1. Introduction
Cancer is characterized as a disease with the uncontrolled division of cancerous cells, which often metastasize into other organs [
1]. It is the second leading cause of death globally and holds responsibility for an estimated 9.6 million deaths in 2018 [
2]. Most commonly diagnosed cancer is lung cancer, followed by female breast cancer, prostate cancer, colorectal cancer, stomach cancer, liver cancer, and skin melanoma [
2]. Different chronic infections, including human papilloma virus (HPV),
Helicobacter pylori, hepatitis B, and hepatitis C virus, are also risk factors for cancer and have a major relevance in low- and middle-income countries [
2]. Slovenia is in the top third of European countries in terms of death rates due to cancer [
3]; aging population, lack of physical activity, tobacco and alcohol use, high body mass index, and polluted environment are one of the main reasons why more and more people are suffering from cancer. Second plant metabolites are already considered as preventive agents in terms of cardiovascular, neurodegenerative diseases, and cancer. This fact is derived from epidemiologic data as well as from in-vitro and in-vivo studies [
4,
5,
6,
7] and results in respective nutritional recommendations.
Additionally, the activity of numerous enzymes and cell receptors may be affected by phenolic compounds. The aim of the study was to evaluate possible substances derived from the natural materials that are widely available in the phytochemical region of Central Europe. These substances should represent an interesting product for the pharmaceutical industry. As already known, taxol (paclitaxel) is a natural compound used as a chemotherapy drug used to treat different cancers, including ovarian, breast, and non-small cell lung cancer. However, undoubtedly, several pre-clinical and later clinical studies are needed so that the substance could be applied for medical treatment. Thus, the identification, research, and development of compounds with such properties has become a major area of experimental cancer research. Phenolic compounds are known of their safety and low toxicity, and in general, are an important constituent of the human diet. Several studies involving polyphenols isolated from different plant species have recently been related to modulation of carcinogenesis [
8]. Bioproperties of
S. tectorum have attracted limited scientific investigation in terms of antioxidant, anti-inflammatory, cytostatic, and cytotoxic activities of crude extracts or of pure components [
9,
10,
11]. Despite the information about
S. tectorum’s anticancer properties, such as effect of extracts on the metabolic activity of human melanoma cells, the literature regarding it is very poor, especially concerning the method of extract preparation, antioxidant activity, and the content of phenolic compounds. Melanoma cells have been taken under research since melanoma is a skin tumor whose diffusion is rapidly increasing in the world and whose malignancy is reinforced by its high resistance to cytotoxic agents; hence, the availability of new cytotoxic drugs would be very helpful to improve melanoma prognosis. There are two common approaches for obtaining biological substances. The first approach to obtain mentioned substances is through different extraction methods from plant materials, while the second uses biotechnological tools to produce desired compounds from plants. The substances of natural origin and with anti-cancer properties come from different groups of compounds, including alkaloids, peptides, proteins, diterpenes, macrocyclic polyethers, purine-based compounds, etc. Many studies have shown that many of the substances that were considered as anti-cancer agents exhibit anti-microbial activities as well. Said microorganisms can reside within plants without injuring them or causing any diseases but also have the ability to shield plants from colonization by pathogenic microorganisms. For this reason, our primary interest was to investigate how the extracts of the plant
S. tectorum (common houseleek) affect the metabolic activity of human melanoma cell line WM-266-4.
S. tectorum is an evergreen plant belonging to a large family of Crassulaceae [
12]. It is easily recognized by its distinctive fleshy and pointed leaves, which form a rosette and prevent water from evaporating [
12]. It is not sensitive to cold or drought and can grow on sandy soils even in the worst climatic conditions [
13]. S. tectorum is widely known as a folk medicine, and its juice is still used today for healing wounds, burns, and insect bites as a refrigerant and astringent [
14]. Tea can be made from the prepared leaves, which is recommended for the treatment of ulcers [
14]. In Italy, the beaten aerial part of leaves placed on the brow has been reported to reduce the intensity of headaches [
15]. However, the most common use of
S. tectorum is to relieve ear infections and to soften earwax [
16].
In our study, the utilized techniques for extraction of S. tectorum contained conventional methods, such as Soxhlet, cold solvent, and ultrasound extraction, and high-pressure methods, including supercritical fluid extraction method with CO2. Conventional extraction methods are based on evaporation of solvent (Soxhlet extraction), which has an indirect contact with material, material/solvent stirring (extraction with cold solvent), and formation of the bubbles (ultrasound extraction), which disrupt cellulosic cell walls from plants and consequently acquire active components easily. In conventional extraction methods, various solvents were used, including ethanol, methanol, water, and acetone. For supercritical extraction, CO2 was used, which represents a common usage in supercritical methods and can extract active compounds with similar polarity. The obtained extracts with different contained compounds were compared based on their antioxidativity as well as contents of polyphenols and proanthocyanins.
Human melanoma cells represent one of the most aggressive forms of cancer cells. The ability of these cells is to invade different tissues [
17]. As a useful experimental model tissue for determining genetic and biochemical changes in tumor development recently, metastatic human melanoma was utilized [
18]. Although melanoma is curable in the early stages, surgical interventions are practically ineffective in preventing metastatic disease and death in patients with advanced stages of malignant melanoma [
19]. These findings highlight the need for improved therapeutic approaches to treat patients with metastatic melanoma more effectively [
19]. In our study, the WM-266-4 metastatic cell line was used.
Research of
S. tectorum leaves has reported lipid-lowering effect in rats [
20]. In other studies, promising antioxidative properties were shown [
10,
21]. Its liver-protecting and membrane-stabilizing effects, which have been attributed to antioxidant activity, have been described [
22,
23]. M. Florin et al. described the protective effects of aqueous extract of
S. tectorum on aluminum-induced oxidative stress in rat blood, indicating its good antioxidant properties [
24]. Some articles have shown that leaf juice from
S. tectorum contains polyphenol compositions [
16] and high flavonoid content [
25]. F. Cattaneo et al. demonstrated the therapeutic effects of common houseleek, and also wound healing activity was measured [
12]. Recently, there were several studies of antinociceptive activity [
16], and both antimicrobial [
26] and antibacterial [
27] effect on various microorganisms was reported. However, no research on the influence of the extracts of
S.tectorum on human melanoma cells has been found in the literature. Therefore, we wanted to test whether the extracts obtained during our research had an effect on the metabolic activity and inhibited the growth of cancer cells, specifically on human metastatic cell line WM-266 4.
The aim of this study was to obtain extracts from S. tectorum leaves using Soxhlet, cold solvent, ultrasound, and supercritical extraction methods with different solvents. We wanted to show the differences in yield and their composition, especially in content of active components, such as polyphenols, proanthocyanins, and antioxidants. Our conventional and supercritical extracts from SLO lyophilized S. tectorum were compared to CHI dried S. tectorum. Contents of active compounds were determined with UV-VIS spectrophotometer and DPPH, TPC, and PAC assays. Lastly, selected extracts were used for WST-8 test analysis to study the possible inhibitory effects on metabolic activity of human melanoma cells WM-266-4.
For the best of our knowledge, up to now, there has been no research done about the influence of S.tectorum extracts on metabolic activity of human melanoma cells. Our group was the first to check and confirm the inhibitory effect of S. tectorum extracts on WM-266-4 human melanoma cells, which gives our study extremely high importance. Though it is unclear which components of S. tectorum possess inhibitory effects on metabolic activity of melanoma cells, our work presents a novel, natural chemotherapeutic agent that may be extended to other chemo-resistant cancer lines. The study is a preliminary screening of substances that can be isolated from the materials, which are available in the nature. These substances may pose several biological effects, but much more must be done to realize the final application in humane medicine due to all pre-clinical and clinical studies required before authorization for use in human medicine.
2. Materials and Methods
2.1. Chemicals and Plant Material
Samples of S. tectorum from two different regions were examined. The first half was cultivated and dried in Shanghai, China. The second half of plant material was collected locally in early summer and lyophilized at −30 °C and 5 Pa with Kambič Lio 2000 PNS.
Methanol, ethanol, and acetone (purity 99.9%), used as solvents for conventional extraction, were purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Gallic acid, Folin-Ciocalteu reagent (FCR), sodium carbonate, n-butanol, hydrochloric acid, iron sulfate heptahydrate, and 2,2-diphenyl-1-picrylhydrazyl (DPPH), used for spectrophotometric analysis, were supplied from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Colorimetric cell viability kit I (CCVK-1), HEPES buffered saline solution, and trypsin neutralizing solution (TNS) for analysis of metabolic activity were obtained from PromoCell GmbH (Heidelberg, Germany).
2.2. Lyophilization Process
Lyophilization, also known as freeze-drying, is known as a low-temperature dehydration process, which differs from most conventional drying methods that evaporate water with the use of heat. It consists of three major steps, including freezing the product, lowering pressure, and lastly removing the formed ice by sublimation. It shows many advantages compared to heat-drying processes, such as the drying ability of thermolabile substances, maintaining the chemical stability and biological activity of the substances, and tolerating temperature changes of lyophilized products [
28].
For the research, S. tectorum from two different locations (Shanghai CHI and Maribor SLO) was used. They both differed in preparation method: CHI material was air dried, while SLO S. tectorum was lyophilized. At the beginning, the material was put into the big steel plates and placed into the lyophilizer Kambič Lio 2000 PNS. First and foremost, temperature was lowered to −30 °C. With the temperature condition met, the pressure was then lowered to 0.05 mbar and the plant material was left to lyophilize at these parameters for two weeks. Lastly, the pressure was slowly increased to atmospheric value, and the lyophilized S. tectorum leaves were taken out of steel plates and were carefully stirred in a blender.
Lyophilization is ideal for drying the materials containing components that are thermosensitive and could be denatured with the use of drying processes depending on heat [
29].
2.3. Conventional Extraction Methods
2.3.1. Soxhlet Extrction
Soxhlet extraction was performed using ISOLAB NS29-32 Soxhlet apparatus. To obtain the extract from Soxhlet extraction,
S. tectorum leaves (10 g) were extracted with the solvent of choice (150 mL); the material:solvent ratio was 1:15. Ethanol, methanol, hexane, acetone, and two mixtures of ethanol and water (EtOH:H
2O = 7:3 and 1:1) were used for extraction since we wanted to compare total phenol and proanthocyanin contents between the extracts acquired with different solvents. The heating temperature during the extraction was adjusted to the used solvent, and the extraction time was 5 h [
30].
2.3.2. Extraction with Cold Solvent
Extraction by pouring the cold solvent on the material was executed with the magnetic stirrer. Unlike the Soxhlet extraction, in this case, the material was in direct contact with the solvent. A total of 10 g of
S. tectorum leaves were weighed and were put in the glass flask. Then, 150 mL of selected solvent was poured over the plant material and was mixed on the magnetic stirrer for 5 h [
31].
2.3.3. Ultrasound Extraction
Ultrasound extraction was carried out using VEVOR Digital Ultrasonic Bath Digital Pro+. The same amount of
S. tectorum leaves and solvent were used for each extraction, as in both previous extraction methods. The extraction proceeded at room temperature for 1 h [
32].
2.3.4. Supercritical Extraction with CO2
First and foremost, the continuous high-pressure apparatus was filled with S. tectorum leaves (10 g) and was put in preheated water bath (40 °C). Autoclave was connected with the gas pump, which pumped the CO2 inside to the desired pressure (15 and 25 MPa). Supercritical conditions were achieved, and consequently, nonpolar components were extracted. The extraction with supercritical CO2 (sc CO2) lasted for 1 h, and the material: solvent ratio was 1:8.
2.4. Sample Preparation for Conventional Extraction Methods
S. tectorum leaves were powdered in a blender and submitted to Soxhlet, cold solvent, and ultrasound extraction methods with different solvents as a comparison. After the certain period of selected conventional extraction, the extracts were filtered, and then the filtrates were collected. The solvent was evaporated from the filtrate with rotary evaporator BÜCHI Waterbath B-480. Extracts were stored at −20 °C until they were used for further analysis. Powdered leaves were also used for supercritical extraction with CO2 as the chosen solvent. Gathered pure extracts, obtained with sc CO2, were stored at the same conditions as conventional extracts.
2.5. Solution Preparation for Cell Assay
For metabolic activity assay, 7 extract solutions of concentrations—100, 50, 20, 10, 5, 1, and 0.1 mg/mL—were prepared. For the first solution 100 mg of extract was weighed, and then 1000 µL of distilled water was added. Then we pipetted 250, 100, 50, and 25 µL from the first solution, and afterwards 250, 400, 450, and 475 µL of distilled water was added. Consequently, the second, third, fourth, and fifth solutions were obtained. For the sixth solution, 50 µL was pipetted from the fourth solution, and after that, 450 µL of distilled water was added. Finally, for the seventh solution, 50 µL was pipetted from the sixth solution, and thereafter, 450 µL of distilled water was added.
2.6. Cell Cultivation
After sufficient cell multiplication, the cell culture medium was removed. Initially, 15 mL of HEPES buffer was added to the cell line and removed after 10 s. Thereafter, 15 mL of trypsin solution was added. Finally, the 15 mL of TNS solution that neutralized trypsin was added. Centrifugation of the suspension for 7 min at 1500 rpm followed. As a result, the cells settled to the bottom to form a sediment, and TNS and trypsin remained in the liquid phase. Liquid phase was removed, and 4 mL of fresh medium was added to the cells.
After 24-h incubation, cell medium from wells was removed, and diluted extracts were added. The solutions of the extracts were further diluted with cell medium to prevent cell death in a short time because of the shock at high extract concentrations. As we pipetted 120 µL of extract solution, 480 µL of cell medium was added, and therefore, new extract concentrations were obtained: 20, 10, 4, 2, 1, 0.2, and 0.02 mg/mL. Solutions (marked with letters from A to H) represent the type of extract that was used for cells on 96-well plate, and the number next to the letter (1 to 7) represent a certain extract concentration. For the control solution, where only cell medium was added to the cells, only 100 µL of cell medium was pipetted into wells and was marked with letter K. It served as a metabolic activity comparison to the cells containing the extracts.
Subsequently, 100 µL of the diluted extract solution was pipetted into each well on the 96-well plate. For repeatability of the results, the same concentration and amount of diluted extract solution was pipetted into 5 consecutive wells (pentaplicate). The blue line indicates wells where cells were not present, and only 100 µL of distilled water was pipetted.
2.7. Analytical Methods
2.7.1. Total Phenolic Content
The total phenolic content (TPC) of extracts was expressed as gallic acid (GA) equivalent (mg GA/g of raw material) and determined by UV spectrophotometry (Varian Cary 50 UV-VIS) using the Folin–Ciocalteu (FC) method completely described by Anokwuru et al. [
33].
2.7.2. Proanthocyanin Content
The content of proanthocyanins (PAC) in extracts was determined by UV spectrophotometry method (Varian Cary 50 UV-VIS) based on acid hydrolysis and color formation as reported by Škerget et al. [
34].
2.7.3. Antioxidative Activity with the Radical Method
The antioxidative activity of the extracts was determined using 2,2 diphenyl picrylhydrazyl (DPPH) free radical described by Knez M. et al. (2016) [
35].
2.7.4. WST Test
WST-8 test (Promo Kine, Germany) was used to determine the metabolic activity of human melanoma cells WM-266-4. For this analysis, the tetrazolium salt of WST-8 (2-(2-methoxy-4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium-sodium salt) was used. In the presence of an electron carrier, the colorimetric salt permeating into the cytosol of a metabolically active cell is reduced to a water-soluble formazan of yellow color, which is soluble in the tissue culture medium. The number of cells seeded per well was 1 × 10
4. The number of living cells is thus directly proportional to the amount of formazan produced by the addition of tetrazolium salt of WST-8. CCVK-1 reagent was used since the solution mixing was not necessary and therefore was added directly to the cells [
36].
After 24 h of incubation with extract solution, washing with fresh cell medium, pipetting CCVK-1 reagent to each well, and following 2-h incubation, the absorbance was measured at 450 nm with spectrophotometer (Biotek SYNERGY 2). Our reference was measured at 650 nm. The results were presented as percentage of metabolic activity according to the control sample [
36].
2.8. Statistical Analysis
The distribution of TPC, PAC, and antioxidant activity measurements, carried out in triplicates, was verified by Shapiro–Wilk statistical test. Based on the results, nonparametric statistical tests were selected for the analysis of differences between material origin and extraction methods. Statistical differences between Slovenian lyophilized S. tectorum and Chinese dried S. tectorum were verified using the Mann–Whitney Wilcoxon nonparametric statistical test, while statistical differences by extraction methods were verified using the Kruskal–Wallis Statistical Test.
(Numerical variables are described by mean (±standard deviation) and in case of abnormal distribution, by median (interquartile range).)
2.8.1. Total Phenols
The Mann–Whitney Wilcoxon test showed a significant difference (W = 43, p < 0.001) between TPC found in lyophilized Slovenian S. Tectorum and Chinese dried material. The p-value of the Kruskal–Wallis is less than the significance level (χ2 = 12.222, p < 0.001), which confirms significant differences between TPC content by different extraction methods.
2.8.2. Proanthocyanins
Mann–Whitney Wilcoxon test confirmed a significant difference (W = 4, p < 0.001) between PAC found in lyophilized Slovenian S. Tectorum and Chinese dried material. The Krukal–Wallis test showed a significant difference between PAC in extracts obtained by different extraction methods (χ2 = 23.455, p < 0.001).
2.8.3. Antioxidant Activity
A Significant difference between antioxidant activity determined in lyophilized Slovenian S. Tectorum and Chinese dried material was confirmed with Mann–Whitney Wilcoxon test (W = 18, p < 0.001). While differences between the antioxidant activity of extracts by extraction groups were confirmed with Kruskal–Wallis test (χ2 = 18.984, p < 0.001).
3. Results and Discussion
S. tectorum from two different locations (Shanghai CHI and Maribor SLO) was used. They both differed in preparation method: CHI material was air dried, while the local SLO S. tectorum was lyophilized. Heat drying is commonly known as a very fast method, but it can cause denaturation of active components because of the possible present high temperatures. Therefore, it is recommended that only thermostable components for heat drying are used. On the other hand, the reason why we used freeze-drying is because its drying is very effective and does not use high temperatures. Additionally, we wanted to make a comparison in content of active components, including polyphenols, proantocyanidins, and antioxidants, from both plants, which were dried with two different processes.
3.1. Total Phenolic Content
Polyphenolic compounds are aromatic hydroxylated compounds that can be found in many foods, such as fruits, vegetables, tea, coffee, etc. The most known polyphenols are flavonoids, anthocyanides, and resveratrol. Total phenolic content was determined mainly due to its high antimicrobial, antioxidant, and antitumor activity. Polyphenols from
S. tectorum can contribute to the research as components for preventing and treating well known diseases, such as cancer [
35]. For this reason, the content of polyphenols in our plant extracts was determined.
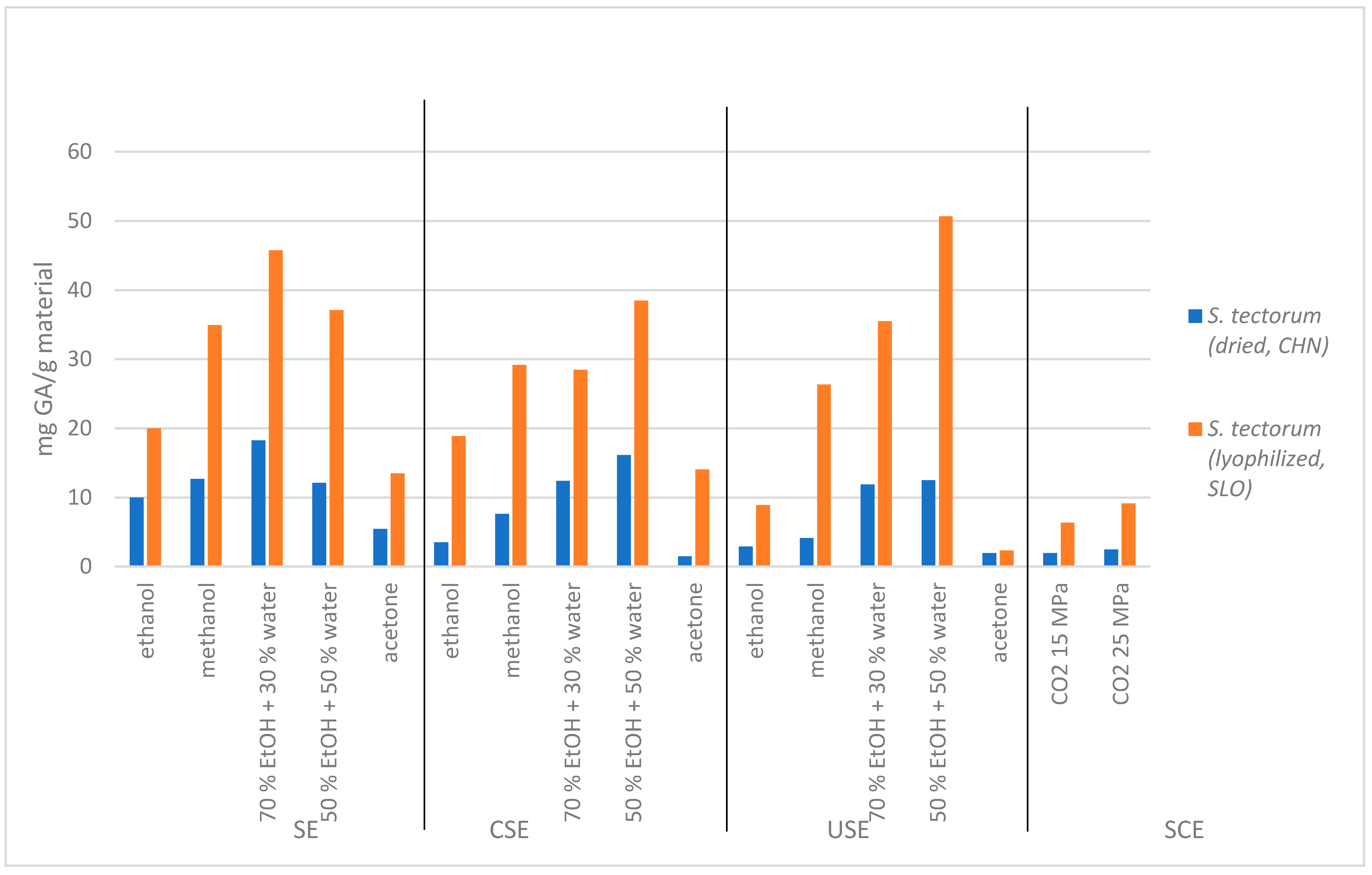
The total phenolic content (TPC) in
S. tectorum extracts acquired with Soxhlet, cold solvent, ultrasound, and supercritical extraction methods and solvents can be seen in
Figure 1. The results show that the highest TPC was found in lyophilized SLO
S. tectorum (up to 50.66 mg GA/g material), while in CHI dried material, there was significantly lower content of total phenols (up to 18.24 mg GA/g material). The lowest TPC in CHI
S. tectorum was obtained with cold solvent extraction using acetone, which yielded 1.46 mg GA/g material. Supercritical extracts of CHI dried
S. tectorum also showed very low TPC (1.96 mg GA/g material for extraction at 15 MPa and 2.47 mg/g material for 25 MPa) in comparison to supercritical extracts obtained from SLO
S. tectorum (6.33 mg GA/g material for extraction at 15 MPa and 9.15 mg GA/g material at 25 MPa). Supercritical extracts in general showed very low TPC compared to extracts obtained with conventional methods. This proves that extracts obtained with polar solvents were much richer in polyphenolic components than extracts obtained with nonpolar solvent. In addition, extraction yields obtained by supercritical extraction were low, which means that the content of nonpolar substances in
S. tectorum is very low. With the comparison of TPC in supercritical extracts gained with different pressure parameters, we assumed that slightly higher extract yields and consequently higher TPC can be obtained with higher pressure of CO
2 (25 MPa) than lower one (15 MPa) for both CHI and SLO
S. tectorum extracts but still not enough to be worth continued study. On the other hand, the highest amount of TPC in the dried material, namely 18.24 mg GA/g material, was determined in extract retrieved with Soxhlet extraction method and mixture ethanol:water = 7:3. In common, all of SLO
S. tectorum extracts showed significantly higher contents of polyphenols compared to the CHI extracts acquired with the same extraction methods and solvents. Our results show that the best choice to achieve the highest amount TPC is the use of ultrasound extraction method and mixture ethanol:water = 1:1, which is ideal since these solvents are most preferable in food applications. In the case of ultrasound extraction with acetone as a solvent, the lowest content of total phenols was obtained (2.32 mg GA/g material); therefore, this is not the appropriate alternative for extraction of polyphenolic compounds.
We assume that CHI dried material showed lower TPC because of the different climatic conditions where the plant was cultivated. This can be the main reason why a certain plant has enormous differences in the amount of certain active substances. Preparation process can be the other reason why there was such a big difference in total phenolic content. With drying at high temperature, the active compounds disintegrated, while with lyophilization high temperatures were not used, and therefore, most compounds retained their original activity. We may confirm that the optimal choice of the sample preparation is lyophilization process.
Rovčanin et al. determined TPC in
S. tectorum extracts (solvent extraction, solvent ethanol:water = 7:3) and in other plants in 2015 using Folin–Ciocalteu assay [
27]. TPC for
S. tectorum was 16.00 mg GA/g extract. Compared to this result, in our study, much higher TPC was determined with all three conventional extraction methods using the same solvent, namely 138.85 mg GA/g extract for Soxhlet extraction, 127.13 mg GA/g extract for extraction with cold solvent, and 139.42 mg GA/g extract for ultrasound extraction. Probably one of the reasons is the material preparation, as the leaves of
S. tectorum in their study were air dried at 25–28 °C, while ours were lyophilized and could potentially receive higher TPC. The other reason could be attributed to climate and where
S. tectorum plant was grown, since their plant was cultivated in Shanghai (CHI), while ours was in Maribor (SLO). This was proven by both Stockham et al. and Biniari et al. by comparing the TPC of grapes from different growing regions and climate change [
37,
38]. To the best of our knowledge, there exists no study in the literature to describe TPC in supercritical extracts. Hence, we can state that we were the first to check and confirm the TPC in dried
S. tectorum extracts. Supercritical fluid extractions have been performed with SC CO
2 without a cosolvent that would modify the non-polar nature of CO
2. The aim was to completely avoid application of organic solvents (cosolvents) and attain a pure extract that would need no further purification. The water that was still present in material (a part of material was dried, the other part lyophilized) could contribute to hydrolyze the bond, as the majority of polyphenols in plants exist as glycosides. However, we could conclude that, overall, more lyophilic compounds were isolated by SCE since there was no polar modifier. This demonstrated that the phenolic compounds, soluble in polar solvents, contribute to the antioxidant and the decreased metabolic activity of the melanoma cells.
3.2. Proanthocyanidin Content
Proanthocyanidins (PAC) are a class of oligomeric or polymeric polyphenols found in a variety of plants. Most of them are oligomers of catechin, epicatechin, and their gallic acid esters. Just like polyphenols, they also have various biological activities, such as antioxidative, antienzymatic, and antitumor potential. However, their biological abilities are mainly based on their structure [
39]. Nowadays, PAC has gained a great deal of interest in the biomedical industry, such as medicine for certain cancer diseases, since it was proven by Kawahara et al. that epicatechin pentamer and hexamer inhibited cancer metastasis and invasion [
40]. Therefore, it has garnered great interest in potential for finding a possible cure for various cancers as chemopreventive agents.
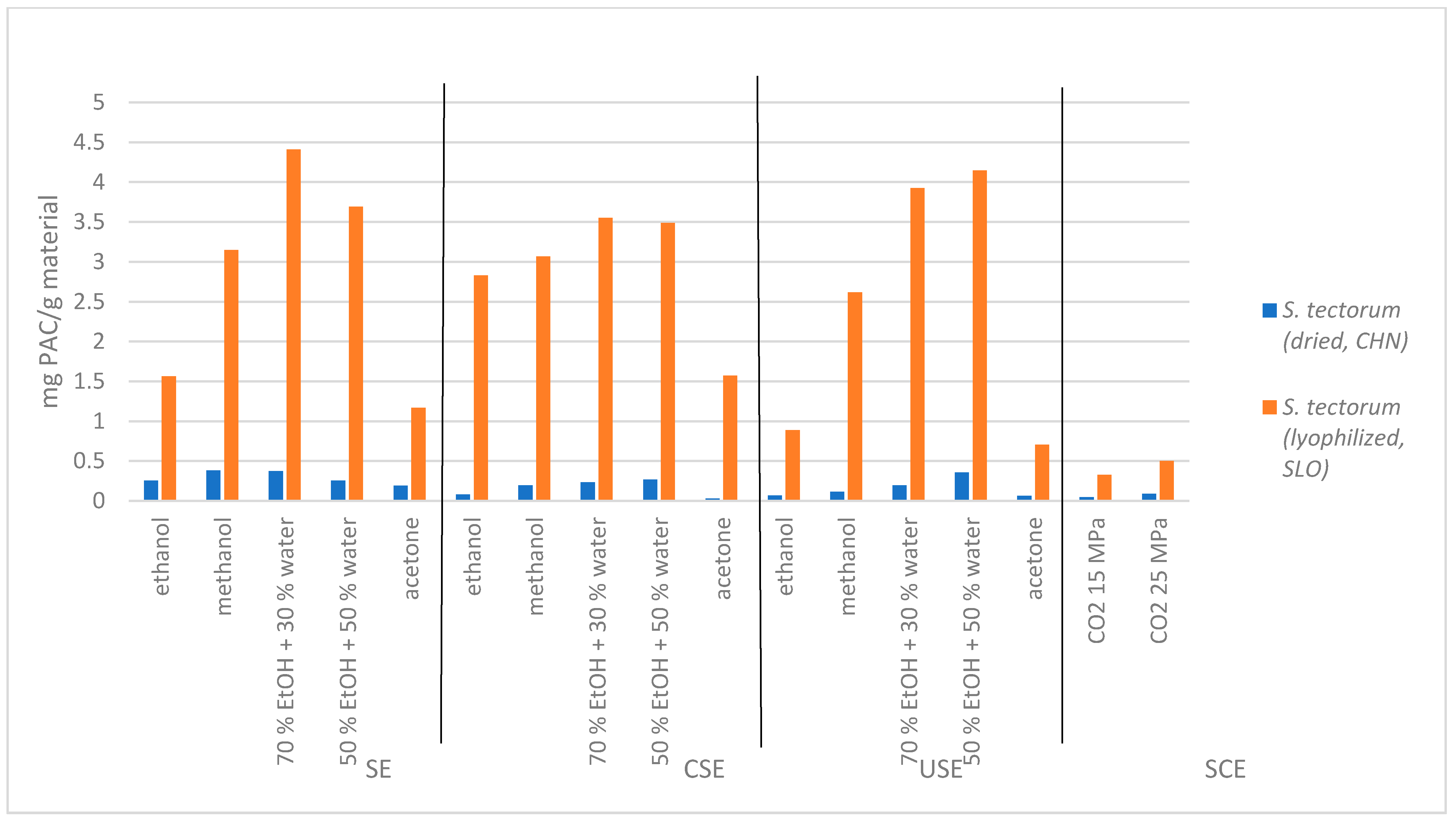
In
Figure 2, the PAC content in
S. tectorum extracts is presented. The results show that the highest PAC content was obtained in SLO lyophilized
S. tectorum material (up to 4.41 mg PAC/g material) using Soxhlet extraction and mixture of ethanol:water = 30:70. In CHI dried material, a lower content of PAC (up to 0.38 mg PAC/g material) was detected. Dried
S. tectorum extracts obtained with supercritical extraction method also hold low PAC contents (0.04 mg PAC/g material for 15 MPa and 0.09 mg PAC/g material for 25 MPa) compared to supercritical extracts obtained from SLO
S. tectorum (0.33 mg PAC/g material from 15 MPa and 0.50 mg PAC/g material for 25 MPa). However, all supercritical extracts showed lower PAC in comparison to extracts from both dried and lyophilized material obtained with conventional extraction methods. Additionally, all conclusions that have been stated for TPC content in supercritical extracts can be attributed to their PAC content. As for the pressure change, more experiments need to be done to further confirm the correlation with PAC content, but the PAC content is too small to be worth proceeding with further experiments. The highest PAC content in extracts from SLO
S. tectorum using all three conventional extraction methods were obtained as mentioned above with ethanol:water mixture (both, 7:3 and 1:1), followed by extracts obtained with methanol, ethanol, and lastly, acetone. Therefore, the optimal selection of extraction method and solvent for obtaining the highest content of PAC is Soxhlet extraction of
S. tectorum with ethanol:water mixture (7:3), respectively. As for the CHI dried
S. tectorum extracts results showed remarkably lower PAC contents (lower than 0.5 mg PAC/mg material) compared to extracts obtained from SLO
S. tectorum.
Again, we anticipate that PAC compounds in extracts from dried material degraded due to the high temperatures during the drying process. For this reason, their analysis showed very low PAC content compared to lyophilized S. tectorum extracts. The lyophilization process itself once again turned out to be ideal for the preparation of a given material since the active component did not disintegrate during the process.
Beside TPC, Rovčanin et al. also confirmed PAC content with the expression of the results as mg of catechin equivalent (CE)/g of extract [
27]. For ethanol:water (7:3) mixture, they obtained 0.9 mg of CE/g of extract. With the same solvent mixture in our study, for all three conventional extraction methods, the following results were obtained: 13.40 mg PAC/g extract for Soxhlet extraction, 15.89 mg PAC/g extract for extraction with cold solvent, and 15.42 mg PAC/g extract for ultrasound extraction. The main reasons for such significant differences are again different climate and material preparation procedures. The same was already mentioned in the discussion about TPC results. Abram et al. identified two main PAC compounds (4-thiobenzyl-(-)-epigallocatechin and 4-thiobenzyl-(-)-epigallocatechin-3-gallate) in
S. tectorum extracts [
26]. They concluded that procyanidins of B2 type could be the major components of the PAC fraction, but on the other hand, no analysis for PAC content has been made for this plant. Again, for supercritical extracts, no results in articles were found. Accordingly, we can state that we were the first to check and confirm the PAC content in dried
S. tectorum extracts.
3.3. Antioxidative Activity
Antioxidants are compounds that can act as free radical scavengers and therefore break the chain reaction to prevent oxidation [
41]. A large proportion of reactive oxygen species (ROS), such as peroxide, superoxide, singlet oxygen, and hydroxyl radical, are formed with an electron transfer to molecular oxygen [
42]. Presence of ROS can cause oxidative damage to proteins, DNA, lipids, and membranes, which can lead to various tumor and cancer diseases [
43,
44]. For this reason, it is of great importance to evaluate antioxidant properties in different plant species, isolate them, and prevent ROS from causing oxidative stress to human health.
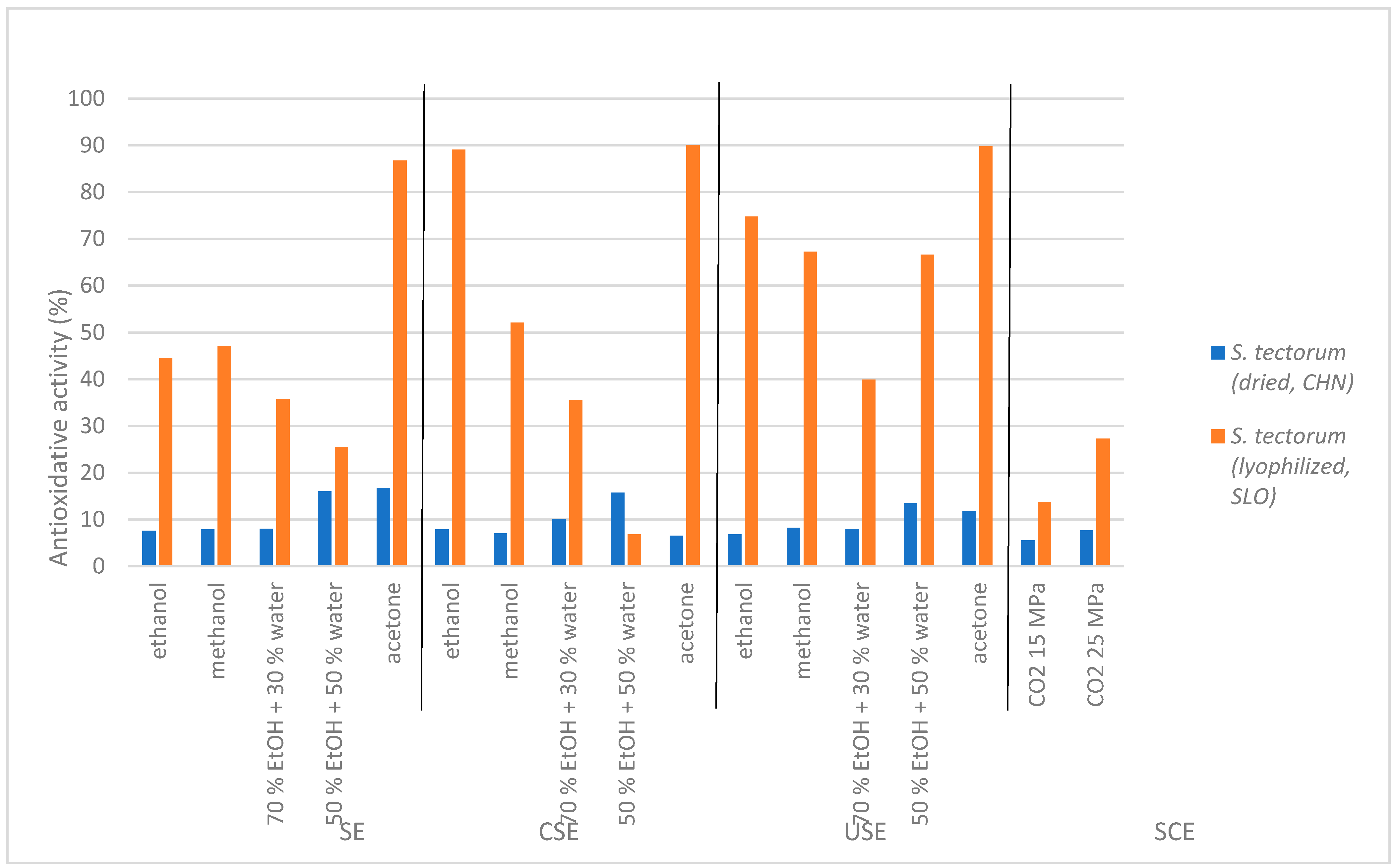
The results of antioxidative activity of
S. tectorum extracts obtained with DPPH assay are presented in
Figure 3. The results clearly indicate that the majority of extracts from SLO lyophilized
S. tectorum significantly inhibited free radicals (up to 90.06%), while extracts from CHI dried
S. tectorum showed much lower antioxidative potential (up to 16.76%). The highest antioxidative capacity in SLO
S. tectorum extracts with all conventional extraction methods was detected when using acetone as a solvent of choice. Slightly lower antioxidative potential was obtained with ethanol extracts, followed by methanol extracts and, lastly, with those obtained with both ethanol:water mixtures. Therefore, the best solvent choice for achieving the highest number of compounds with antioxidative activity is acetone since it showed the best results for all three conventional extraction methods. On the other hand, ethanol can also be used as a second solvent choice due to its promising antioxidative potential, especially with ultrasound and cold solvent extraction methods. With supercritical extracts, low antioxidative potential was obtained since there were also small yields of nonpolar components from
S. tectorum. In comparison, supercritical extracts from SLO
S. tectorum obtained slightly higher antioxidative potential (13.74% for 15 MPa and 27.29% for 25 MPa) than CHI
S. tectorum supercritical extracts (5.54% for 15 MPa and 7.68 MPa for 25 MPa). This could be due to number of polyphenols in the plant, which correlated with the TPC results since it is known that they also have antioxidative activity.
Again, extracts from dried CHI S. tectorum displayed notably lower antioxidative potentials opposed to extracts from SLO lyophilized plant material. However, the lower antioxidant activities were mainly due to the drying process of S. tectorum. Since the material was dried at high temperatures to which active substances are mostly sensitive, much lower percentages of inhibition were obtained.
Once more, the lyophilization process turned out to be the best process to preserve all the active substances contained in S. tectorum and consequently yielded the highest antioxidant potentials.
In the literature, only one article with the results on antioxidant activity of
S. tectorum was found, written by Šentjurc et al. [
10] Antioxidative potential for the separated individual compounds of
S. tectorum extract was described. In our study, antioxidative activity of the whole, unseparated extracts was determined. If we compare the antioxidative values between our extract and their individual compounds, it can be seen that their percent contribution of free radical adduct was between 30 and 82%, while ours varies between 6.8 and 90%. Similar results show that
S. tectorum plant indeed has a high content of polyphenolic compounds that tend to have high antioxidative potential. With the proper extraction method and solvent, it is possible to extract maximum amount of antioxidative compounds, which are widely known for their anticancer properties. According to study by Šentjurc et al., the compounds that tend to have the highest antioxidant potential are oligomeric polyphenols. Blazovics et al. examined in their experiments the superoxide scavenger activity of
S. tectorum extracts and inhibition of iron-induced lipid peroxidation induced by ascorbic acid. However, no antioxidant activity was determined in this study. As for supercritical extracts, since no results and articles have been found for antioxidative measurements, no comparison could be done. In addition, we can state that we were the first to check and confirm the antioxidative potential in dried
S. tectorum extracts.
3.4. Effect of Extracts on the Metabolic Activity of Human Melanoma Cells
WM-266-4 is a metastatic human melanoma cell line with small flat mesenchymal morphology. This cell line was derived from the same patient as the cell lines WM115, WM293A, and WM165-1. The subject (55-year-old female) displayed VGP with a Clark level III tumor with thickness of 2.24 mm. This cell line also expresses PTEN loss of function, including hemizygous deletion. WM-266-4 cells produce xenograft tumors when injected into immunocompromised mice.
Table 1 shows the extracts with attributed labels to which we referred in the following results. We chose the extracts based on their previous results of antioxidative activity. Y. K. Mahmoud et al. described that antioxidative potential plays a major role in the inhibitory effect on cancer cells since these are the most known for their anticancer activity [
45]. Extracts obtained with conventional methods were mainly used for these assays since nonpolar compounds acquired with sc CO
2 extraction did not show high potential in antioxidativity, TPC, and PAC, which has a major inhibitory role and effect in human melanoma cells. In addition, lower yields in sc CO
2 extraction were obtained, which further corresponds to low nonpolar compound content in
S. tectorum and consequently were not tested in metabolic activity assay of WM-266-4 cell line.
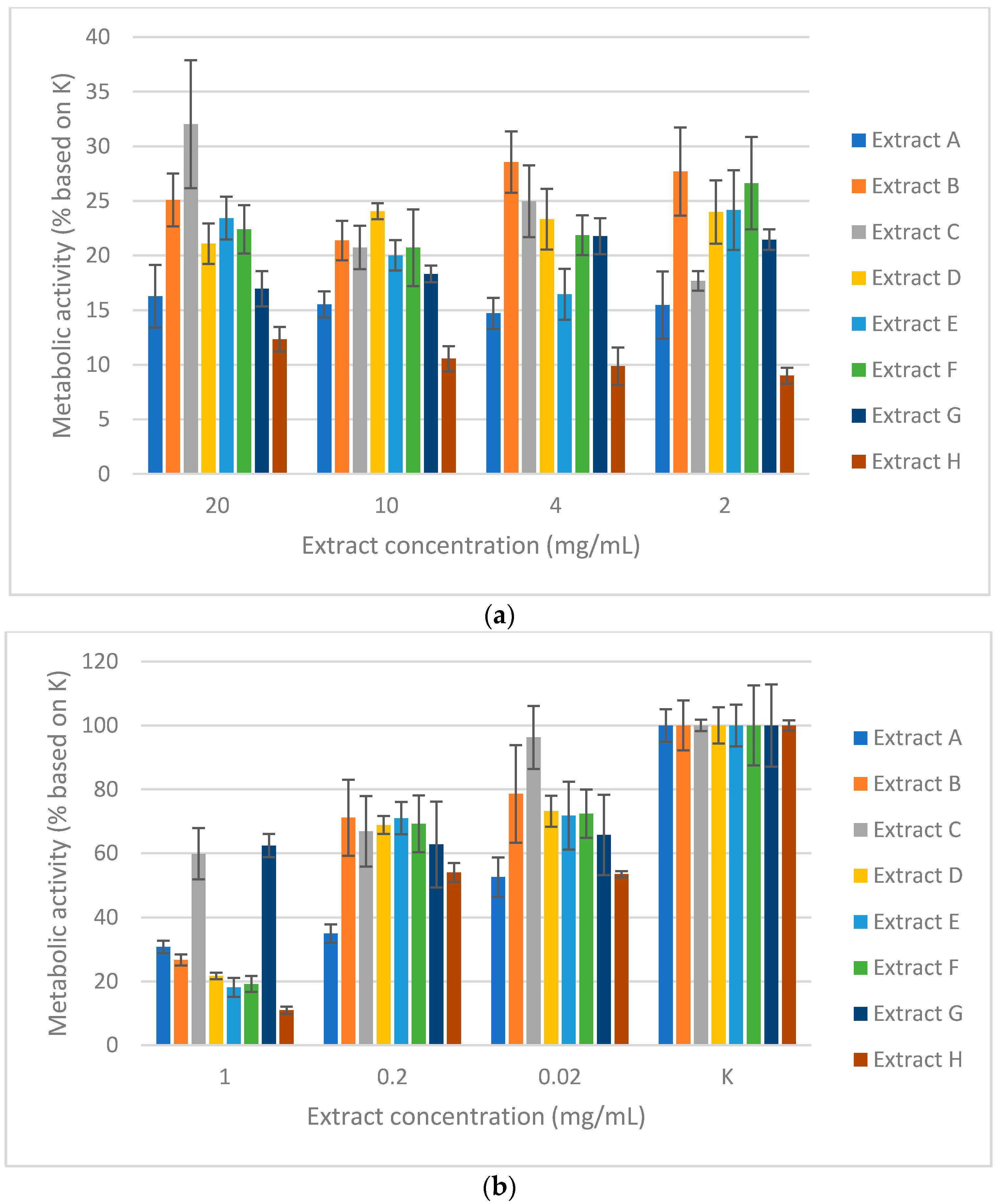
Figure 4a,b summarize the results of the inhibitory effects for all studied extracts on WM-266-4 human melanoma cells. As predicted, higher concentrations (20 to 2 mg/mL) strongly and effectively inhibited the metabolic activity of human melanoma cells WM-266-4. At a concentration of 1 mg/mL, inhibition began to decrease for particular extracts, while for lower concentrations (0.2 to 0.02 mg/mL), the effect of extracts on the cells was minimal; probably because of the high dilution of the extracts, the concentration of active substances was too low.
The best results showed both extract A (SLO lyophilized
S. tectorum, acetone, and ultrasound extraction) and extract H (CHI dried
S. tectorum, 50% EtOH + 50% H
2O, Soxhlet extraction). At higher concentrations (20 to 2 mg/mL), a range from 14.71 to 16.27% of metabolic activity was measured for extract A and from 8.99 to 12.34% for extract H (
Figure 4a). Therefore, both strongly inhibited the growth of human melanoma cells. In addition, at the lowest concentration (0.02 mg/mL), both extracts showed moderate inhibition (48.45% for A and 46.55% for H) of WM-266-4 metastatic cell line growth. On the other hand, for extract B (SLO lyophilized
S. tectorum, ethanol, and Soxhlet extraction) and C (SLO lyophilized
S. tectorum, acetone, and Soxhlet extraction) at their high concentrations, lower inhibition values were obtained. In comparison to other extracts, both showed the lowest inhibition of cells at higher concentrations (74.90% for B and 67.97% for C). At 0.02 mg/mL, the growth of human melanoma cells was only slightly inhibited (21.41% for B and 3.73% for C).
The main interest of this study was SLO
S. tectorum; therefore, the best extract from this plant was further described, even though extract H (CHI
S. tectorum) showed slightly better results than extract A (SLO
S. tectorum). The reason for high inhibitory effect of CHI
S. tectorum probably lies in polyphenols since there was higher content of polyphenols in extract H than in extract A. Some studies have already stated that polyphenols have the ability to prevent cancer with the reduction of harmful effects caused by free radicals and diversity of biological functions, which show the relation to control mechanisms of carcinogenesis [
35,
46]. Their main advantage is that they can give protection to normal cells, while on the other side show cytotoxic effects on cancerous cells. On this basis, we concluded that extract H showed better inhibitory effect of human melanoma cells WM-266-4 than extract A due to the difference in TPC.
With these results, we proved that within 24 h of incubation, chosen extracts at concentrations of 20, 10, 4, 2, 1, 0.2, and 0.02 mg/mL inhibited the growth of human melanoma cells WM-266-4 and their metabolic activity.
We separately described the acquired results for extract A, obtained with ultrasound extraction using acetone as a solvent since it gave the suitable results for inhibition on this human metastatic cell line, although extract H (CHI dried
S. tectorum) showed better results as extract A (SLO lyophilized
S. tectorum). The focus of this research was SLO
S. tectorum, hence the reason we have chosen the best result shown by extract A.
Figure 5 shows the results of the inhibitory effect of extract A, which significantly inhibited the metabolic activity (up to 85% according to the control sample (C)) of WM-266-4 melanoma cells at all studied concentrations. At the lowest concentration (0.02 mg/mL), there was 48% inhibition of human melanoma cells WM-266-4 compared to the control sample, which indicates that even at lower concentrations, this extract had a certain effect on their metabolic activity.
For comparison,
Figure 6 shows the cells shape of a sample without the extract solution (control) and with the certain extract solution, which were observed with FLoid Cell Imaging Station microscope under 200× magnification. Presence of the extract solution blocked the clear view of the melanoma cells; therefore, a blurred image is seen. In the observation process, melanoma cells changed their shape from spindle-shaped to spherical at high concentrations (20, 10, 4, and 2 mg/mL) of present extract. The same results were obtained for all previous mentioned extracts (
Table 1) used on human melanoma cells WM-266-4.
No research or results have been found regarding the metabolic activity of S. tectorum extracts on this specific human metastatic cell line. This is the reason that our study on confirmation of the inhibitory effect of S. tectorum extracts on human melanoma cells WM-266-4 offers an important contribution to the research in this field.
3.5. Statistical Analysis
Mann–Whitney U test showed a significant difference (W = 43, p < 0.001) between TPC found in lyophilized SLO S. Tectorum and CHI dried material. The median TPC of SLO material was 26.32 (13.48, 35.49) mg GA/g compared to 7.62 (2.90, 12.38) GA/mg of CHN material.
As the p-value of the Kruskal–Wallis is less than the significance level (χ2 = 12.222, p < 0.001), we can conclude that there are significant differences between the content of total phenolics in extracts obtained by different extraction methods. Mann–Whitney U test showed a significant difference (W = 4, p < 0.001) between PAC determined in lyophilized materials from both regions. The median PAC content of Slovenian material was 02.62 (0.88, 3.93) mg CA/g compared to 0.12 (0.07, 0.25) CA/mg in the Chinese material. As the p-value of the Kruskal–Wallis is less than the significance level (χ2 = 23.455, p < 0.001), we can conclude significant differences between the content of proanthocyanins in extracts obtained by different extraction methods. Mann–Whitney U test showed a significant difference (W = 18, p < 0.001) between antioxidant activity of the extracts of lyophilized S. Tectorum from Slovenian region and dried material from China. The median antioxidant activity of Slovenian material was 47.10 (35.54, 74.78)% compared to the one of the Chinese, which was significantly lower; 7.94 (7.60, 11.75)%.