Analysis of the Anti-Corrosion Performance of Dextrin and Its Graft Copolymer on J55 Steel in Acid Solution
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Experimental Chemicals and Materials
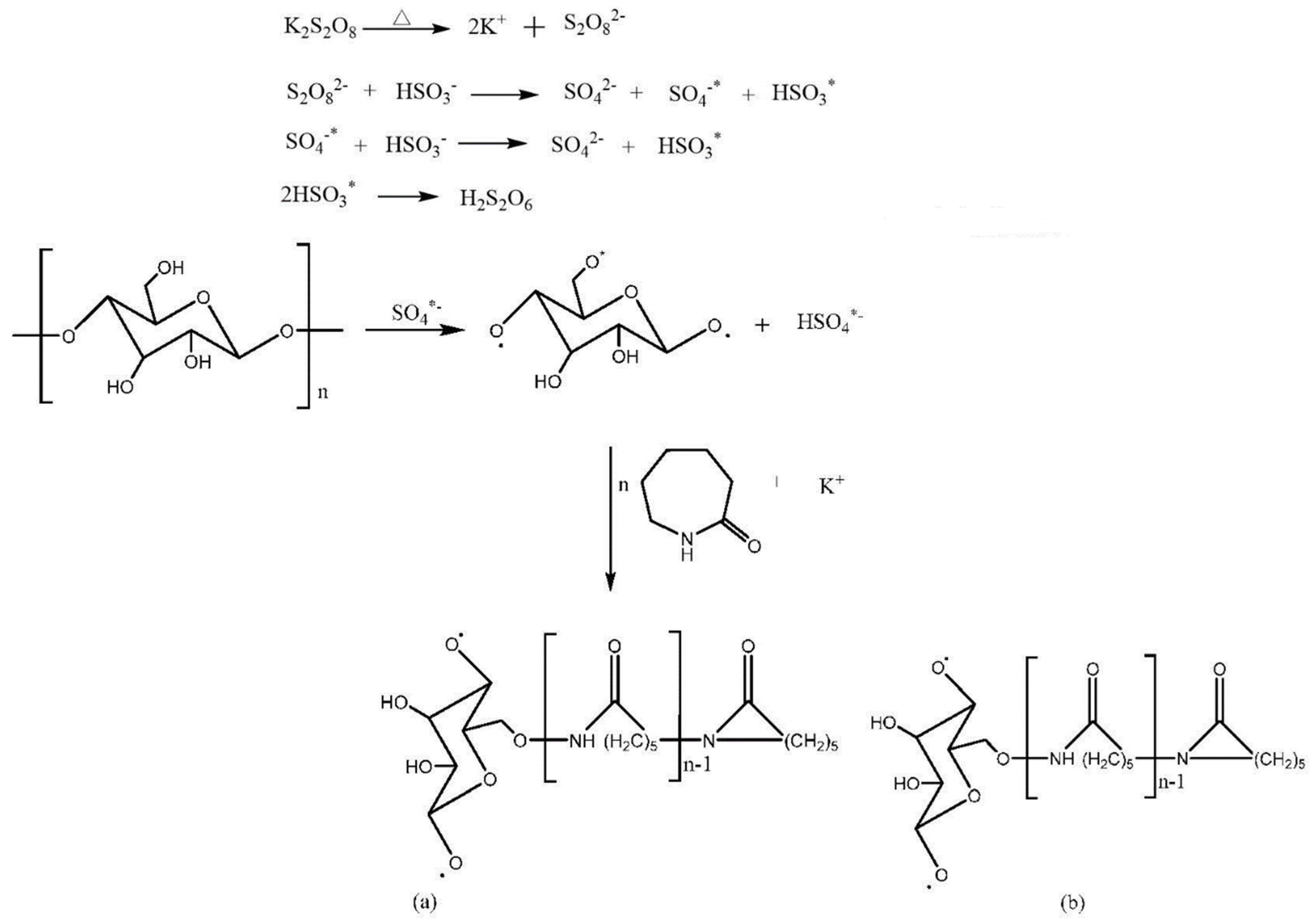
2.2. Synthesis of Dextrin Graft Copolymer
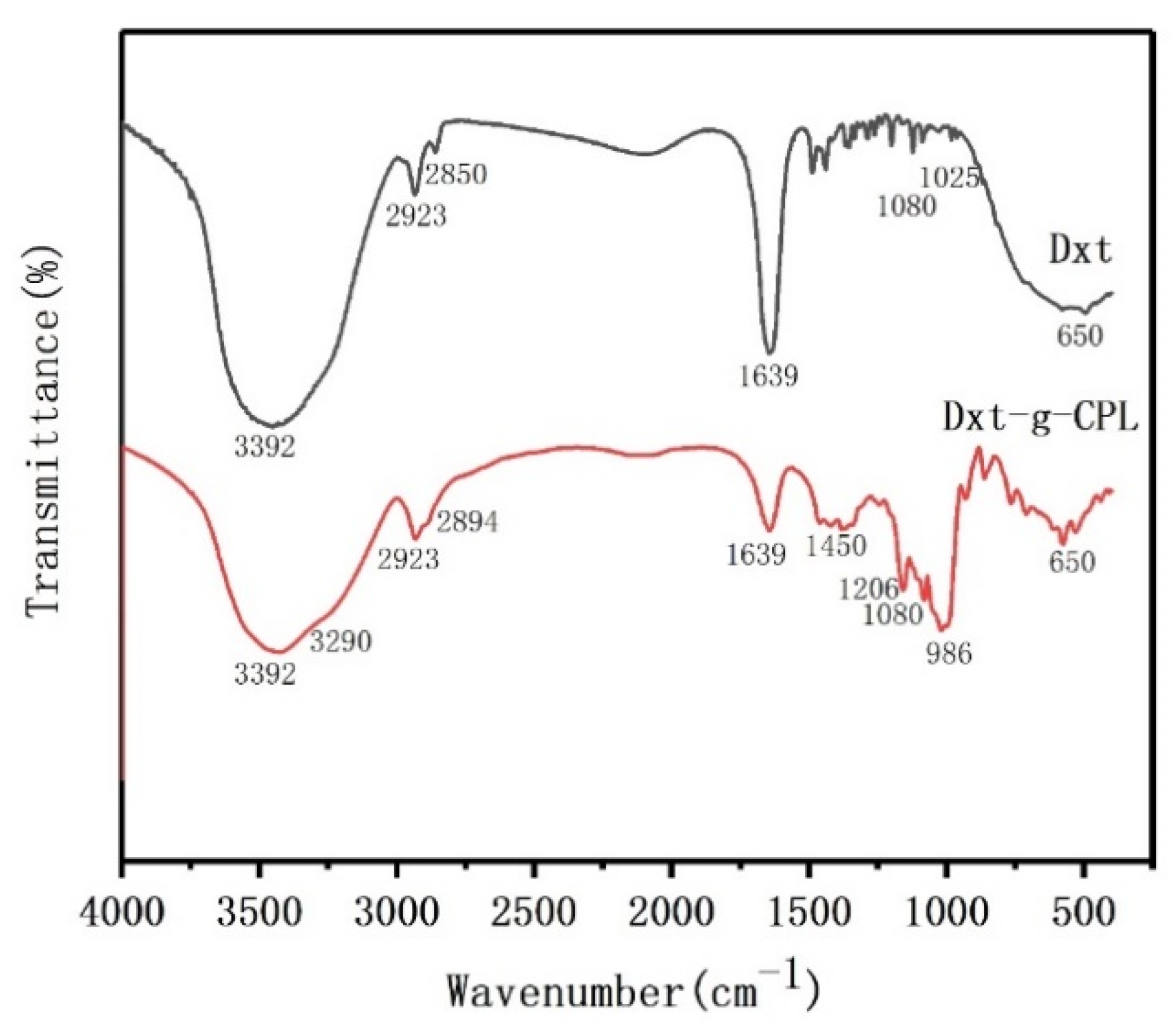
2.3. Structural Characterization of the Copolymer
2.4. Copolymer Corrosion Inhibition Performance Test
2.4.1. Static Weightlessness Test
2.4.2. Electrochemical Test
2.4.3. Scanning Electrochemical Microscope
2.4.4. Scanning Electron Microscope–Energy Spectrum Test
2.4.5. Contact Angle Test
3. Results and Discussion
3.1. Characterization of Dextrin Grafted Caprolactam Copolymer
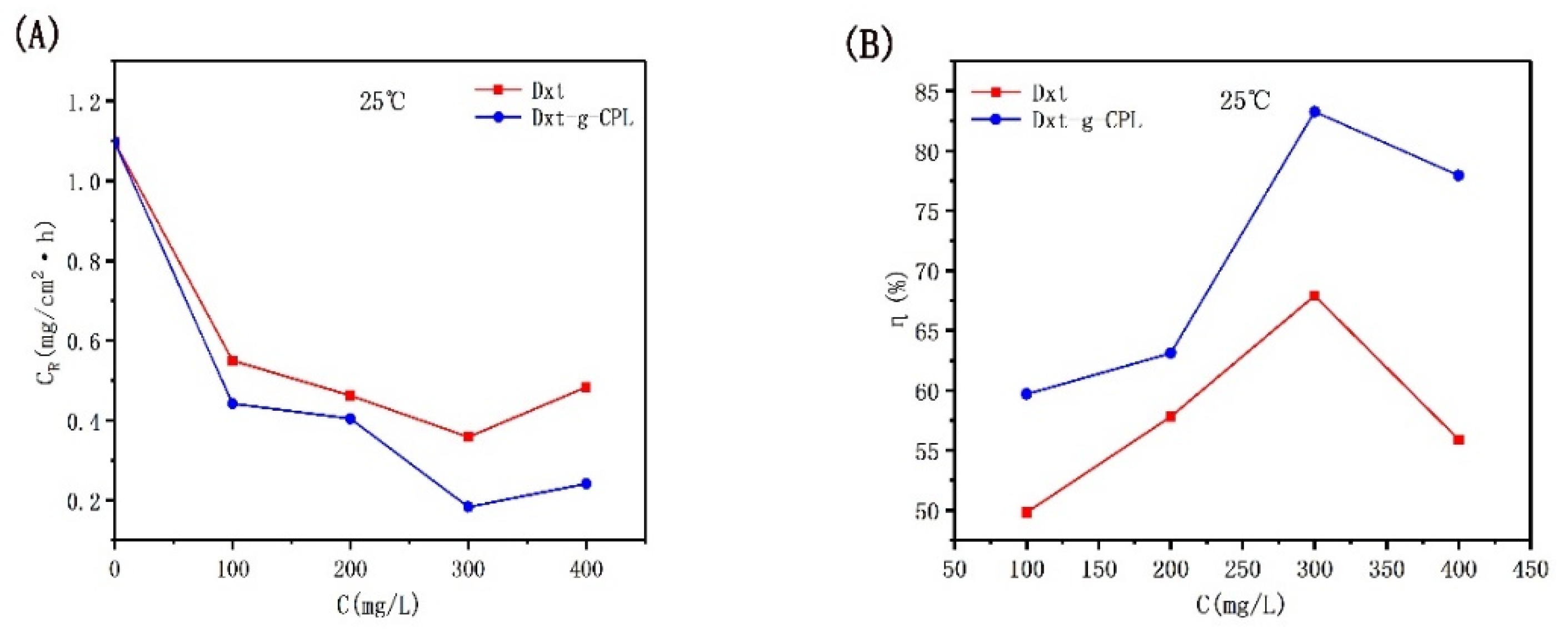
3.2. Weightlessness Experiment
3.2.1. The effect of Concentration
3.2.2. The Influence of Temperature
3.3. Electrochemical Studies
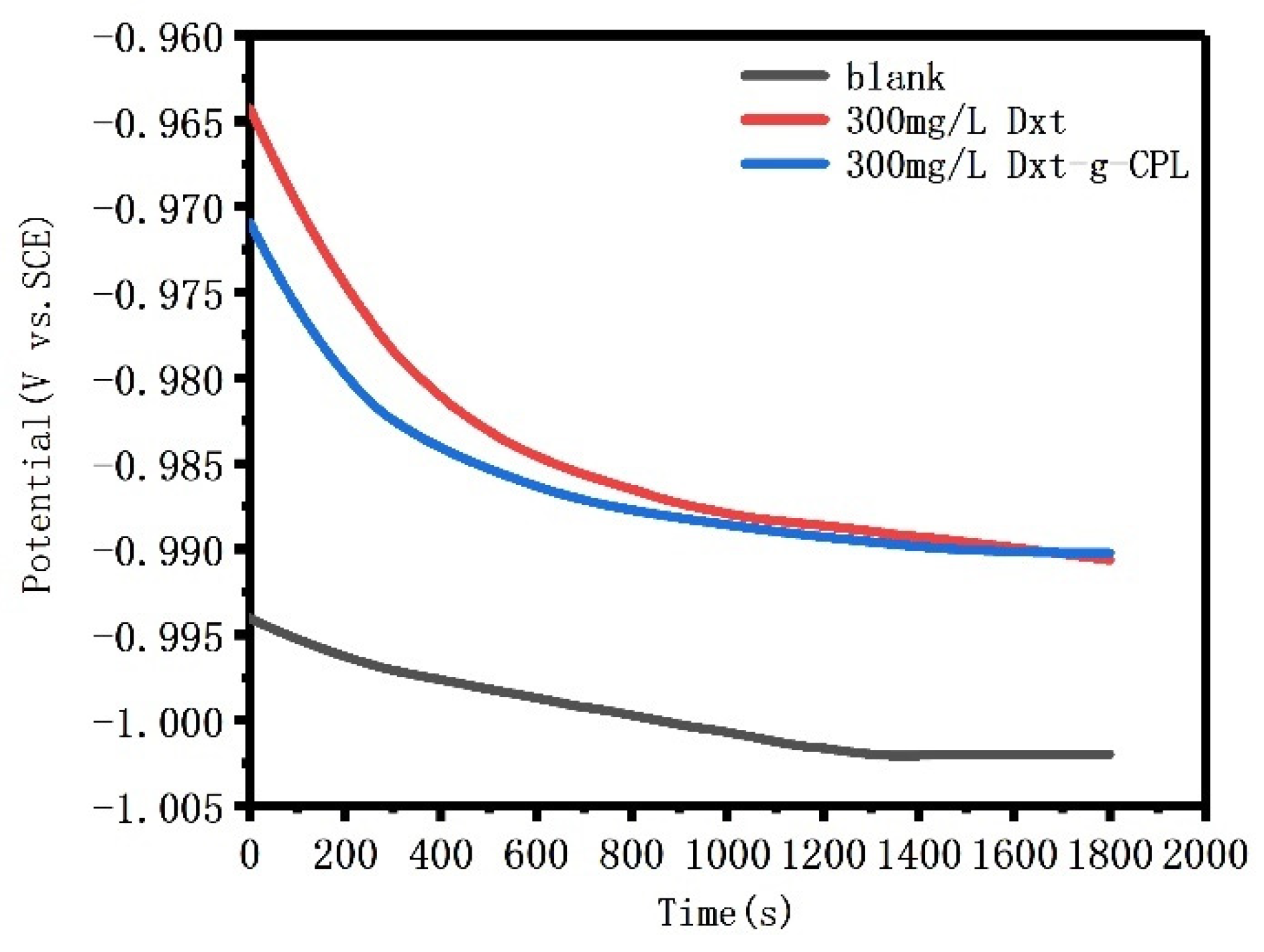
3.3.1. Open circuit Potential Analysis
3.3.2. Electrochemical Impedance Spectroscopy
3.3.3. Potentiodynamic Polarization Measurements
3.4. Scanning Electrochemical Microscope
3.5. Scanning Electron Microscope
3.6. Contact Angle Measurement
4. Analysis of Adsorption Mechanism
4.1. Adsorption Isotherm
4.2. Analysis of Corrosion Inhibition Mechanism
- (1)
- The electrostatic interaction between the corrosion inhibitor molecules and the metal surface makes the corrosion inhibitor molecules physically adsorb onto the metal surface;
- (2)
- The d orbitals in the atoms on the metal surface, and the electrons of the O and N atoms in the corrosion inhibitor molecules easily form coordination bonds, so that the corrosion inhibitor molecules are chemically adsorbed onto the metal surface;
- (3)
- The corrosion inhibitor molecule contains functional groups, such as hydroxyl and carbonyl, which can form coordination bonds with the metal surface.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achary, G.; Naik, Y.A.; Kumar, S.V.; Venkatesha, T.; Sherigara, B. An electroactive co-polymer as corrosion inhibitor for steel in sulphuric acid medium. Appl. Surf. Sci. 2008, 254, 5569–5573. [Google Scholar] [CrossRef]
- De la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Jeeva, M.; Prabhu, G.V.; Boobalan, M.S.; Rajesh, C.M. Interactions and Inhibition Effect of Urea-Derived Mannich Bases on a Mild Steel Surface in HCl. J. Phys. Chem. C 2015, 119, 22025–22043. [Google Scholar] [CrossRef]
- Döner, A.; Solmaz, R.; Özcan, M.; Kardaş, G. Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros. Sci. 2011, 53, 2902–2913. [Google Scholar] [CrossRef]
- Lahiri, A.K. Applied Metallurgy and Corrosion Control: A Handbook for the Petrochemical Industry Indian Institute of Metals Series; Springer: Berlin/Heidelberg, Germany, 2017; p. 276. [Google Scholar]
- Barmatov, E.; Geddes, J.; Hughes, T.; Nagl, M. Research on corrosion inhibitors for acid stimulation. In Proceedings of the CORROSION 2012, Salt Lake City, UT, USA, 11–15 March 2012. [Google Scholar]
- Papavinasam, S. Corrosion inhibitors. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; Wiley: Hoboken, NJ, USA, 2011; p. 1021e32. [Google Scholar]
- Dawson, J.L. Chemical treating in oil and gas production. In Shreir’s Corrosion; Richardson, T., Ed.; Elsevier: Oxford, UK, 2010; p. 2900e29. [Google Scholar]
- Abd El-Lateef, H.M.; Abbasov, V.M.; Aliyeva, L.I.; Ismayilov, T.A. Corrosion protection of steel pipelines against CO2 corrosion—A review. Chemother J. 2012, 2, 52. [Google Scholar]
- Balaskas, A.C.; Kartsonakis, I.A.; Snihirova, D.; Montemor, M.F.; Kordas, G. Improving the corrosion protection properties of organically modified silicate-epoxy coatings by incorporation of organic and inorganic inhibitors. Prog. Org. Coat. 2011, 72, 653. [Google Scholar] [CrossRef]
- Yuce, A.O.; Mert, B.D.; Kardas, G.; Yazıcı, B. Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2014, 83, 310–316. [Google Scholar] [CrossRef]
- Helal, N.H.; Badawy, W.A. Environmentally safe corrosion inhibition of Mg–Al–Zn alloy in chloride free neutral solutions by amino acids. Electrochim. Acta 2011, 56, 6581–6587. [Google Scholar] [CrossRef]
- Singh, A.; Soni, N.; Deyuan, Y.; Kumar, A. A combined electrochemical and theoretical analysis of environmentally benign polymer for corrosion protection of N80 steel in sweet corrosive environment. Results Physics. 2019, 13, 102–116. [Google Scholar] [CrossRef]
- Atta, A.; El-Azabawy, O.; Ismail, H.; Hegazy, M. Novel dispersed magnetite core–shell nanogel polymers as corrosion inhibitors for carbon steel in acidic medium. Corros. Sci. 2011, 53, 1680–1689. [Google Scholar] [CrossRef]
- Zou, C.; Yan, X.; Qin, Y.; Wang, M.; Liu, Y. Inhibiting evaluation of b-Cyclodextrin-modified acrylamide polymer on alloy steel in sulfuric solution. Corros. Sci. 2014, 85, 445–454. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 2019, 282, 366–384. [Google Scholar] [CrossRef]
- Okafor, P.; Ikpi, M.; Uwah, I.; Ebenso, E.; Ekpe, U.; Umoren, S. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2018, 69, 18–31. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel: Electrochemical and theoretical studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Mobin, M.; Basik, M.; Aslam, J. Pineapple stem extract (Bromelain) as an environmental friendly novel corrosion inhibitor for low carbon steel in 1 M HCl. Measurement 2018, 134, 595–605. [Google Scholar] [CrossRef]
- Benarioua, M.; Mihi, A.; Bouzeghaia, N.; Naoun, M. Mild steel corrosion inhibition by Parsley (Petroselium Sativum) extract in acidic media. Egypt. J. Pet. 2019, 28, 155–159. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Wang, F.; Jin, X.; Yang, T.; Cai, Z.; Zhang, J. Corrosion inhibition of mild steel in hydrochloric acid solution by quaternary ammonium salt derivatives of corn stalk polysaccharide (QAPS). Desalination 2015, 372, 57–66. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Ferreira, M.G.S. Chitosan as a smart coating for corrosion protection of aluminium alloy 2024: A review. Prog. Org. Coat. 2015, 89, 348–356. [Google Scholar] [CrossRef]
- Kumar, S.; Vashisht, H.; Olasunkanmi, L.; Bahadur, I.; Verma, H.; Singh, G.; Obot, I.B.; Ebenso, E.E. Experimental and theoretical studies on inhibition of mild steel corrosion by some synthesized polyurethane tri-block co-polymers. Sci. Rep. 2016, 6, 30937. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Eduok, U. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.E.; Jonathan, A.; Ameh, P.O.; Anya, C. A review on the assessment of polymeric materials used as corrosion inhibitor of metals and alloys. Int. J. Ind. Chem. 2013, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Biswas, A.; Das, D.; Lgaz, H.; Pal, S.; Udayabhanu, U.G. Biopolymer dextrin and poly (vinyl acetate) based graft copolymer as an efficient corrosion inhibitor for mild steel in hydrochloric acid: Electrochemical, surface morphological and theoretical studies. J. Mol. Liq. 2018, 275, 867–878. [Google Scholar] [CrossRef]
- Das, D.; Mukherjee, S.; Pal, A.; Das, R.; Sahu, S.G.; Pal, S. Synthesis and characterization of biodegradable copolymer derived from dextrin and poly(vinyl acetate) via atom transfer radical polymerization. RSC Adv. 2015, 6, 9352–9359. [Google Scholar] [CrossRef]
- Biswas, A.; Mourya, P.; Mondal, D.; Pal, S.; Udayabhanu, G. Grafting effect of gum acacia on mild steel corrosion in acidic medium: Gravimetric and electrochemical study. J. Mol. Liq. 2018, 251, 470–479. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Abdallah, M. Guar Gum as Corrosion Inhibitor for Carbon Steel in Sulfuric Acid Solutions. Port. Electrochimica Acta 2004, 22, 161–175. [Google Scholar] [CrossRef]
- Singh, A.; Mohamed, H.S.; Singh, S.; Yu, H.; Lin, Y. Corrosion inhibition using guar gum grafted 2-acrylamido-2-methylpropanesulfonic acid (GG-AMPS) in tubular steel joints. Constr. Build. Mater. 2020, 258, 119728. [Google Scholar] [CrossRef]
- Fiori-Bimbi, M.V.; Alvarez, P.E.; Vaca, H.; Gervasi, C.A. Corrosion inhibition of mild steel in HCL solution by pectin. Corros. Sci. 2015, 92, 192–199. [Google Scholar] [CrossRef]
- Solomon, M.; Umoren, S.; Udosoro, I.; Udoh, A. Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—inhibition of castiron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.S.; Ye, F.; Carrillo, E.A.T.; Leliopoulos, C.; Savaloni, H.; Dutta, J. Chitosan nanocomposite coatings with enhanced corrosion inhibition effects for copper. Int. J. Biol. Macromol. 2020, 162, 1566–1577. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A. Inhibition effect of natural polysaccharide composite on hydrogen evolution and P110 steel corrosion in 3.5 wt% NaCl solution saturated with CO2: Combination of experimental and surface analysis. Int. J. Hydrog. Energy 2020, 45, 25398–25408. [Google Scholar] [CrossRef]
- Kumar, K.V.; Appa Rao, V.B. Phosphorylated xanthan gum, an environment-friendly, efficient inhibitor for mild steel corrosion in aqueous 200 ppm NaCl. Mater. Today Proc. 2019, 15, 155–165. [Google Scholar] [CrossRef]
- Bertalan, G.; Ruszna, I.; Anna, P.; Boros-lvicz, M.; Marosi, G. Mechanism and kinetics of hydrochloric acid initiated e-caprolactam polymerization: 1. The role of stereoelectronic control and acid catalysis. Polym. Bull. 1988, 19, 539–546. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Ituen, E.; Guo, L.; Wahab, A.; Quraishi, M.; Kong, X.; Lin, Y. A new series of synthesized compounds as corrosion mitigator for storage tanks: Detailed electrochemical and theoretical investigations. Constr. Build. Mater. 2020, 259, 120421. [Google Scholar] [CrossRef]
- Daoud, D.; Douadi, T.; Hamani, H.; Chafaa, S.; Al-Noaimi, M. Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study. Corros. Sci. 2015, 94, 21–37. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Jeyaprabha, B.; Prakash, P. Adsorption and corrosion inhibiting behavior of Lanneacoromandelica leaf extract on mild steel corrosion. Arab. J. Chem. 2017, 10, 2343–2354. [Google Scholar] [CrossRef] [Green Version]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl Cellulose/Silver Nanoparticles Composite: Synthesis, Characterization and Application as a Benign Corrosion Inhibitor for St37 Steel in 15% H2SO4 Medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6379. [Google Scholar] [CrossRef]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochimica Acta 2003, 48, 2635–2640. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, Y.; Cao, Z. Performance and theoretical study on corros inhibition of 2-(4-pyridyl)-benzimidazole for mild steel in hydrochloric acid. Corros. Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Ituen, E.; Akaranta, O.; James, A.; Sun, S. Green and sustainable local biomaterials for oilfield chemicals Griffonia simplicifolia extract as steel corrosion inhibitor in hydrochloric acid. Sustain. Mater. Technol. 2017, 11, 12–18. [Google Scholar] [CrossRef]
- Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadi, M. Enhanced corrosion resistance of mild steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros. Sci. 2017, 114, 133–145. [Google Scholar] [CrossRef]


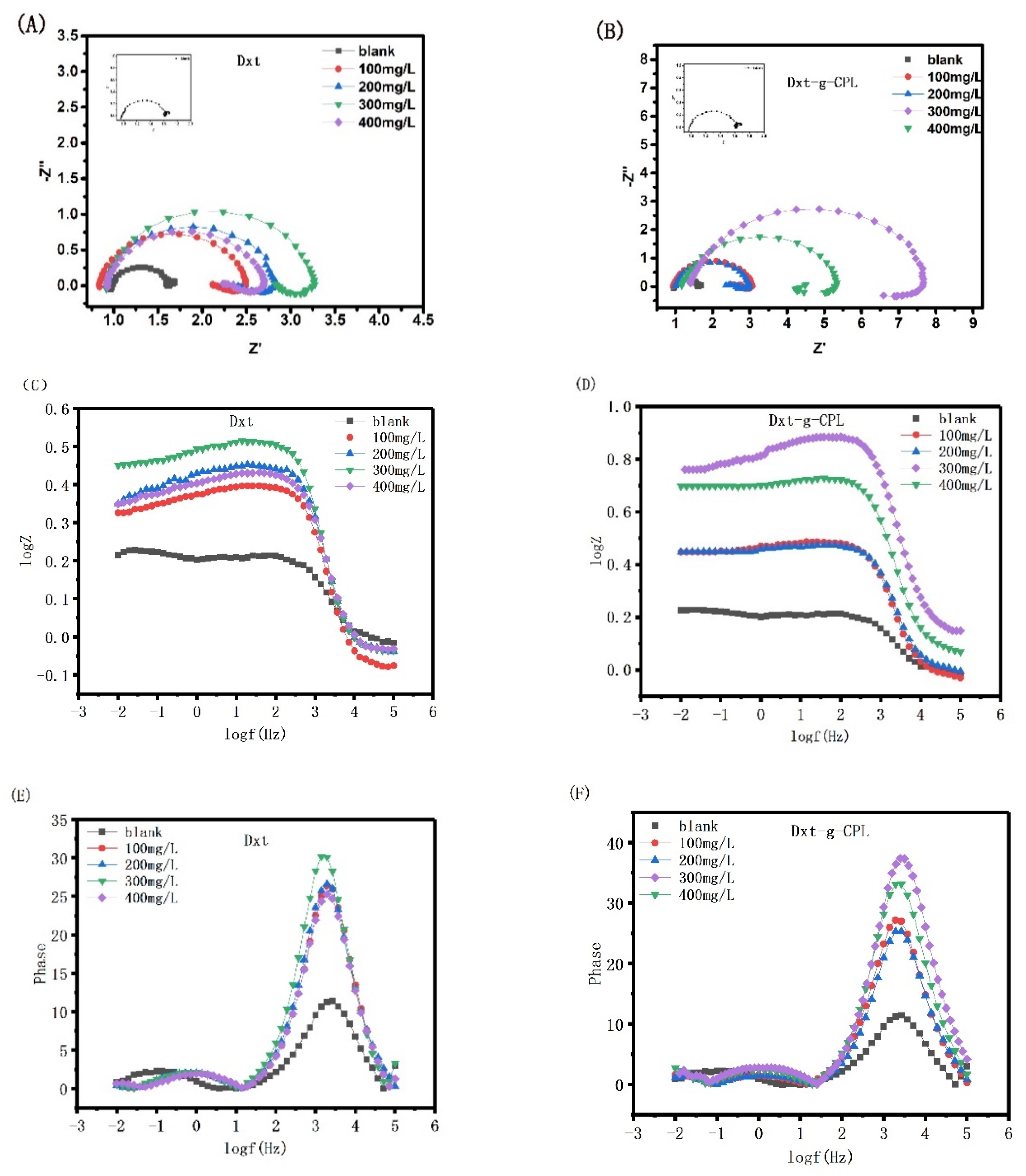

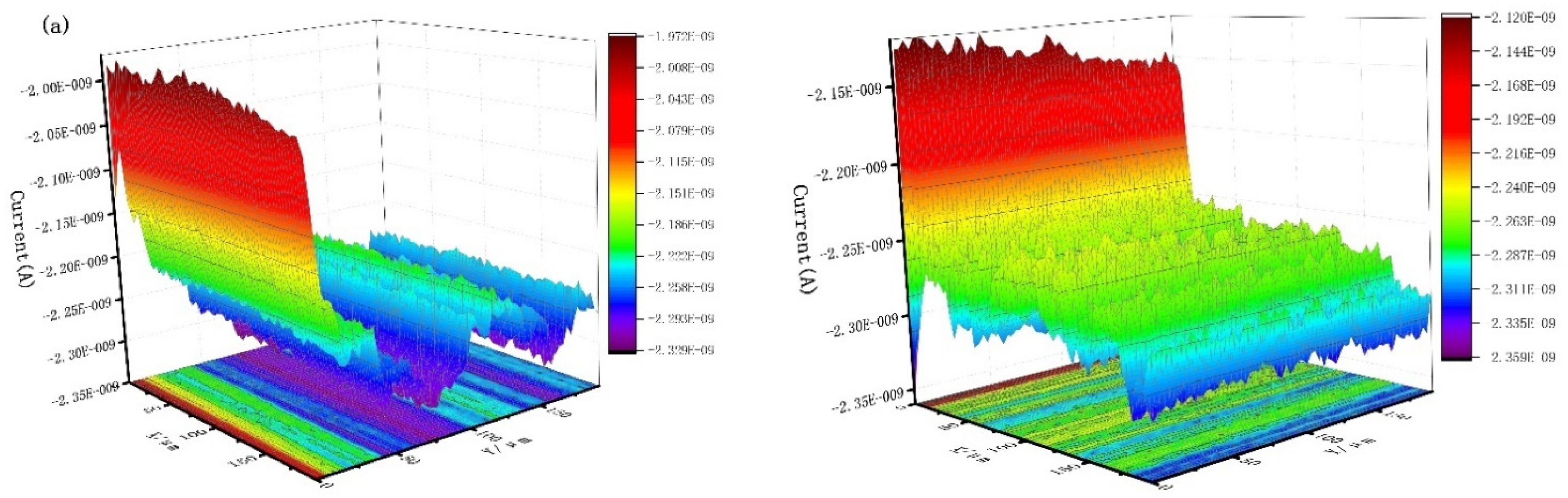
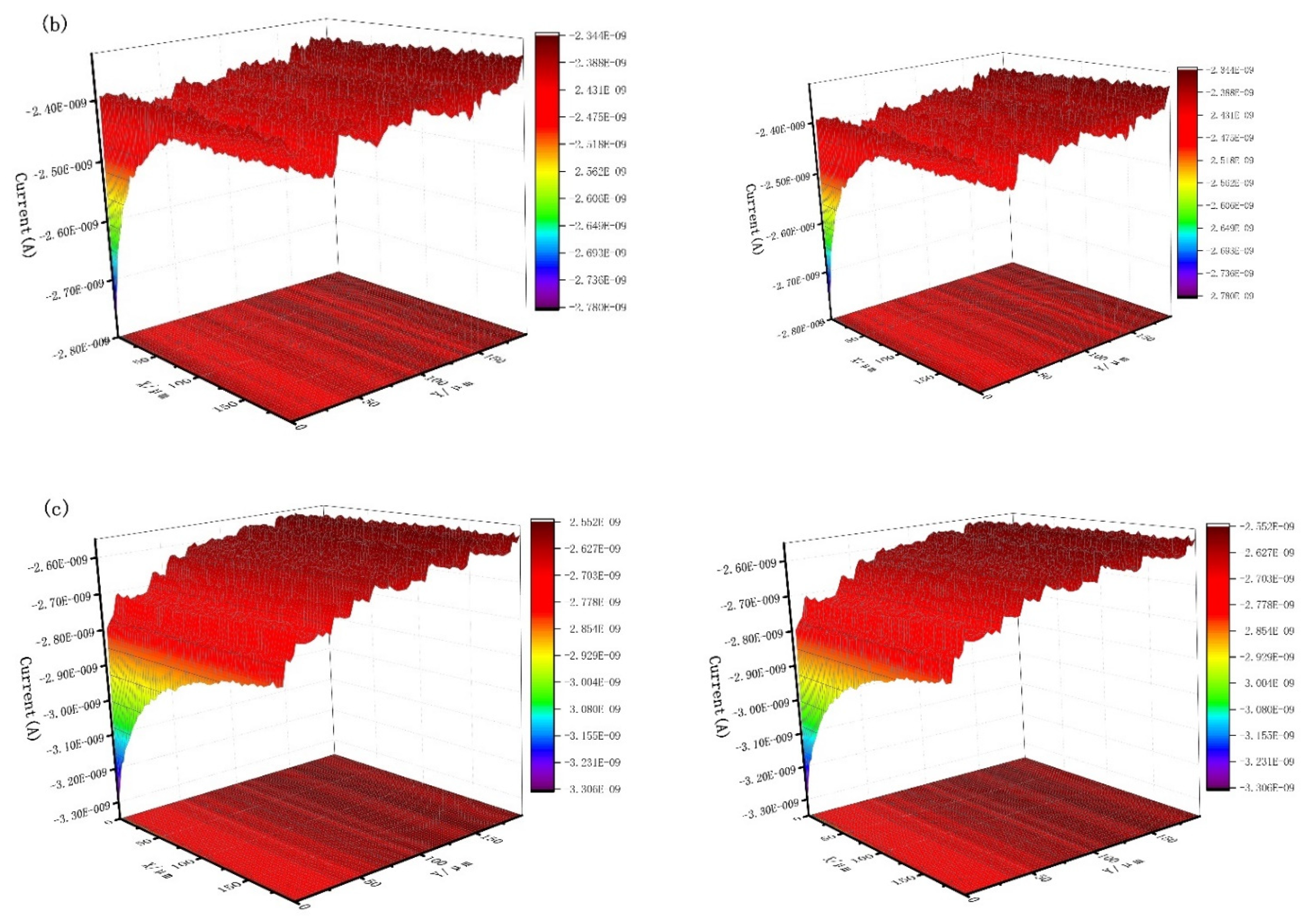
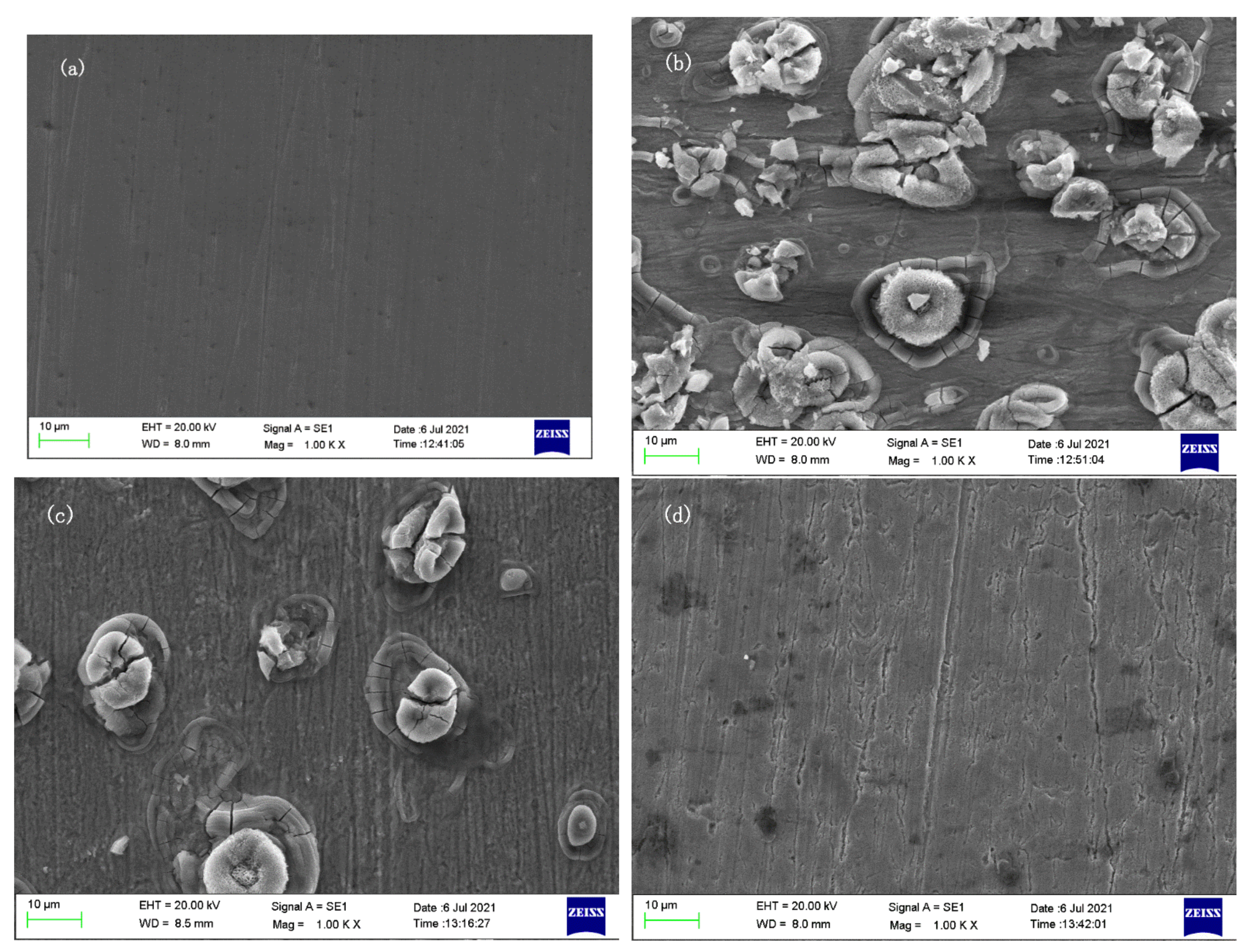


| Concentration (mg/L) | Rs (Ωcm2) | CPE | Rct (Ωcm2) | RL (Ωcm2) | L (H cm2) | ηR (%) | ||
|---|---|---|---|---|---|---|---|---|
| Q (mΩ−1 S n cm−2) | n | |||||||
| blank | 0.9811 | 0.6729 | 0.8527 | 0.6583 | 0.1635 | 0.00001168 | - | |
| Dxt | 100 mg/L | 0.8383 | 0.1644 | 0.9248 | 1.364 | 0.2732 | 0.06177 | 51.71 |
| 200 mg/L | 0.9205 | 0.1583 | 0.9237 | 1.502 | 0.3696 | 0.1143 | 56.17 | |
| 300 mg/L | 0.9227 | 0.1428 | 0.935 | 1.965 | 0.3519 | 0.07441 | 66.49 | |
| 400 mg/L | 0.9245 | 0.171 | 0.915 | 1.432 | 0.3141 | 0.05934 | 54.03 | |
| Dxt-g-CPL | 100 mg/L | 0.9484 | 0.1644 | 0.8994 | 1.606 | 0.2852 | 0.05247 | 59.01 |
| 200 mg/L | 1.004 | 0.1423 | 0.9073 | 1.811 | 0.3471 | 0.08547 | 63.65 | |
| 300 mg/L | 1.41 | 0.0463 | 0.9148 | 3.736 | 2.267 | 0.5813 | 82.38 | |
| 400 mg/L | 1.191 | 0.09234 | 0.8977 | 3.023 | 1.057 | 0.2105 | 78.22 | |
| Concentration(mg/L) | ba | bc | Ecorr(V/SCE) | Icorr(A/cm2) | ηR (%) | Surface Coverage(θ) | |
|---|---|---|---|---|---|---|---|
| blank | 0.1279 | 0.2638 | −1.010 | 0.0105 | |||
| Dxt | 100 mg/L | 0.1224 | 0.2133 | −0.997 | 0.00498 | 52.57 | 0.5257 |
| 200 mg/L | 0.1134 | 0.2099 | −0.997 | 0.00475 | 54.76 | 0.5476 | |
| 300 mg/L | 0.0903 | 0.1822 | −0.997 | 0.00338 | 67.81 | 0.6781 | |
| 400 mg/L | 0.1336 | 0.2022 | −0.998 | 0.00425 | 59.52 | 0.5952 | |
| Dxt-g-CPL | 100 mg/L | 0.1239 | 0.1996 | −0.999 | 0.0045 | 57.14 | 0.5714 |
| 200 mg/L | 0.1035 | 0.1823 | −0.999 | 0.00383 | 63.52 | 0.6352 | |
| 300 mg/L | 0.1392 | 0.1945 | −1.000 | 0.00196 | 81.33 | 0.8133 | |
| 400 mg/L | 0.0978 | 0.1661 | −1.010 | 0.00233 | 77.81 | 0.7781 | |
| Slope | R2 | Intercept | Kads | △GkJ/mol | |
|---|---|---|---|---|---|
| EIS | 1.0757 | 0.9691 | 70.88 | 0.01411 | −23.68 |
| TAFEL | 1.0714 | 0.9733 | 75.37 | 0.01327 | −23.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Xia, D.; Singh, A.; Lin, Y. Analysis of the Anti-Corrosion Performance of Dextrin and Its Graft Copolymer on J55 Steel in Acid Solution. Processes 2021, 9, 1642. https://doi.org/10.3390/pr9091642
Liu M, Xia D, Singh A, Lin Y. Analysis of the Anti-Corrosion Performance of Dextrin and Its Graft Copolymer on J55 Steel in Acid Solution. Processes. 2021; 9(9):1642. https://doi.org/10.3390/pr9091642
Chicago/Turabian StyleLiu, Mingxing, Dayu Xia, Ambrish Singh, and Yuanhua Lin. 2021. "Analysis of the Anti-Corrosion Performance of Dextrin and Its Graft Copolymer on J55 Steel in Acid Solution" Processes 9, no. 9: 1642. https://doi.org/10.3390/pr9091642





