene (beta globulin negative). The remaining 464 samples were specifically amplified with a pair of generic primers and HPV genotyping was performed using the Roche linear array HPV genotyping test kit (Roche Diagnostics). From the 464 samples, 62 (13.36%) were HPV positive with one or more HPV types.
The different HPV types identified were 25 (5.39) HR-HPV and 37 (7.97%) LR-HPV. The HR-HPV types identified included HPV 16 (6, 24%), HPV 18 (8, 36.4%), HPV 33 (2, 9.1%), HPV 39 (2, 9.1%), and HPV 45 (7, 28%) as presented in Figure 1. The LR-HPV types (Figure 1) included HPV 6 (3, 8.1%), HPV 11 (6, 16.2%). HPV 42 (4, 10.8%), HPV 53 (7, 18.9%), HPV 61 (1, 2.7%), HPV 66 (7, 18.9%) and HPV 67 (9, 24.3%). ASC-US, ASC-H, LSILs and HSILs represent high degrees of HPV related precancerous lesions. The HPV prevalence increased with the severity of cervical lesions, ranging from 9.7% in ASC-US, 22.6% in ASC-H, 29% in LSIL and 38.7% in HSIL.
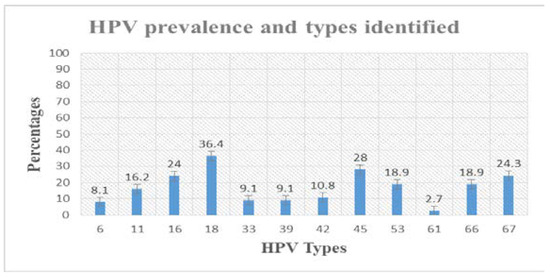
Figure 1.
HR-HPV and LR-HPV types identified in cervical swabs. Different prominent HR-HPV types identified included HPV 16 (6, 24%), HPV 18 (8, 36.4%), HPV 33 (2, 9.1%), HPV 39 (2, 9.1%), and HPV 45 (7, 28%). The LR-HPV types included HPV 6 (3, 8.1%), HPV 11 (6, 16.2%). HPV 42 (4, 10.8%), HPV 53 (7, 18.9%), HPV 61 (1, 2.7%), HPV 66 (7, 18.9%) and HPV 67 (9, 24.3%).
Multiple HPV infection was observed in this study. From the 62 women positive with both HR and LR-HPV, 26 (42%) had one genotype, 31 (50%) had two genotypes and 5 (8%) had more than three genotypes. Forty-seven (75.8%) had at least one HR-HPV subtype identified. Thirty-eight of the participants out of 62 (61.3%) had HPV types 6, 11, 16 and 18 that are currently covered by the commercial HPV vaccine Gardasil.
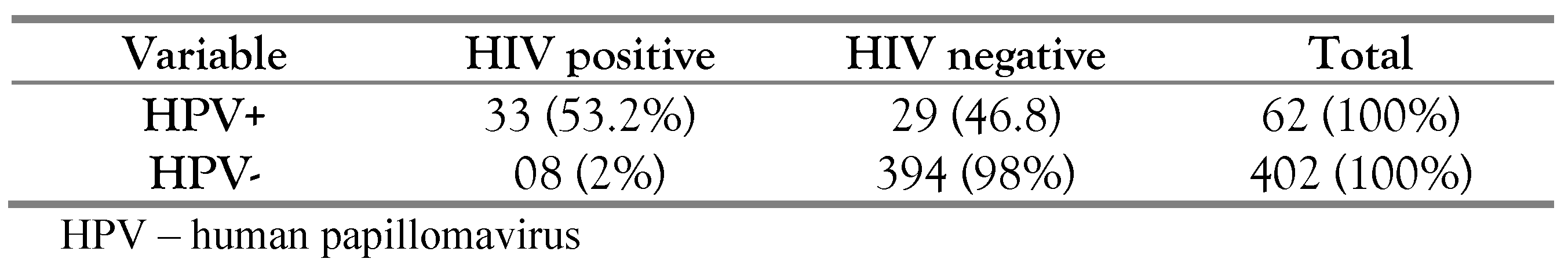
Thirty-three (33, 80.5%) of the 41 HIV positive samples were positive for HPV. Compared to HIV negative participants, HIV positive participants were more prone to be tested for any HPV type, have multiple HPV infection than HIV negative participants as shown in Table 3. HIV positive women were also more likely to have one of the HR-HPV subtypes covered in the current HPV vaccine.

Table 3.
Correlation between HPV infection and HIV serology.
A few varieties of bacteria (Gardnerella vaginalis, lactobacilli and group B streptococci), fungi (Candida albicans) and viruses (herpes simplex virus) were observed in this study. Infection with Trichomonas vaginalis was observed in four cases of HSIL. These ubiquitous microorganisms were present in two cases of LSIL, but this was not significant. Viral cytopathic effect consistent with herpes simplex virus was observed in two cases of LSIL during sample collection and showed correlation with cervical cells lesions (r2=0.8).
Discussion
In this study that aimed to determine prevalent HPV genotypes among pregnant women using exfoliated cells from cervical swabs and determine risk factors responsible for the upsurge of cervical precancerous lesions, the mean age was 27 and the age range 25–29 was predominant. The predominant age range 25–29 was certainly due to the young nature of our study population. Most of the pregnant women in this study were mostly young and single women, as such we could not draw any meaningful conclusion. Cervical abnormality prevalence of 26.6% (128/482) was observed in this study. This prevalence of 26.6% in this study was twice higher than that observed in other studies from different countries [1,10,12,13,14,15]. This could probably be due to the small sample size used in this study.
In this study, only a small fraction of the pregnant women (32, 6.6%) reported ever had regular cervical cancer screening. Among those that had ever been screened for cervical cancer, 07/32 (21.9%) had been screen for cervical cancer more than once in their lifetime. One of the reasons that these women are not regularly screened just like other women in most part of the African continent can be due to limited access to health services or providers and limited financial resources [10,16].
The number of sexual partners, early intercourse, and age at first pregnancy were some of the risk factors that influenced the etiology of preinvasive cervical lesions and were found to be statistically significant risk factors in the development of preinvasive cervical lesions among pregnant women in this study. These risk factors are interrelated and linked; they are associated with the development of cervical lesions and with the acquisition of HPV infection [17,18,19]. There exists a link between cervical lesions and the number of sexual partners [20,21,22]. The high rate of poverty in most African countries causes women to be financially dependent on their husbands [19]. For single or unmarried women with low or no income, sexual promiscuity (increase in number of sexual partners) may tend to be the sole means to gain financial income and as such the prevalence of cervical cancer may be elevated. Studies have reported that married women or those living with a partner are less likely to be infected with HR-HPV and to consequently develop cervical lesions compared to single or separated women [17,18].
Early intercourse and age at first pregnancy have been reported to be associated with an increased risk of developing cervical lesions and subsequently cervical cancer [17,20,23]. These two factors are strongly interrelated in most African countries. Early intercourse is an important risk factor for the acquisition of HR-HPV infection and other related sexually transmitted infections, responsible for most cases of cervical lesions and cervical cancer [20]. Sexual behavior (multiple sexual partners, having multiple unprotected sex) therefore determine the risk of having HR-HPV and related diseases, early intercourse therefore is important as it is associated with sexual behavior [20]. There is also a hypothesis that an immature cervix is more vulnerable to HR-HPV infection than a mature cervix, hence adolescents are more susceptible to HR-HPV persistent infections and consequently the development of cervical lesions [20].
Most of the participants, n=347, (78.7%) were HIV negative. There was an association between cervical lesions and HIV status. HIV positive participants were more likely than HIV negative participants to have cervical abnormal lesions on cytology probably due to immunosuppression caused by HIV. Other studies have reported similar findings [12,24,26]. Unfortunately, our study did not include HIV viral load and CD4 counts measurements to better investigate this aspect. Most of the HIV positive pregnant women in our study didn’t have such testing.
A significant percentage of HR-HPV (40.3%, 25/62) were detected in this study. This can be explained by the fact that most of the samples collected were cervical swabs and HR-HPV types are more prevalent in the columnar or metaplastic tissue of the cervix compared to LR-HPV types that have a predilection for the vaginal squamous cell epithelium [22]. HR-HPV 18 was the most prevalent genotype (36.4%), followed by HPV 45 (28%) and HPV 16 (24%), belonging to the most frequently identified HR-HPV. HPV genotypes can vary even within the same country and this might have serious implication for vaccine efficiency [27]. For example, HPV 16 was not the most identified genotype in this study among pre-cancerous and non-pre-cancerous pregnant women. This is in contrast to previous studies that identified HPV 16 and 18 as the most predominant HR-HPV types in Cameroon [15,28]. Due to the small sample size in this study, we could not really draw any significant conclusion with respect to this.
The increased risk of developing HPV infection in pregnant women is about 1.4 fold as calculated in a meta-analysis study [29]. With respect to this risk, the HPV prevalence of 13.36% observed in this study is lower than the 33.3% prevalence rate described in pregnant women with normal and abnormal cytology in Ghana [22]. This prevalence is also lower than the 25%, 27% and 28.7% prevalence observed in non-pregnant women in Nigeria and most West African countries [16,19]. In Cameroon, Desruisseau et al., 2009, had a prevalence of 67.2% in 65 HIV positive and negative fertile women [21], Jerome et al., 2014, observed 38.5% prevalence in 846 women, Rosa et al., 2016, had 39.0% in 838 women and Sando et al., 2013, had 83.8% prevalence in 37 endocervical secretions of high grade squamous intraepithelial lesions [15,23,30]. These studies are limited to their study framework and may not reflect the general reality. Findings from these studies greatly support the need for increased efforts to determine prominent HR-HPV types that are contributing to the development of cervical cancer in Cameroonian women. Due to the disparity in our study population and other studies carried out in Cameroon, and the small sample size in this study, we could not really compare and contrast the results obtained to other studies and draw any significant conclusion.
The LR-HPV genotypes were prevalent in this study, with a prevalence of 59.68%. Prominent among the LR-HPV were HR-HPV 53 (18.9%) and HPV 67 (24.3%). HPV 67 belongs to the same species (alpha 9) with the carcinogenic HPV16. So far HPV 67 has never been taken into consideration or considered as carcinogenic, nor has it been included in the nonavalent HPV vaccine, which is considered as the HPV vaccine of the future. This might be due to the fact that HPV 67 has been less reported in most studies. Marco et al., 2016, reported similar trends in HPV 67 prevalence among pregnant women with normal and invasive cervical cancer as in this study [22]. The risk of infection with HPV 67 should be taken into consideration.
There were several cases of multiple infection with one or more HR-HPV and or one or more LR-HPV types and HR-HPV with LR-HPV types. From the 62 women positive for HR or LR-HPV, 26 (42%) had one genotype, 31 (50%) had two genotypes and 5 (8%) had more than three genotypes. Forty-seven (75.8%) had at least one HR-HPV subtype identified. Thirty-eight of the participants out of 62 (61.3%) had HPV types 6, 11, 16 and 18 that are currently covered by the commercial HPV vaccine—Gardasil. From previous studies [15,22,28], women infected with one or more HR-HPV types are more prone to having cervical abnormalities than women with LR-HPV only. A significant proportion of women with ASC-US, ASCUS-H and LSIL were infected with one or more types of HR-HPV and LR-HPV. The outcome of this co-infection could be very informative if these women were followed up.
Thirty-three (80.5%) of the 41 HIV positive patients were also positive for HPV. There was an association between cervical lesions and HIV status. Compared to HIV negative participants, HIV positive participants were more prone to be tested for any HPV type, have multiple HPV infection than HIV negative participants. HIV positive women were also more likely to have one of the HR-HPV subtypes covered in the current HPV vaccine probably due to immunosuppression caused by the concomitant infection of HIV and HPV. Studies have shown that HPV infection and related disease is high in individuals infected with HIV, and cervical cancer deaths account for approximately 70% of the deaths of people living with HIV worldwide [30,31]. HIV has been shown to exacerbate the burden of cervical cancer. Current evidence suggests that HIV and HPV infections may interact in multiple ways [30,31]. Both viruses infect the anogenital organs and share similar risk factors such as number of sexual partners, and there is evidence for direct biological and immunological interactions between HPV and HIV [30].
Unfortunately, this study did not include HIV viral load and CD4 counts measurements to better investigate this aspect. Most of the HIV positive pregnant women in our study did not have such testing and as such, detailed analyses between HPV and HIV progression rate were not possible.
The HPV prevalence increases with the severity of cervical lesions, ranging from 9.7% in ASC-US to 38.7% in HSIL in this study. HR-HPV is usually incriminated in almost 100% of all cervical cancer cases, with HPV 16 and 18 contributing to about 70% of all cases of cervical cancer [32,33], HR-HPV 16 and 18 contribute between 41% and 67% of HSIL and 16% to 32% of LSIL.
Gardnerella vaginalis, lactobacilli and group B streptococci, Candida albicans, herpes simplex virus and Trichomonas vaginalis were observed in this study. These ubiquitous microorganisms are important cofactors capable of amplifying the upsurge of cervical precancerous lesions [23].
Limitations of the study
Most of the participants in this study were single and mostly between the ages of 25–30; this made it difficult to draw significant conclusion with respect to age, marital status and cervical lesions. Also, the small sample size used in this study made it challenging to compare the prevalence rate with other studies.
Conclusions
Prevalent HPV types identified in our study were HPV 16, 18, 45, 53 and 67. Number of sexual partners, early intercourse, and age at first pregnancy were some of the risk factors that influence the etiology of preinvasive cervical lesions. Organizing effective screening programs in prenatal care facilities is crucial in order to detect precursors for cervical lesions. More attention should be paid to precancerous lesions during pregnancy as it could be a positive signal for the development of cervical cancer. With high endemicity of HIV, the Cameroon National Program for the fight against cancer guidelines should be in line with the National HIV/AIDS program to offer Pap smears to all HIV positive women during routine prenatal HIV screening. The addition of HPV vaccination in the national immunization program with focus on the different epidemiological HPV genotypes circulating in the country is essential.
Author Contributions
MS, GBJ, GMI and GD. conceived the study. AEO, MM and CF, contributed to study design, study selection and screening. MM, GMI and GD contributed to data extraction, data analysis, and drafted the manuscript. EM contributed in study design, study selection, screening and contributed in data extraction, data analysis. GBJ conceived the study, contributed in data selection and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the Poliomyelitis Research Foundation (PRF) of South Africa.
Acknowledgments
The authors acknowledge the cytologist, Mr. Banai Thomas for reading the slides before further confirmation by G.M.I.
Conflicts of Interest
None to declare.
References
- Bakari, F.; Abdul, M.A.; Ahmed, S.A. The prevalence and course of preinvasive cervical lesions during pregnancy in a Northern Nigerian Teaching Hospital. Ann. Afr. Med. 2017, 16, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sheets, E.E. Management of abnormal Pap test. In Danforth’s Obstetrics and Gynecology, 9th ed.; Scott, J.R., Gibbs, R.S., Karlan, B.Y., Haney, A.E., Eds.; Lippincott Williams and Wilkins: Philadephia, PA, USA, 2003; pp. 59–66. [Google Scholar]
- Insinga, R.P.; Glass, A.G.; Rush, B.B. Diagnoses and outcomes in cervical cancer screening: A population-based study. Am. J. Obstet. Gynecol. 2004, 191, 105–113. [Google Scholar] [CrossRef]
- Dempsey, A.F. Human papillomavirus: The usefulness of risk factors in determining who should get vaccinated. Rev. Obstet. Gynecol. 2008, 1, 122–128. [Google Scholar]
- Zhao, S.; Zhao, X.L.; Hu, S.Y.; et al. [Comparison of high-risk human papillomavirus infection rate and genotype distribution between Han and Mongolian women]. Zhonghua Liu Xing Bing Xue Za Zhi 2019, 40, 1439–1444. [Google Scholar]
- Jordão, P.M.; Russomano, F.B.; Gerbauld, G.T.; Andrade, C.V.; Osorio, C.F.E.M. Accuracy of endocervical cytological tests in diagnosing preinvasive lesions of the cervical canal in patients with type 3 transformation zone: A retrospective observational study. Sao Paulo Med. J. 2020, 138, 47–53. [Google Scholar] [CrossRef]
- Nkfusai, N.C.; Mubah, T.M.; Yankam, B.M.; Tambe, T.A.; Cumber, S.N. Prevalence of precancerous cervical lesions in women attending Mezam Polyclinic Bamenda, Cameroon. Pan Afr. Med. J. 2019, 32, 174. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; et al.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre) Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 15 June 2020).
- World Health Organization. Comprehensive cervical cancer prevention and control programme guidance for countries. Cad. UNFPA 2011, 1, 20. Available online: https://www.unfpa.org/webdav/site/global/shared/E NGLISH-Cervical Cancer Guidance.pdf (accessed on 15 June 2020).
- Catarino, R.; Petignat, P.; Dongui, G.; Vassilakos, P. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J. Clin. Oncol. 2015, 6, 281–290. [Google Scholar] [CrossRef]
- Soler, M.E.; Gaffikin, L.; Blumenthal, P.D. Cervical cancer screening in developing countries. Prim. Care Update Ob. Gyns. 2000, 7, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Zeier, M.D. The effect of highly active antiretroviral therapy on human papilloma virus infection and cervical dysplasia in women living with HIV. PhD Thesis, Stellenbosch University, 2014. [Google Scholar]
- Ifenne, D.I.; Shittu, S.O.; Ekwempu, C.C. Cervical smear in pregnancy: Zaria experience. Niger. J. Surg. Res. 2001, 3, 81–84. [Google Scholar] [CrossRef]
- Adekunle, O.O.; Samaila, M.O. Prevalence of cervical intraepithelial neoplasia in Zaria. Ann. Afr. Med. 2010, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Júnior, J.C.; Dufloth, R.M.; do Vale, D.B.; Tavares, T.A.; Zeferino, L.C. High-grade squamous intraepithelial lesions in pregnant and non-pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 103–106. [Google Scholar] [CrossRef]
- Stonehocker, J. Cervical cancer screening in pregnancy. Obstet. Gynecol. Clin. North. Am. 2013, 40, 269–282. [Google Scholar] [CrossRef]
- Mujuni, F.; Mirambo, M.M.; Rambau, P.; Klaus, K.; Andreas, M.; Matovelo, D.; Majigo, M.; Kasang, C.; Mshana, S.E. Variability of high risk HPV genotypes among HIV infected women in Mwanza, Tanzania-the need for evaluation of current vaccine effectiveness in developing countries. Infect. Agent. Cancer. 2016, 11, 49. [Google Scholar] [CrossRef][Green Version]
- Thomas, J.O.; Herrero, R.; Omigbodun, A.A.; Ojemakinde, K.; Ajayi, I.O.; Fawole, A.; Oladepo, O.; Smith, J.S.; Arslan, A.; Muñoz, N.; Snijders, P.J.; Meijer, C.J.; Franceschi, S. Prevalence of papillomavirus infection in women in Ibadan, Nigeria: A population-based study. Br. J. Cancer 2004, 90, 638–645. [Google Scholar] [CrossRef]
- Pirek, D.; Petignat, P.; Vassilakos, P.; et al. Human papillomavirus genotype distribution among Cameroonian women with invasive cervical cancer: A retrospective study. Sex Transm. Infect. 2015, 91, 440–444. [Google Scholar] [CrossRef]
- Crofts, V.; Flahault, E.; Tebeu, P.M.; et al. Education efforts may contribute to wider acceptance of human papillomavirus self-sampling. Int. J. Womens Health 2015, 7, 149–154. [Google Scholar] [CrossRef]
- Bigoni, J.; Gundar, M.; Tebeu, P.M.; et al. Cervical cancer screening in sub-Saharan Africa: A randomized trial of VIA versus cytology for triage of HPV-positive women. Int. J. Cancer 2015, 137, 127–134. [Google Scholar] [CrossRef]
- Desruisseau, A.J.; Schmidt-Grimminger, D.; Welty, E. Epidemiology of HPV in HIV-positive and HIV-negative fertile women in Cameroon, West Africa. Infect. Dis. Obstet. Gynecol. 2009, 2009, 810596. [Google Scholar] [CrossRef] [PubMed]
- Enyegue Elisée Libert, E.; Mogtomo Martin Luther, K.; Thomas, B.; et al. Dynamics of factors responsible for the resurgence of cervical cancer lesions in women in developing countries. J. Appl. Life Sci. Int. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Aballéa, S.; Beck, E.; Cheng, X.; et al. Risk factors for cervical cancer in women in China: A meta-model. Womens Health 2020, 16, 1745506520940875. [Google Scholar] [CrossRef]
- Shrestha, A.D.; Neupane, D.; Vedsted, P.; Kallestrup, P. Cervical cancer prevalence, incidence and mortality in low and middle income countries: A systematic review. Asian Pac. J. Cancer Prev. 2018, 19, 319–324. [Google Scholar] [CrossRef]
- Louie, K.S.; de Sanjose, S.; Diaz, M.; et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br. J. Cancer. 2009, 100, 1191–1197. [Google Scholar] [CrossRef]
- Atashili, J.; Miller, W.C.; Smith, J.S.; et al. Age trends in the prevalence of cervical squamous intraepithelial lesions among HIV-positive women in Cameroon: A cross-sectional study. BMC Res. Notes 2012, 5, 590. [Google Scholar] [CrossRef] [PubMed]
- Atashili, J. Cervical precancerous lesions in HIV-positive women in cameroon: Prevalence, predictors and potential impact of screening. Ph.D. Thesis, University of North Carolina at Chapel Hill, 2009. [Google Scholar]
- Yar, D.D.; Salifu, S.P.; Darko, S.N.; et al. Genotypic characterisation of human papillomavirus infections among persons living with HIV infection; a case-control study in Kumasi, Ghana. Trop. Med. Int. Health 2016, 21, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Black, E.; Richmond, R. Prevention of cervical cancer in Sub-Saharan Africa: The advantages and challenges of HPV vaccination. Vaccines 2018, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, L.; Sun, Y.; Wang, Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol. Infect. 2014, 142, 1567–1578. [Google Scholar] [CrossRef]
- Ogembo, R.K.; Gona, P.N.; Seymour, A.J. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: A systematic review and meta-analysis. PLoS ONE. 2015, 10, e0122488. [Google Scholar] [CrossRef]
- Catarino, R.; Vassilakos, P.; Tebeu, P.M.; Schäfer, S.; Bongoe, A.; Petignat, P. Risk factors associated with human papillomavirus prevalence and cervical neoplasia among Cameroonian women. Cancer Epidemiol. 2016, 40, 60–66. [Google Scholar] [CrossRef]
© GERMS 2021.