The Prevalence of HCV RNA Positivity in Anti-HCV Antibodies-Negative Hemodialysis Patients in Thrace Region. Multicentral Study
Abstract
Introduction
Methods
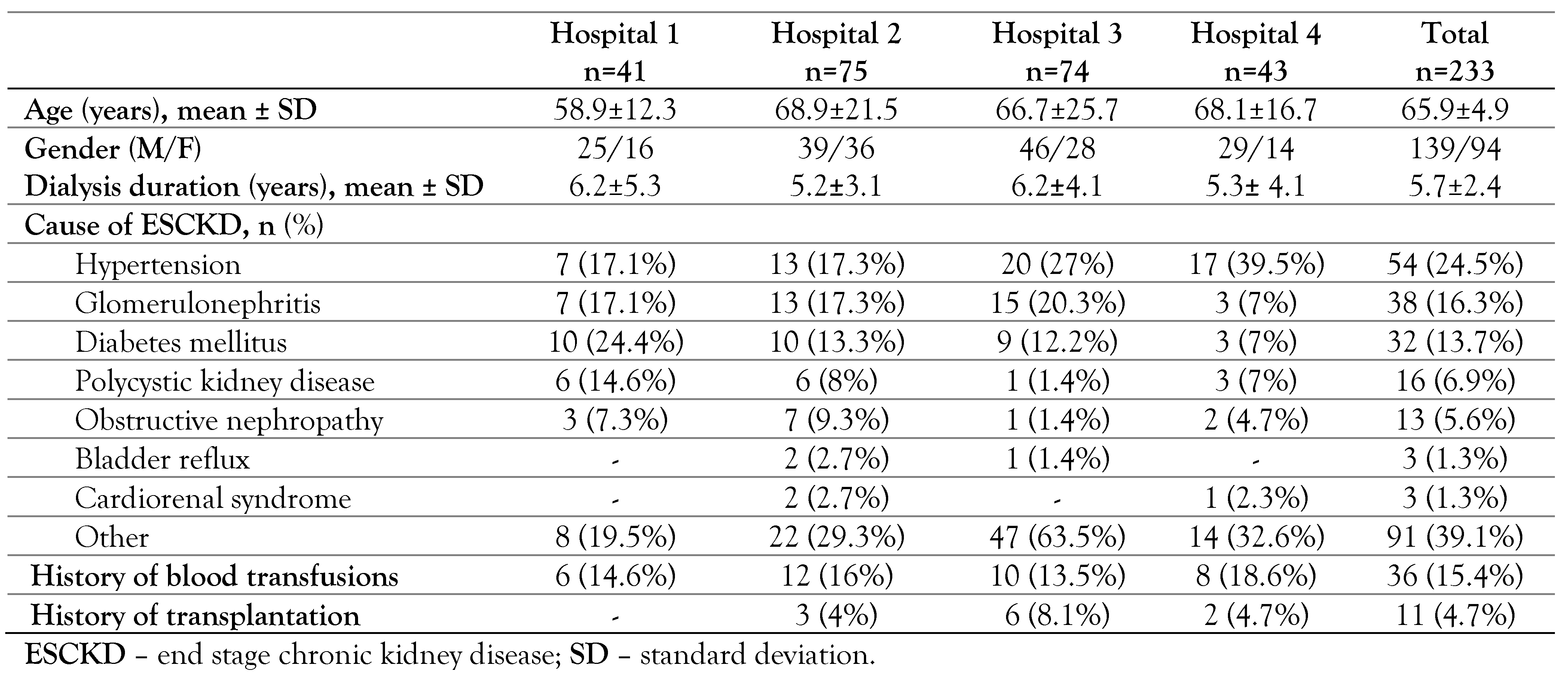
Study population
- Patients who did not give a written consent for participation in this study or were unable to do so.
- Age under 18 years.
- Patients with a history of chronic hepatitis C.
- Patients treated for chronic hepatitis C.
- Patients on peritoneal dialysis.
HCV core antigen test
Molecular detection of HCV RNA
Dialysis process
Statistical analysis
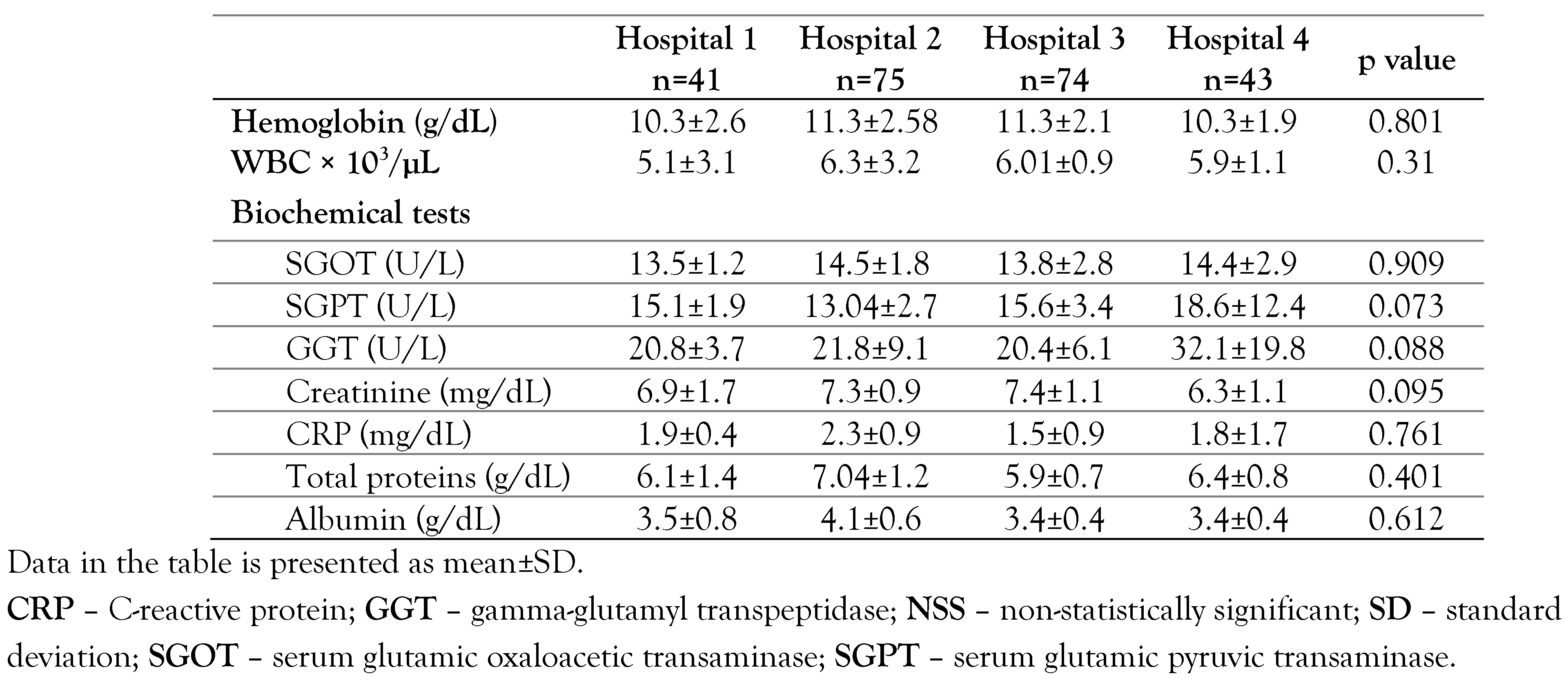
Results
HCV core antigen
HCV RNA test
Discussion
Conclusions
Author Contributions
Funding
Conflicts of interest
References
- World Health Organization. Hepatitis C. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 25 September 2020).
- Triantos, C.; Konstantakis, C.; Tselekouni, P.; Kalafateli, M.; Aggeletopoulou, I.; Manolakopoulos, S. Epidemiology of hepatitis C in Greece. World J Gastroenterol. 2016, 22, 8094–8102. [Google Scholar] [CrossRef]
- Leblebicioglu, H.; Arends, J.E.; Ozaras, R.; et al. Availability of hepatitis C diagnostics and therapeutics in European and Eurasia countries. Antiviral Res. 2018, 150, 9–14. [Google Scholar] [CrossRef]
- Akhtar, S.; Nasir, J.A.; Usman, M.; Sarwar, A.; Majeed, R.; Billah, B. The prevalence of hepatitis C virus in hemodialysis patients in Pakistan: A systematic review and meta-analysis. PLoS One. 2020, 15, e0232931. [Google Scholar] [CrossRef]
- Akabayashi, A.; Nakazawa, E.; Ino, H.; Ozeki-Hayashi, R.; Jecker, N.S. Sacrificing the Fukushima 50 again? J Public Health (Oxf). 2020, 42, 194–197. [Google Scholar] [CrossRef]
- Jakupi, X.; Mlakar, J.; Lunar, M.M.; et al. A very high prevalence of hepatitis C virus infection among patients undergoing hemodialysis in Kosovo: a nationwide study. BMC Nephrol. 2018, 19, 304. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2018 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl (2011) 2018, 8, 91–165. [CrossRef]
- Kalantar-Zadeh, K.; Miller, L.G.; Daar, E.S. Diagnostic discordance for hepatitis C virus infection in hemodialysis patients. Am J Kidney Dis. 2005, 46, 290–300. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Griveas, I.; Sveroni, E.; et al. HCV viraemia in anti-HCV-negative haemodialysis patients: do we need HCV RNA detection test? Int J Artif Organs. 2018, 41, 168–170. [Google Scholar] [CrossRef]
- Galli, C.; Julicher, P.; Plebani, M. HCV core antigen comes of age: a new opportunity for the diagnosis of hepatitis C virus infection. Clin Chem Lab Med. 2018, 56, 880–888. [Google Scholar] [CrossRef]
- Moini, M.; Ziyaeyan, M.; Aghaei, S.; et al. Hepatitis C virus (HCV) infection rate among seronegative hemodialysis patients screened by two methods; HCV core antigen polymerase chain reaction. Hepat Mon. 2013, 13, e9147. [Google Scholar] [CrossRef]
- Chevaliez, S.; Soulier, A.; Poiteau, L.; Bouvier-Alias, M.; Pawlotsky, J.M. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis. J Clin Virol. 2014, 61, 145–148. [Google Scholar] [CrossRef]
- Ali, N.; Hussain, W.; Hayat, A.; et al. Prevalence and risk factors of hepatitis B and C viruses among haemodialysis patients: a multicentric study. Eur J Gastroenterol Hepatol. 2019, 31, 29–33. [Google Scholar] [CrossRef]
- Kataruka, M.; Gupta, S.; Ramchandran, R.; Singh, M.; Dhiman, R.K.; Lal Gupta, K. Incidence and risk factors for hepatitis C virus and hepatitis B virus seroconversion in end-stage renal failure patients on maintenance hemodialysis. J Clin Exp Hepatol. 2020, 10, 316–321. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Martins, R.M.; Teles, S.A.; et al. Hepatitis C prevalence and risk factors in hemodialysis patients in Central Brazil: a survey by polymerase chain reaction and serological methods. Mem Inst Oswaldo Cruz. 2001, 96, 765–769. [Google Scholar] [CrossRef][Green Version]
- Hwang, J.C.; Jiang, M.Y.; Lu, Y.H.; Weng, S.F. Impact of HCV infection on diabetes patients for the risk of end-stage renal failure. Medicine (Baltimore). 2016, 95, e2431. [Google Scholar] [CrossRef]
- Johannessen, I.; Danial, J.; Smith, D.B.; et al. Molecular and epidemiological evidence of patient-to-patient hepatitis C virus transmission in a Scottish emergency department. J Hosp Infect. 2018, 98, 412–418. [Google Scholar] [CrossRef]
- Dalekos, G.N.; Boumba, D.S.; Katopodis, K.; et al. Absence of HCV viraemia in anti-HCV-negative haemodialysis patients. Nephrol Dial Transplant. 1998, 13, 1804–1806. [Google Scholar] [CrossRef][Green Version]
- Rigopoulou, E.I.; Stefanidis, I.; Liaskos, C.; et al. HCV-RNA qualitative assay based on transcription mediated amplification improves the detection of hepatitis C virus infection in patients on hemodialysis: results from five hemodialysis units in central Greece. J Clin Virol. 2005, 34, 81–85. [Google Scholar] [CrossRef]
- Sakamoto, N.; Enomoto, N.; Marumo, F.; Sato, C. Prevalence of hepatitis C virus infection among long- term hemodialysis patients: detection of hepatitis C virus RNA in plasma. J Med Virol. 1993, 39, 11–15. [Google Scholar] [CrossRef]
- Kiani, I.G.; Khan, A.N.; Butt, B.; Sabir, S.; Ejaz, S.; Perveen, A.; Ghani, E. HCV-RNA PCR positivity in HCV antibody negative patients undergoing haemodialysis. J Ayub Med Coll Abbottabad. 2018, 30, 397–400. [Google Scholar]
- Ozer Etik, D.; Ocal, S.; Boyacioglu, A.S. Hepatitis C infection in hemodialysis patients: A review. World J Hepatol. 2015, 7, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, R.; Zazgornik, J.; Gabriel, C.; Garbeis, B.; Blauhut, B. Hepatitis C virus – does it penetrate the haemodialysis membrane? PCR analysis of haemodialysis ultrafiltrate and whole blood. Nephrol Dial Transplant. 1995, 10, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Potthoff, A.; Meyer-Olson, D.; et al. HCV core antigen testing in HIV- and HBV-coinfected patients, and in HCV-infected patients on hemodialysis. J Clin Virol. 2012, 53, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sauné, K.; Kamar, N.; Miédougé, M.; et al. Decreased prevalence and incidence of HCV markers in haemodialysis units: a multicentric French survey. Nephrol Dial Transplant. 2011, 26, 2309–2316. [Google Scholar] [CrossRef]
 |
 |
© GERMS 2021.
Share and Cite
Konstantinidou, E.I.; Kontekaki, E.G.; Kefas, A.; Konstantinidis, T.; Romanidou, G.; Fotiadou, E.; Rekari, V.; Triantafyllidou, E.; Zisaki, S.; Kasmeridou, E.; et al. The Prevalence of HCV RNA Positivity in Anti-HCV Antibodies-Negative Hemodialysis Patients in Thrace Region. Multicentral Study. Germs 2021, 11, 52-58. https://doi.org/10.18683/germs.2021.1240
Konstantinidou EI, Kontekaki EG, Kefas A, Konstantinidis T, Romanidou G, Fotiadou E, Rekari V, Triantafyllidou E, Zisaki S, Kasmeridou E, et al. The Prevalence of HCV RNA Positivity in Anti-HCV Antibodies-Negative Hemodialysis Patients in Thrace Region. Multicentral Study. Germs. 2021; 11(1):52-58. https://doi.org/10.18683/germs.2021.1240
Chicago/Turabian StyleKonstantinidou, Eleni I., Eftychia G. Kontekaki, Aristidis Kefas, Theocharis Konstantinidis, Gioulia Romanidou, Eleni Fotiadou, Viki Rekari, Eleni Triantafyllidou, Stavroula Zisaki, Evi Kasmeridou, and et al. 2021. "The Prevalence of HCV RNA Positivity in Anti-HCV Antibodies-Negative Hemodialysis Patients in Thrace Region. Multicentral Study" Germs 11, no. 1: 52-58. https://doi.org/10.18683/germs.2021.1240
APA StyleKonstantinidou, E. I., Kontekaki, E. G., Kefas, A., Konstantinidis, T., Romanidou, G., Fotiadou, E., Rekari, V., Triantafyllidou, E., Zisaki, S., Kasmeridou, E., Andreadou, M., Kantartzi, K., Mavromatidis, K., Martinis, G., Cassimos, D., Thodis, E., Panopoulou, M., & Mimidis, K. (2021). The Prevalence of HCV RNA Positivity in Anti-HCV Antibodies-Negative Hemodialysis Patients in Thrace Region. Multicentral Study. Germs, 11(1), 52-58. https://doi.org/10.18683/germs.2021.1240




