Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strains and Culture Conditions
2.2. Microalgal Cultivation in Photobioreactors
2.3. Analytical Methods
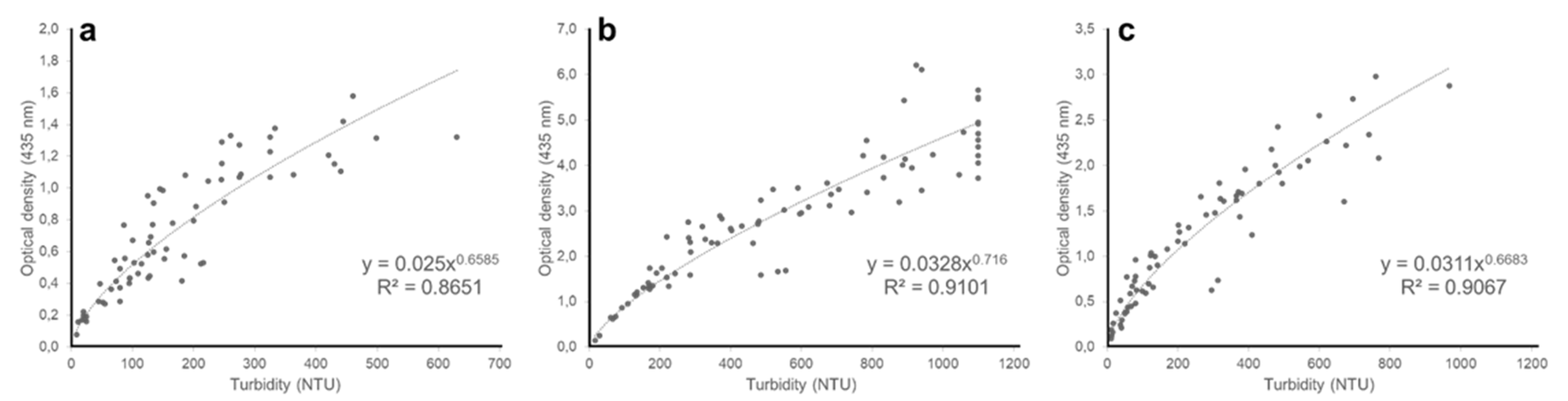
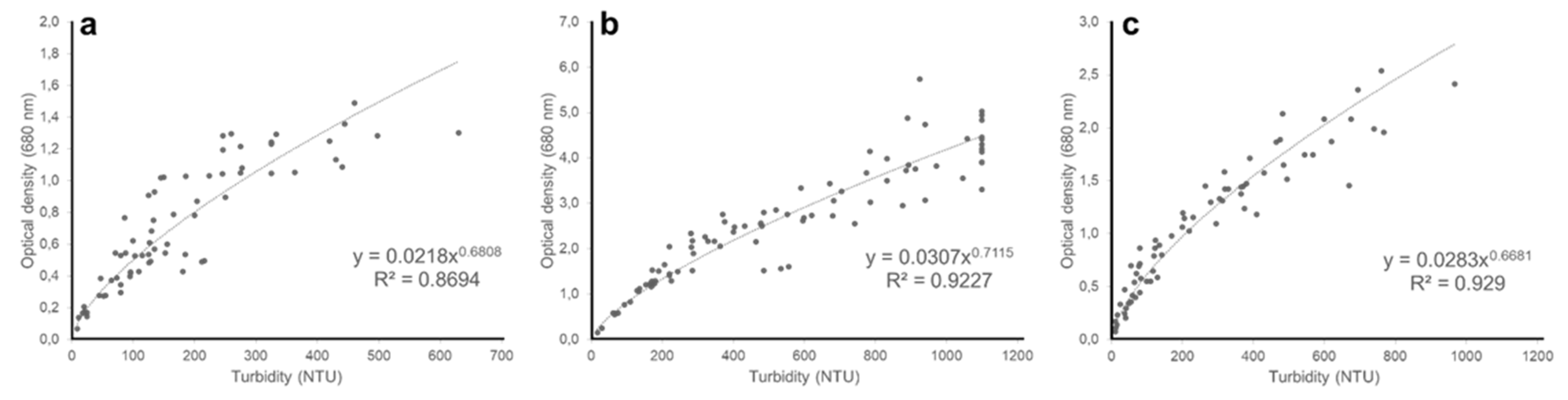
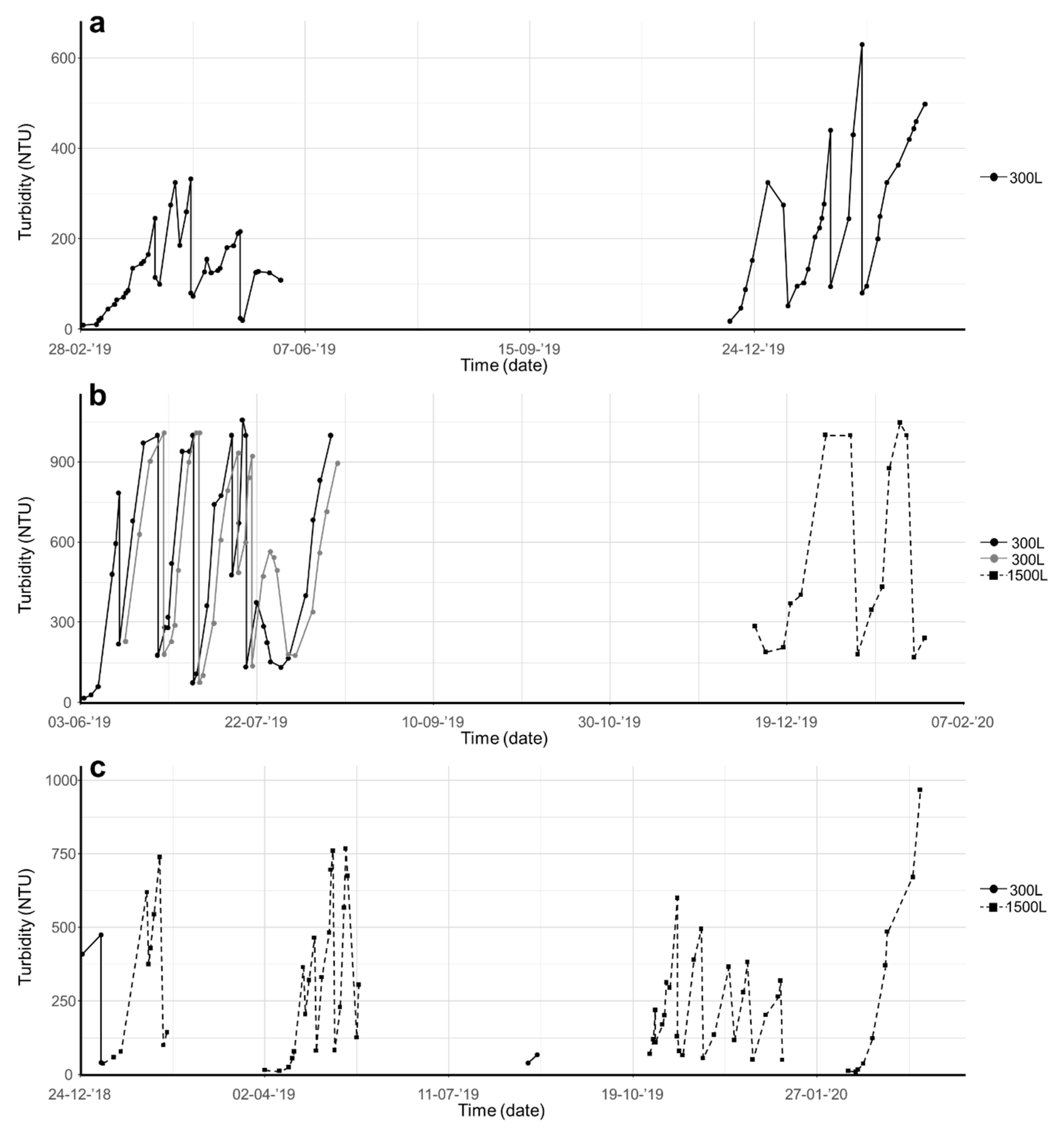
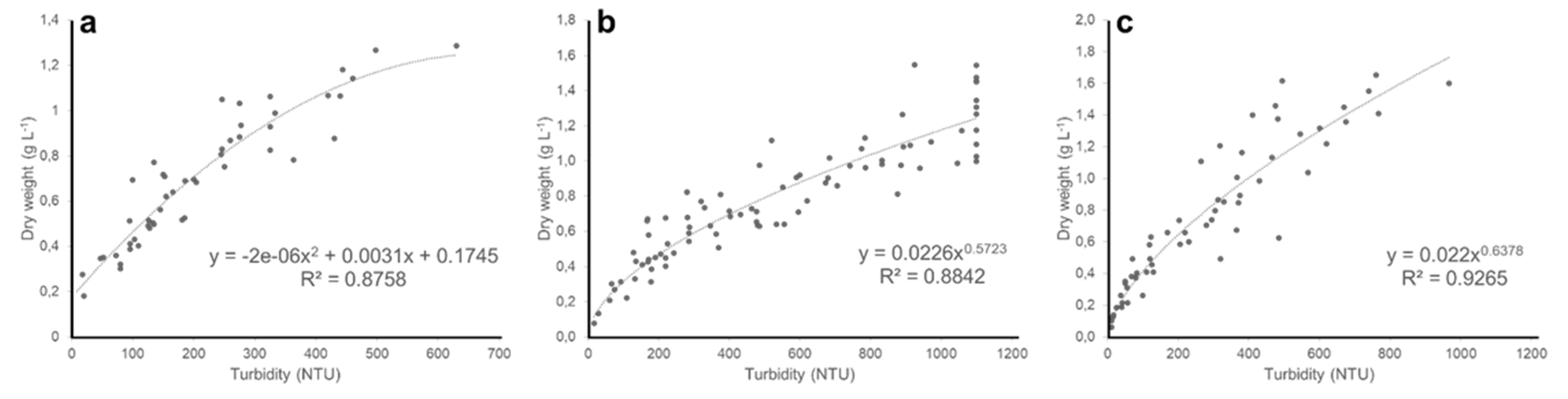
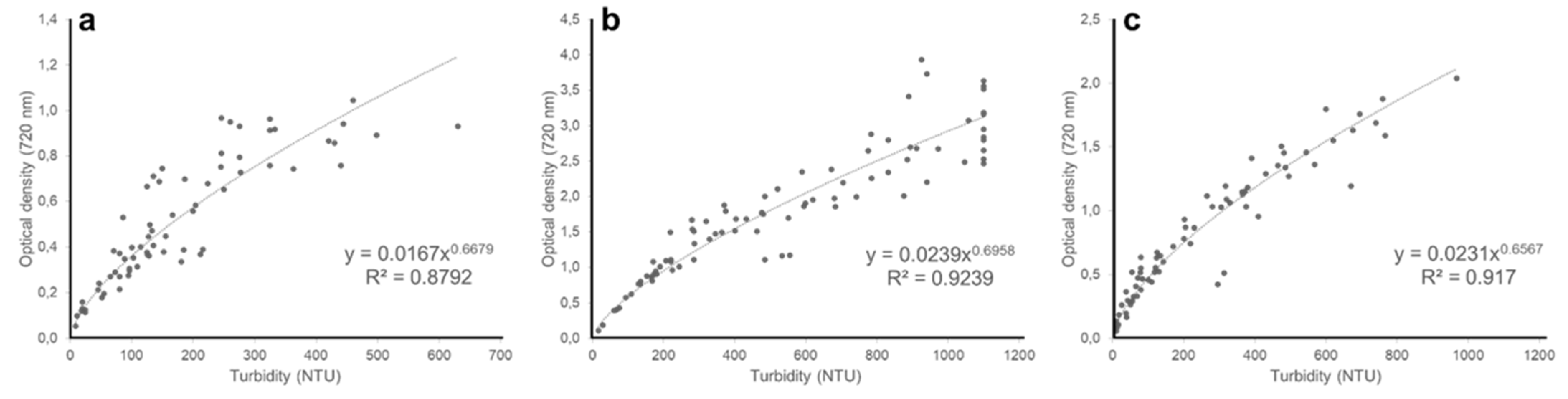
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Rösch, C.; Roßmann, M.; Weickert, S. Microalgae for integrated food and fuel production. GCB Bioenergy 2019, 11, 326–334. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Schoeters, F.; De Cuyper, A.; Vleugels, R.; Noyens, I.; Bleyen, P.; Van Miert, S. Waste Is the New Wealth—Recovering Resources from Poultry Wastewater for Multifunctional Microalgae Feedstock. Front. Environ. Sci. 2021, 9, 679917. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: Application of chlorophyll fluorescence techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Havlik, I.; Linder, P.; Scheper, T.; Reardon, K.F. On-line monitoring of large cultivations of microalgae and cyanobacteria. Trends Biotechnol. 2013, 31, 406–414. [Google Scholar] [CrossRef]
- Calmes, B.; Strittmatter, M.; Jacquemin, B.; Perrineau, M.; Rousseau, C.; Badis, Y.; Cock, J.M.; Destombe, C.; Valero, M.; Gachon, C.M.M. Parallelisable non-invasive biomass, fitness and growth measurement of macroalgae and other protists with nephelometry. Algal Res. 2020, 46, 101762. [Google Scholar] [CrossRef]
- Ferrando, N.S.; Benítez, H.H.; Gabellone, N.A.; Claps, M.C.; Altamirano, P.R. A quick and effective estimation of algal density by turbidimetry developed with Chlorella vulgaris cultures. Limnetica 2015, 34, 397–406. [Google Scholar] [CrossRef]
- Embleton, K.V.; Gibson, C.E.; Heaney, S.I. Automated counting of phytoplankton by pattern recognition: A comparison with a manual counting method. J. Plankton Res. 2003, 25, 669–681. [Google Scholar] [CrossRef] [Green Version]
- Jia, F.; Kacira, M.; Ogden, K.L. Multi-wavelength based optical density sensor for autonomous monitoring of microalgae. Sensors 2015, 15, 22234–22248. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D.S. Turbidity as a water quality standard for salmonid habitats in Alaska. N. Am. J. Fish. Manag. 1987, 7, 34–35. [Google Scholar] [CrossRef]
- Goehring, L.S.; Kessels, B.G.F.; Van Maanen, C.; Voorbij, H.A.M.; Sloet Van Oldruitenborgh-Oosterbaan, M.M. Evaluation of nephelometry for albumin measurement in serum and cerebrospinal fluid: Experiences with an indwelling subarachnoidal catheter system for repetitive cerebrospinal fluid collection in horses. J. Vet. Diagn. Investig. 2006, 18, 251–256. [Google Scholar] [CrossRef]
- Butterwick, C.; Heaney, S.I.; Talling, J.F. A comparison of eight methods for estimating the biomass and growth of planktonic algae. Br. Phycol. J. 1982, 17, 69–79. [Google Scholar] [CrossRef]
- Bosma, R.; van Zessen, E.; Reith, J.H.; Tramper, J.; Wijffels, R.H. Prediction of Volumetric Productivity of an Outdoor Photobioreactor. Biotechnol. Bioeng. 2007, 97, 1108–1120. [Google Scholar] [CrossRef]
- Joubert, A.; Calmes, B.; Berruyer, R.; Pihet, M.; Bouchara, J.; Simoneau, P.; Guillemette, T. Laser nephelometry applied in an automated microplate system to study filamentous fungus growth. Biotechniques 2010, 48, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Berberoglu, H.; Pilon, L. Experimental measurements of the radiation characteristics of Anabaena variabilis ATCC 29413-U and Rhodobacter sphaeroides ATCC 49419. Int. J. Hydrog. Energy 2007, 32, 4772–4785. [Google Scholar] [CrossRef]
- Kandilian, R.; Pruvost, J.; Artu, A.; Lemasson, C.; Legrand, J.; Pilon, L. Comparison of experimentally and theoretically determined radiation characteristics of photosynthetic microorganisms. J. Quant. Spectrosc. Radiat. Transf. 2016, 175, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Bin Omar, A.F.; Bin Matjafri, M.Z. Turbidimeter Design and Analysis: A Review on Optical Fiber Sensors for the Measurement of Water Turbidity. Sensors 2009, 9, 8311–8335. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Nguyen, B.T.; Rittmann, B.E. Low-cost optical sensor to automatically monitor and control biomass concentration in microalgal cultivation. Algal Res. 2018, 32, 101–106. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Hara, Y.; Nozaki, H. A taxonomic revision of Chloromonas reticulata (Volvocales, Chlorophyceae), the type species of the genus Chloromonas, based on multigene phylogeny and comparative light and electron microscopy. Phycologia 2012, 51, 74–85. [Google Scholar] [CrossRef]
- Chua, E.T.; Schenk, P.M. A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowitzka, M.A. Biology of Microalgae. In Microalgae in Health and Disease Prevention, 1st ed.; Levine, I.A., Fleurence, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 23–73. [Google Scholar]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon-bercovitch, B.; Bar-zvi, D.; Arad, S.M. Cell-wall formation during the cell cycle of Porphyridium sp. (Rhodophyta). J. Phycol. 1999, 35, 78–83. [Google Scholar] [CrossRef]
- Fisher, R.M.; Bell, T.; West, S.A. Multicellular group formation in response to predators in the alga Chlorella vulgaris. J. Evol. Biol. 2016, 29, 551–559. [Google Scholar] [CrossRef] [Green Version]
- de Carpentier, F.; Lemaire, S.D.; Danon, A. When Unity Is Strength: The Strategies Used by Chlamydomonas to Survive Environmental Stresses. Cells 2019, 8, 1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnurr, P.J.; Allen, D.G. Factors affecting algae biofilm growth and lipid production: A review. Renew. Sustain. Energy Rev. 2015, 52, 418–429. [Google Scholar] [CrossRef]
- Xi, T.; Kim, D.G.; Roh, S.W.; Choi, J.S.; Choi, Y.E. Enhancement of astaxanthin production using Haematococcus pluvialis with novel LED wavelength shift strategy. Appl. Microbiol. Biotechnol. 2016, 100, 6231–6238. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Crawford, S.A.; Dagastine, R.R.; Scales, P.J.; Martin, G.J.O. Nitrogen deprivation of microalgae: Effect on cell size, cell wall thickness, cell strength, and resistance to mechanical disruption. J. Ind. Microbiol. Biotechnol. 2016, 43, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; Orcutt, D.M.; Schwertner, H.A.; Martinez, C.L.; Wickline, H.E. Effects of nitrogen limitation on the growth and composition of unicellular algae in continuous culture. Appl. Microbiol. 1969, 18, 245–250. [Google Scholar] [CrossRef]
- Simionato, D.; Block, M.A.; La Rocca, N.; Jouhet, J.; Maréchal, E.; Finazzi, G.; Morosinotto, T. The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot. Cell 2013, 12, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Havlik, I.; Reardon, K.F.; Ünal, M.; Lindner, P.; Prediger, A.; Babitzky, A.; Beutel, S.; Scheper, T. Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Res. 2013, 2, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Höpfner, T.; Bluma, A.; Rudolph, G.; Lindner, P.; Scheper, T. A review of non-invasive optical-based image analysis systems for continuous bioprocess monitoring. Bioprocess Biosyst. Eng. 2010, 33, 247–256. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoré, E.S.J.; Schoeters, F.; Spit, J.; Van Miert, S. Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry. Processes 2021, 9, 1530. https://doi.org/10.3390/pr9091530
Thoré ESJ, Schoeters F, Spit J, Van Miert S. Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry. Processes. 2021; 9(9):1530. https://doi.org/10.3390/pr9091530
Chicago/Turabian StyleThoré, Eli S. J., Floris Schoeters, Jornt Spit, and Sabine Van Miert. 2021. "Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry" Processes 9, no. 9: 1530. https://doi.org/10.3390/pr9091530
APA StyleThoré, E. S. J., Schoeters, F., Spit, J., & Van Miert, S. (2021). Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry. Processes, 9(9), 1530. https://doi.org/10.3390/pr9091530






