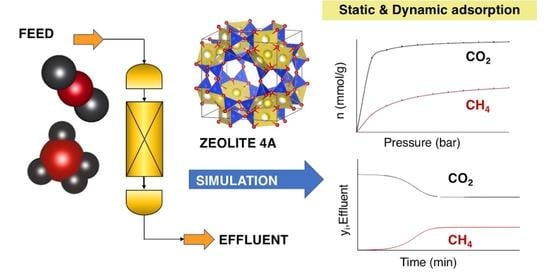
Static and Dynamic Simulation of Single and Binary Component Adsorption of CO2 and CH4 on Fixed Bed Using Molecular Sieve of Zeolite 4A
Abstract
:1. Introduction
2. Materials and Methods
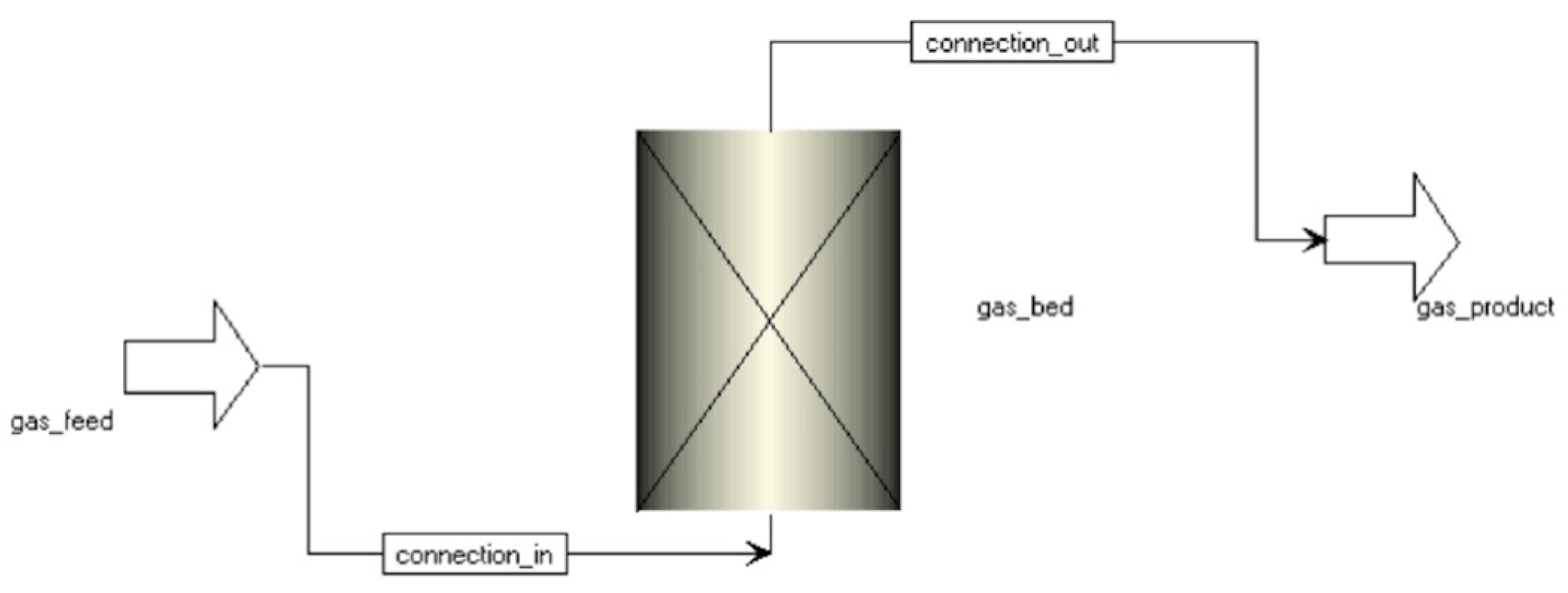
2.1. Simulation Setting
Dynamic Simulation of Adsorption Experiments
2.2. Adsorption Isotherm Model
2.2.1. Pure Component Adsorption Isotherm
2.2.2. Binary Component Adsorption Isotherm
2.3. Modeling of Mass Transfer Coefficient
2.4. Selectivity of CO2 over CH4 in Binary Mixture Gas
2.5. Breakthrough Curves Modeling
2.5.1. Thomas Model
2.5.2. Yoon-Nelson Model
3. Results and Discussion
3.1. Pure Component Adsorption Isotherm
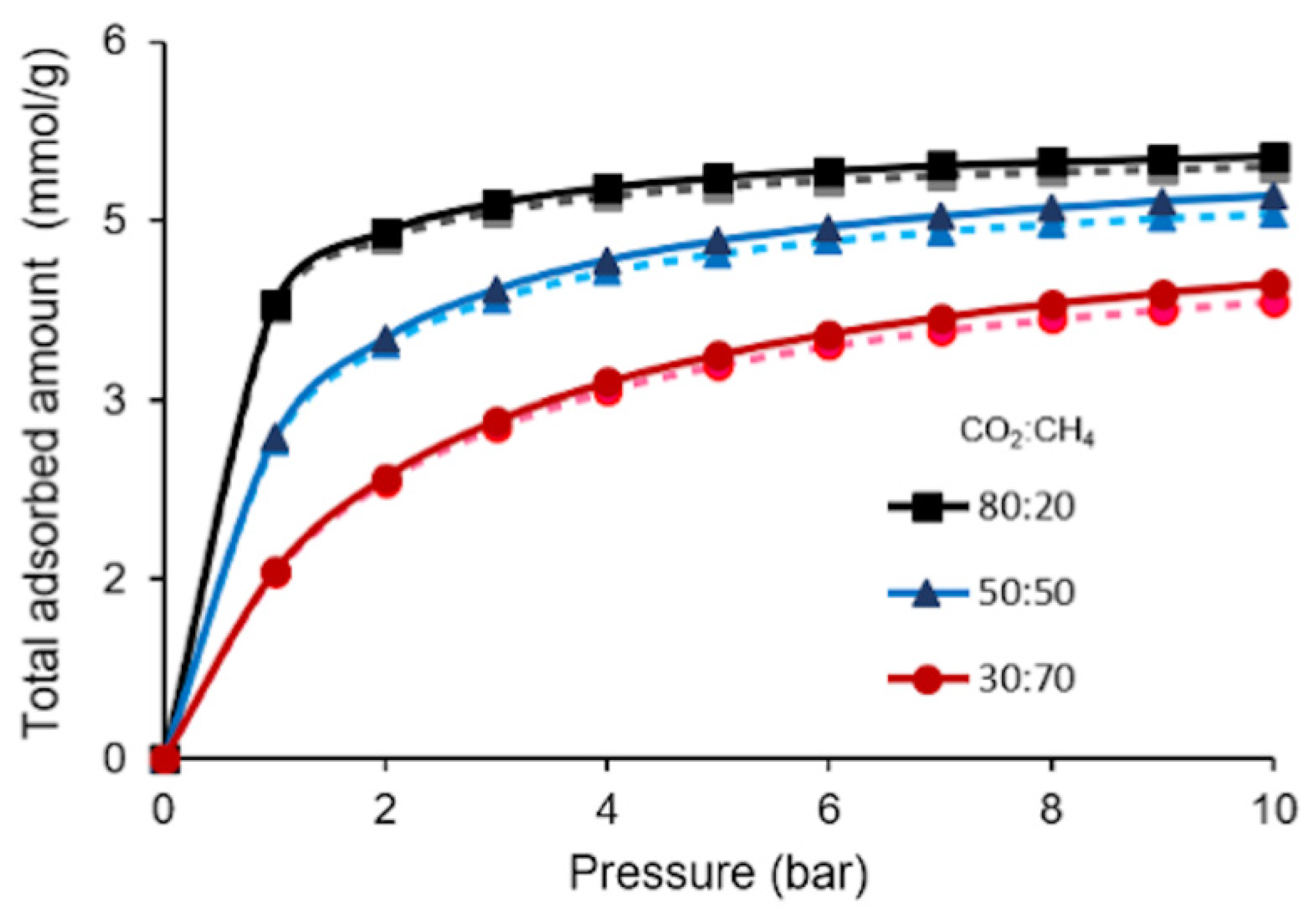
3.2. Binary Component Adsorption Isotherm
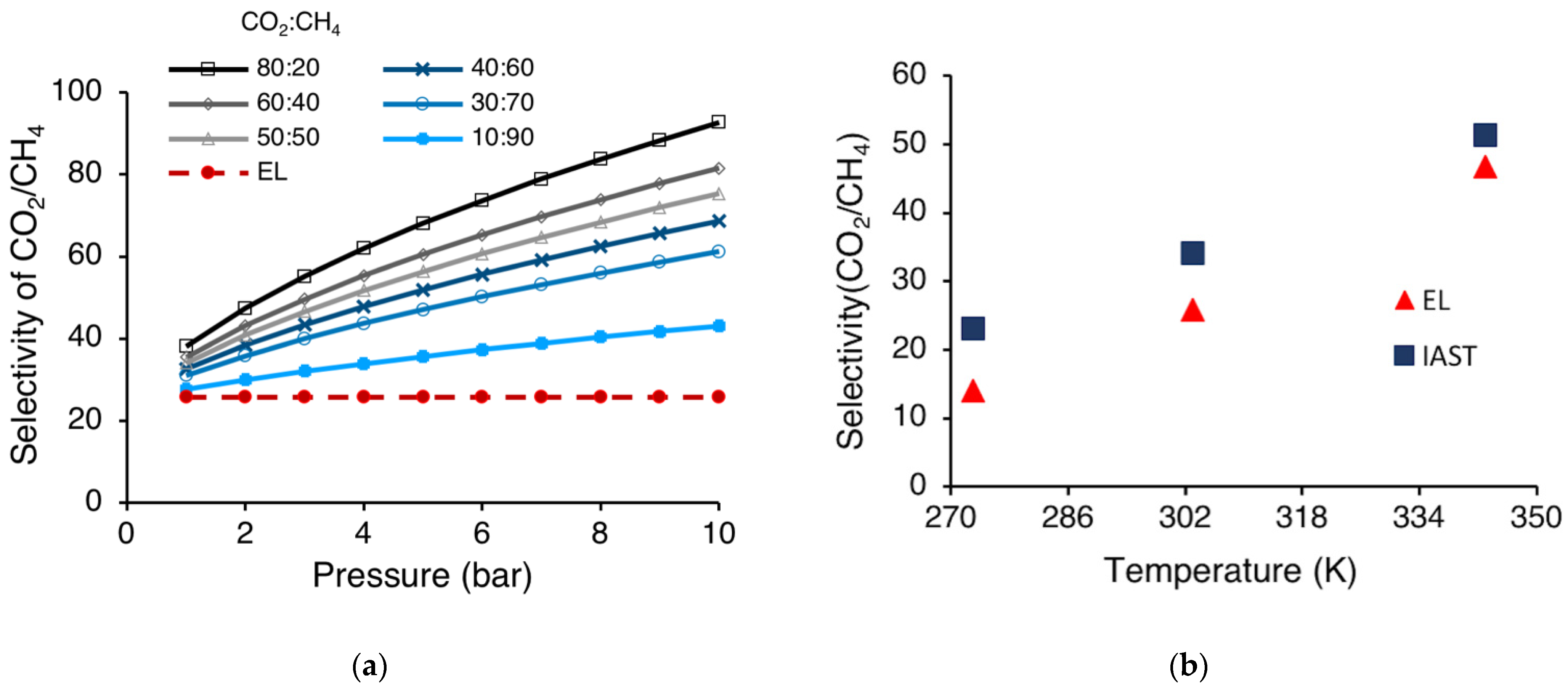
3.3. Selectivity for Separating of CO2-CH4 Mixture
3.4. Dynamic Simulation of Adsorption Experiment
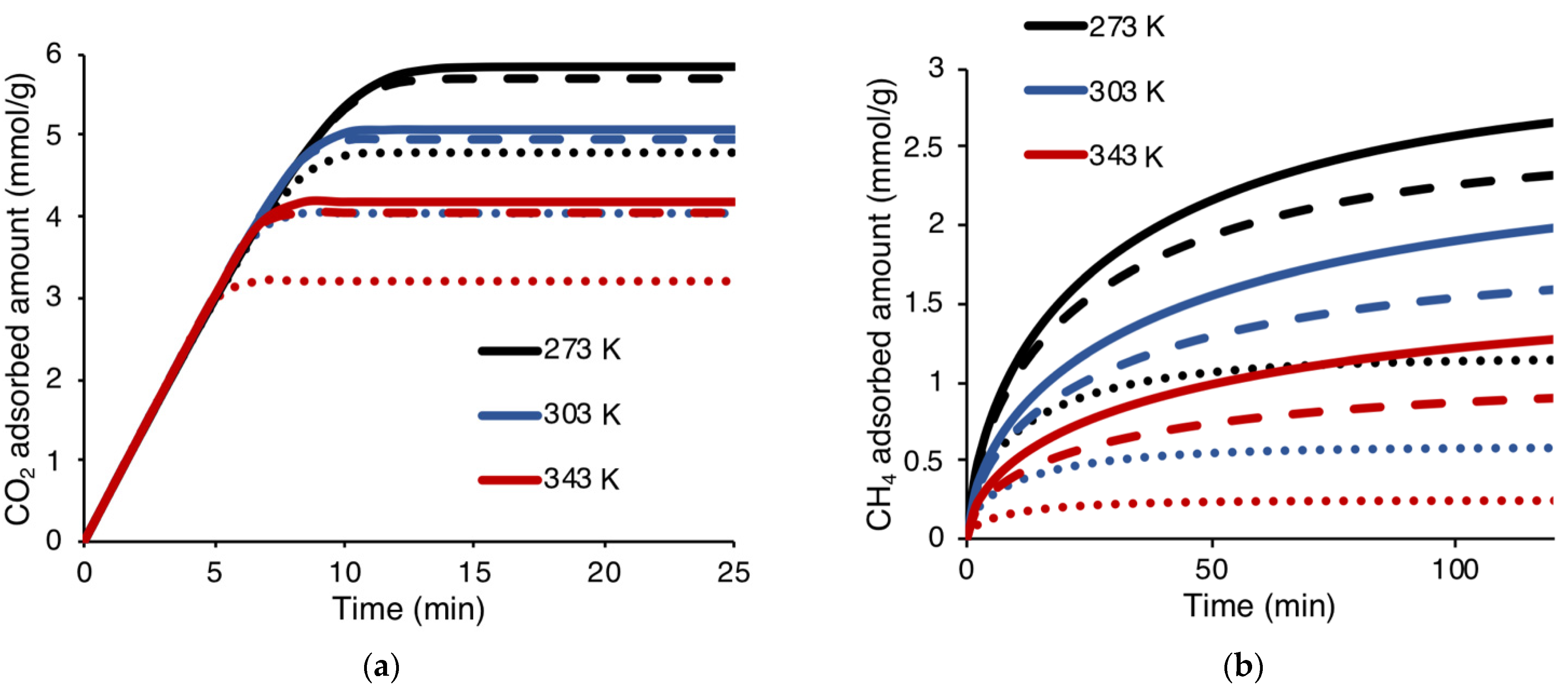
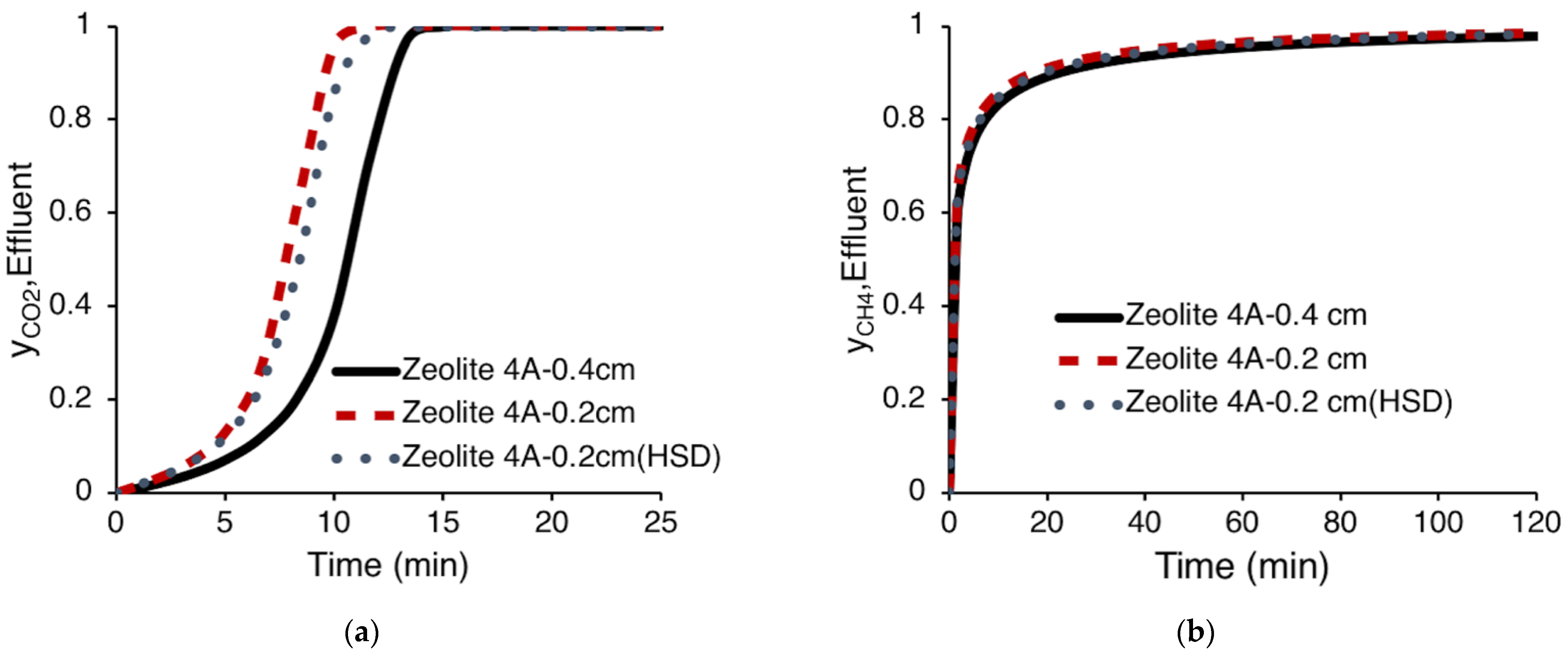
3.4.1. The Effect of the Physical Properties of Zeolite 4A on Kinetic Adsorption
3.4.2. The Effect of Binary CO2-CH4 Mixed Gas
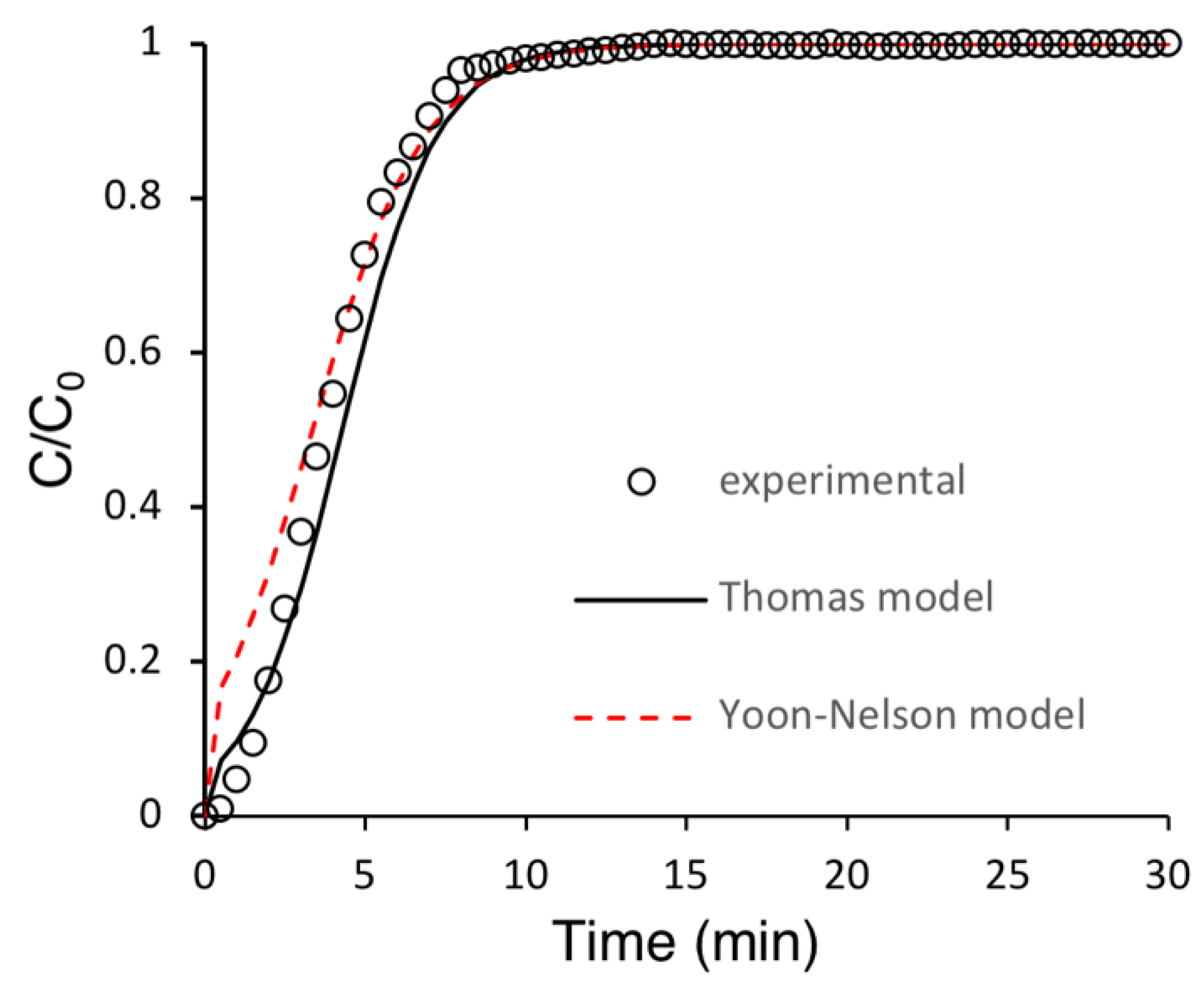
3.4.3. Modeling of Breakthrough Curves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jawaharraj, K.; Shrestha, N.; Chilkoor, G.; Dhiman, S.S.; Islam, J.; Gadhamshetty, V. Valorization of methane from environmental engineering applications: A critical review. Water Res. 2020, 187, 116400. [Google Scholar] [CrossRef]
- Paraschiv, S.; Paraschiv, L.S. Trends of carbon dioxide (CO2) emissions from fossil fuels combustion (coal, gas and oil) in the EU member states from 1960 to 2018. Energy Rep. 2020, 6, 237–242. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X. Conversion of CO2 into Polymers. In Green Chemistry and Chemical Engineering; Han, B., Wu, T., Eds.; Springer: New York, NY, USA, 2019; pp. 323–347. [Google Scholar]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. Low-Temperature Distillation Process for CO2/CH4 Separation: A Study for Avoiding CO2 Freeze-Out. J. Heat Transf. 2017, 140. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Song, X.; Liu, Z.; Li, D.; Zhou, F.; Xie, Y.; Xie, C. Effect of supercritical CO2 extraction on CO2/CH4 competitive adsorption in Yanchang shale. Chem. Eng. J. 2021, 412, 128701. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas. Control. 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Klewiah, I.; Berawala, D.S.; Alexander Walker, H.C.; Andersen, P.Ø.; Nadeau, P.H. Review of experimental sorption studies of CO2 and CH4 in shales. J. Nat. Gas. Sci. Eng. 2020, 73, 103045. [Google Scholar] [CrossRef]
- Wankat, P.C. A review of: “Gas Separation Technology”. Sep. Purif. Methods 1991, 20, 199–200. [Google Scholar] [CrossRef]
- Álvarez-Gutiérrez, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Adsorption performance indicators for the CO2/CH4 separation: Application to biomass-based activated carbons. Fuel Process. Technol. 2016, 142, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Tamnanloo, J.; Fatemi, S.; Golmakani, A. Binary Equilibrium Adsorption Data and Comparison of Zeolites with Activated Carbon for Selective Adsorption of CO2 from CH4. Adsorpt. Sci. Technol. 2014, 32, 707–716. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Lv, C.; Wu, T.; Wen, Y.; He, H.; Yu, S.; Wang, L. Adsorption Separation of CO2/CH4 from Landfill Gas by Ethanolamine-Modified Silica Gel. WaterAir Soil Pollut. 2021, 232. [Google Scholar] [CrossRef]
- Liang, D.; Hu, Y.; Bao, Q.; Zhang, J.; Feng, J.; Sun, P.; Ma, Y.; Zhang, H. A suitable zeolite Rho for separating CO2/CH4 in pressure swing adsorption (PSA) process. Inorg. Chem. Commun. 2021, 127, 108547. [Google Scholar] [CrossRef]
- Verma, M.; Kaur, G. A mini review on zeolite. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef] [Green Version]
- Chong, L.; Myshakin, E.M. Molecular simulations of competitive adsorption of carbon dioxide—Methane mixture on illitic clay surfaces. Fluid Phase Equilibria 2018, 472, 185–195. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Amari, A.; Danish, M.; Al Alwan, B.A.; Shah, M. Simulation study of fixed-bed CO2 adsorption from CO2/N2 mixture using activated carbon. Chem. Eng. Commun. 2020, 1–10. [Google Scholar] [CrossRef]
- Brea, P.; Delgado, J.A.; Águeda, V.I.; Uguina, M.A. Modeling of breakthrough curves of N2, CH4, CO, CO2 and a SMR type off-gas mixture on a fixed bed of BPL activated carbon. Sep. Purif. Technol. 2017, 179, 61–71. [Google Scholar] [CrossRef]
- Seabra, R.; Ribeiro, A.; Gleichmann, K.; Ferreira, A.; Rodrigues, A. Adsorption equilibrium and kinetics of carbon dioxide, methane and nitrogen on binderless zeolite 4A adsorbents. Microporous Mesoporous Mater. 2018, 277. [Google Scholar] [CrossRef]
- Wood, K.; Liu, Y.; Yu, Y. Simulation of Adsorption Processes; Wiley: Hoboken, NJ, USA, 2018; pp. 1–153. [Google Scholar]
- Plaza, M.G.; Durán, I.; Querejeta, N.; Rubiera, F.; Pevida, C. Experimental and Simulation Study of Adsorption in Postcombustion Conditions Using a Microporous Biochar. 1. CO2 and N2 Adsorption. Ind. Eng. Chem. Res. 2016, 55, 3097–3112. [Google Scholar] [CrossRef] [Green Version]
- Azizian, S.; Eris, S. Chapter 6—Adsorption isotherms and kinetics. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 445–509. [Google Scholar]
- Kapoor, A.; Ritter, J.A.; Yang, R.T. An extended Langmuir model for adsorption of gas mixtures on heterogeneous surfaces. Langmuir 1990, 6, 660–664. [Google Scholar] [CrossRef]
- Goel, C.; Bhunia, H.; Bajpai, P. Prediction of Binary Gas Adsorption of CO2 /N2 and Thermodynamic Studies on Nitrogen Enriched Nanostructured Carbon Adsorbents. J. Chem. Eng. Data 2017, 62, 214–225. [Google Scholar] [CrossRef]
- LeVan, M.D.; Vermeulen, T. Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions. J. Phys. Chem. 1981, 85, 3247–3250. [Google Scholar] [CrossRef]
- McIntyre, S.M.; Shan, B.; Wang, R.; Zhong, C.; Liu, J.; Mu, B. Monte Carlo Simulations to Examine the Role of Pore Structure on Ambient Air Separation in Metal–Organic Frameworks. Ind. Eng. Chem. Res. 2018, 57, 9240–9253. [Google Scholar] [CrossRef]
- Hauchhum, S.; Mahanta, P.; De Wilde, J. Capture of CO2 from Flue Gas onto Coconut Fibre-Based Activated Carbon and Zeolites in a Fixed Bed. Transp. Porous Media 2015, 110. [Google Scholar] [CrossRef]
- Krishna, R.; van Baten, J.M. Investigating the validity of the Bosanquet formula for estimation of diffusivities in mesopores. Chem. Eng. Sci. 2012, 69, 684–688. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Res. Chem. Intermed. 2016, 42, 2343–2364. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ. Sci. Pollut. Res. 2017, 24, 7511–7520. [Google Scholar] [CrossRef]
- Khalili, S.; Khoshandam, B.; Jahanshahi, M. A comparative study of CO2 and CH4 adsorption using activated carbon prepared from pine cone by phosphoric acid activation. Korean J. Chem. Eng. 2016, 33, 2943–2952. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; Aguilar-Armenta, G. Adsorption Kinetics and Equilibria of Carbon Dioxide, Ethylene, and Ethane on 4A(CECA) Zeolite. J. Chem. Eng. Data 2010, 55, 3625–3630. [Google Scholar] [CrossRef]
- Harper, R.J.; Stifel, G.R.; Anderson, R.B. Adsorption of gases on 4A synthetic zeolite. Can. J. Chem. 1969, 47, 4661–4670. [Google Scholar] [CrossRef]
- Wei, M.; Yu, Q.; Duan, W.; Hou, L.; Liu, K.; Qin, Q.; Liu, S.; Dai, J. Equilibrium and Kinetics Analysis of CO2 Adsorption on Waste Ion-exchange Resin-based Activated Carbon. J. Taiwan Inst. Chem. Eng. 2017, 77, 161–167. [Google Scholar] [CrossRef]
- Golchoobi, A.; Pahlavanzadeh, H. Molecular simulation, experiments and modelling of single adsorption capacity of 4A molecular sieve for CO2–CH4 separation. Sep. Sci. Technol. 2016, 51, 2318–2325. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Yang, Y.; Kong, X.-M.; Li, P.; Yu, J.-G.; Ribeiro, A.M.; Rodrigues, A.E. Adsorption of Pure and Binary CO2, CH4, and N2 Gas Components on Activated Carbon Beads. J. Chem. Eng. Data 2015, 60, 2684–2693. [Google Scholar] [CrossRef]
- Cornelius, M.-L.U.; Price, L.; Wells, S.A.; Petrik, L.F.; Sartbaeva, A. The steric influence of extra-framework cations on framework flexibility: An LTA case study. Z. Krist. Cryst. Mater. 2019, 234, 461–468. [Google Scholar] [CrossRef]
- Rzepka, P.; Bacsik, Z.; Smeets, S.; Hansen, T.C.; Hedin, N.; Wardecki, D. Site-Specific Adsorption of CO2 in Zeolite NaK-A. J. Phys. Chem. C 2018, 122, 27005–27015. [Google Scholar] [CrossRef]
- Grajciar, L.; Čejka, J.; Zukal, A.; Areán, C.O.; Palomino, G.T.; Nachtigall, P. Controlling the Adsorption Enthalpy of CO2 in Zeolites by Framework Topology and Composition. ChemSusChem 2012, 5, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Zukal, A.; Arean, C.O.; Delgado, M.R.; Nachtigall, P.; Pulido, A.; Mayerová, J.; Čejka, J. Combined volumetric, infrared spectroscopic and theoretical investigation of CO2 adsorption on Na-A zeolite. Microporous Mesoporous Mater. 2011, 146, 97–105. [Google Scholar] [CrossRef]
- Abdul Kareem, F.A.; Shariff, A.M.; Ullah, S.; Mellon, N.; Keong, L.K. Adsorption of pure and predicted binary (CO2:CH4) mixtures on 13X-Zeolite: Equilibrium and kinetic properties at offshore conditions. Microporous Mesoporous Mater. 2018, 267, 221–234. [Google Scholar] [CrossRef]
- Pino, D.; Bessières, D. CH4 /CO2 Mixture Adsorption on a Characterized Activated Carbon. J. Chem. Eng. Data 2017, 62. [Google Scholar] [CrossRef]
- Zhou, F.; Le-Hussain, F.; Guo, Z.; Yanici, S.; Cinar, Y. Adsorption/desorption characteristics for methane, nitrogen and carbon dioxide of coal samples from Southeast Qinshui Basin, China. Energy Explor. Exploit. 2013, 31, 645–666. [Google Scholar] [CrossRef] [Green Version]
- Cheung, O.; Hedin, N. ChemInform Abstract: Zeolites and Related Sorbents with Narrow Pores for CO2 Separation from Flue Gas. ChemInform 2014, 45. [Google Scholar] [CrossRef]
- Rios, R.B.; Stragliotto, F.M.; Peixoto, H.R.; Torres, A.E.B.; Bastos-Neto, M.; Azevedo, D.C.S.; Cavalcante, C.L., Jr. Studies on the adsorption behavior of CO2-CH4 mixtures using activated carbon. Braz. J. Chem. Eng. 2013, 30, 939–951. [Google Scholar] [CrossRef]
- Khalili, S.; Ghoreyshi, A.A.; Jahanshahi, M.; Khoshandam, B. Predictions of the adsorption equilibrium of CO2/O2 mixture on multi-walled carbon nanotube using Ideal Adsorbed Solution Theory. J. Water Environ. Nanotechnol. 2016, 1, 9–17. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E.A. Comparative Studies on CO2 Adsorption Kinetics by Solid Adsorbents. Energy Procedia 2016, 90, 316–325. [Google Scholar] [CrossRef]
- Malekbala, M.; Soltani, S.; Abdul Rashid, S.; Luqman Chuah, A.; Rashid, U.; Imededdine, N.; Choong, T.; Teo, S.H. Optimization the Process of Chemically Modified Carbon Nanofiber Coated Monolith via Response Surface Methodology for CO2 Capture. Materials 2020, 13, 1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, L.; Zacharia, R.; Bénard, P.; Chahine, R. Simulation of binary CO2/CH4 mixture breakthrough profiles in MIL-53(Al). J. Nanomater. 2015. [Google Scholar] [CrossRef] [Green Version]
- Pino, L.; Italiano, C.; Vita, A.; Fabiano, C.; Recupero, V. Sorbents with high efficiency for CO2 capture based on amines-supported carbon for biogas upgrading. J. Environ. Sci. 2016, 48, 138–150. [Google Scholar] [CrossRef]
- Ahn, H.; Moon, J.-H.; Hyun, S.-H.; Lee, C.-H. Diffusion Mechanism of Carbon Dioxide in Zeolite 4A and CaX Pellets. Adsorption 2004, 10, 111–128. [Google Scholar] [CrossRef]
- Arefi Pour, A.; Sharifnia, S.; Neishabori Salehi, R.; Ghodrati, M. Adsorption separation of CO2/CH4 on the synthesized NaA zeolite shaped with montmorillonite clay in natural gas purification process. J. Nat. Gas. Sci. Eng. 2016, 36, 630–643. [Google Scholar] [CrossRef]
- Tobarameekul, P.; Sangsuradet, S.; Chat, N.; Worathanakul, P. Enhancement of CO2 Adsorption Containing Zinc-ion-exchanged Zeolite NaA Synthesized from Rice Husk Ash. Appl. Sci. Eng. Prog. 2020. [Google Scholar] [CrossRef]


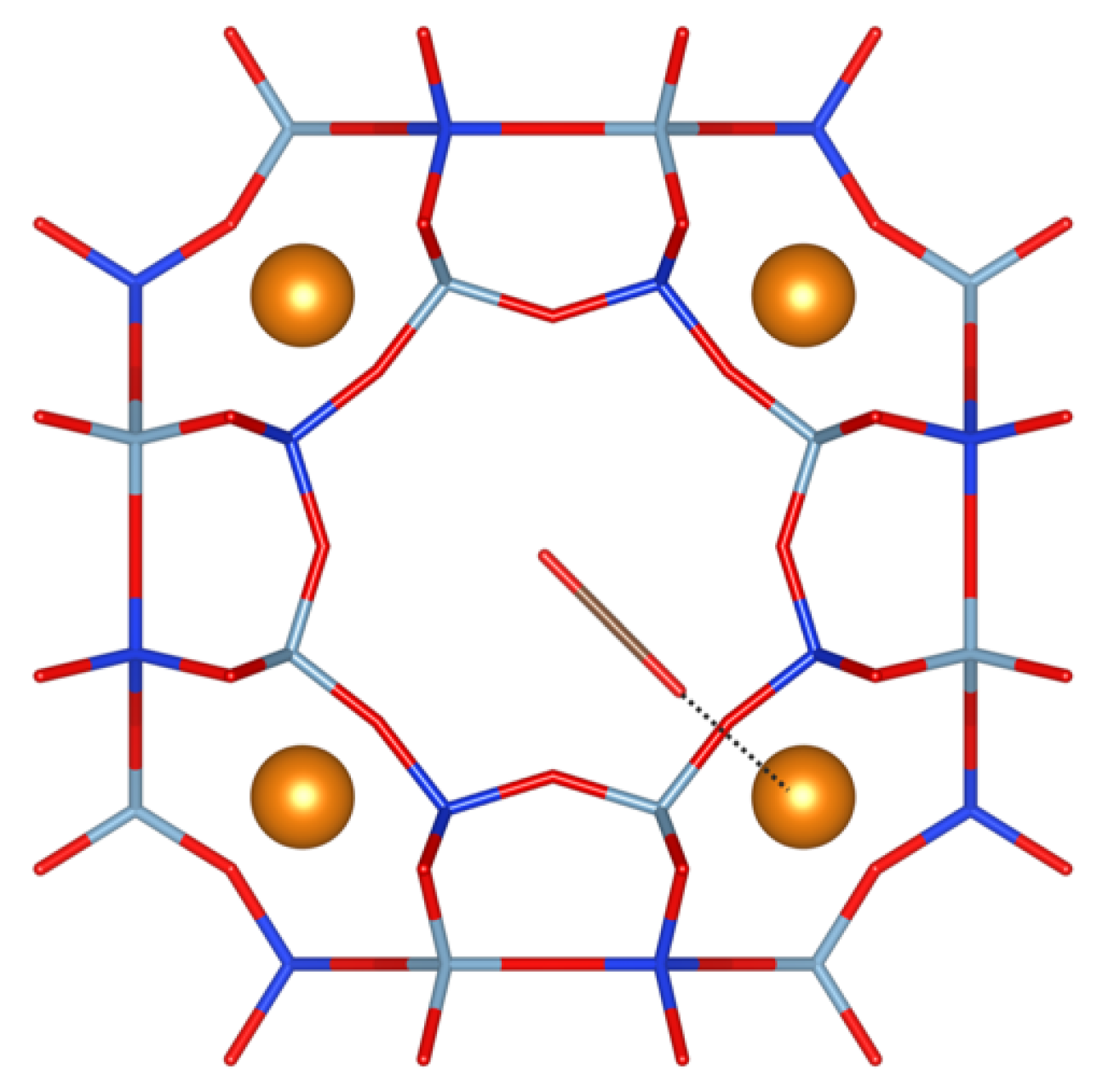


| Parameters | Value |
|---|---|
| Packing length (mm) | 97.5 |
| Internal bed diameter (mm) | 9.1 |
| Particle size (cm) | 0.2 (average diameter) |
| Pellet density (kg/m3) | 1109 |
| Solid density of adsorbent (kg/m3) | 2429 |
| Total intrusion volume (cm3/g) | 0.3147 |
| BET surface area (m2/g) | 501.4 |
| Properties | CO2 | CH4 |
|---|---|---|
| Kinetic diameter (Å) | 3.3 | 3.8 |
| Critical temperature (K) | 304.12 | 190.56 |
| Quadrupole moment (×1026 esu cm2) | 4.30 | 0 |
| Polarizability (×10−25 cm3) | 29.11 | 25.93 |
| Equation | Parameters | CO2 | CH4 |
|---|---|---|---|
| qm,0 (mmol/g) | 3.7881 | 3.4698 | |
| b0 (bar−1) | 0.7078 | 5.88 × 10−5 | |
| Q/R (K) | 470.91 | 2465.8 | |
| X (dimensionless) | 1.7188 | 0.003 | |
| RMS (dimensionless) | 0.0915 | 0.0236 |
| Adsorbent | P (Bar) | CO2:CH4 | (mmol/g) | mmol/g | Ref. |
|---|---|---|---|---|---|
| Zeolite 4A | 6 | 40:60 | 4.076 | 0.159 | This work |
| 10 | 50:50 | 4.400 | 0.102 | ||
| Zeolite 5A | 5.8 | 40:60 | 2.852 | 0.150 | [38] |
| Zeolite 13X | 10 | 50:50 | 4.277 | 0.252 | [44] |
| T (K) | P (Bar) | DP (cm2/s) | k (s−1) | ||
|---|---|---|---|---|---|
| CO2 | CH4 | CO2 | CH4 | ||
| 273 | 1 | 0.0196 | 0.0386 | 0.3222 | 0.6357 |
| 5 | 0.0046 | 0.0093 | 0.0749 | 0.1524 | |
| 10 | 0.0023 | 0.0047 | 0.0382 | 0.0781 | |
| 303 | 1 | 0.0232 | 0.0450 | 0.3820 | 0.7400 |
| 5 | 0.0055 | 0.0102 | 0.0906 | 0.1684 | |
| 10 | 0.0030 | 0.0057 | 0.0489 | 0.0942 | |
| 343 | 1 | 0.0283 | 0.0546 | 0.4662 | 0.8985 |
| 5 | 0.0069 | 0.0127 | 0.1137 | 0.2089 | |
| 10 | 0.0036 | 0.0071 | 0.0584 | 0.1174 | |
| Type of Zeolite | Particle Size (cm) | Pore Volume (cm3/g) | Pore Diameter (nm) | BET Surface (m2/g) |
|---|---|---|---|---|
| Zeolite 4A-0.2 cm | 0.2 | 0.3147 | 361 | 501.4 |
| Zeolite 4A (HSD)-0.2 cm | 0.2 | 0.1606 | 320 | 509.8 |
| Zeolite 4A-0.4 cm | 0.4 | 0.3012 | 314 | 510.4 |
| Type of Zeolite | DP (cm2/s) | k(s−1) | ||
|---|---|---|---|---|
| CO2 | CH4 | CO2 | CH4 | |
| Zeolite 4A-0.2 cm | 0.0030 | 0.0057 | 0.0489 | 0.0942 |
| Zeolite 4A (HSD)-0.2 cm | 0.0028 | 0.0056 | 0.0269 | 0.0546 |
| Zeolite 4A-0.4 cm | 0.0028 | 0.0057 | 0.012 | 0.0244 |
| Model | Parameter | Value |
|---|---|---|
| Thomas | kTh (L/mg min) | 1.232 × 10−3 |
| q0 (mg/g) | 654,490.9 | |
| R2 | 0.948 | |
| Yoon-Nelson | kYN (min−1) | 0.567 |
| τ (min) | 3.347 | |
| R2 | 0.974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parinyakit, S.; Worathanakul, P. Static and Dynamic Simulation of Single and Binary Component Adsorption of CO2 and CH4 on Fixed Bed Using Molecular Sieve of Zeolite 4A. Processes 2021, 9, 1250. https://doi.org/10.3390/pr9071250
Parinyakit S, Worathanakul P. Static and Dynamic Simulation of Single and Binary Component Adsorption of CO2 and CH4 on Fixed Bed Using Molecular Sieve of Zeolite 4A. Processes. 2021; 9(7):1250. https://doi.org/10.3390/pr9071250
Chicago/Turabian StyleParinyakit, Supatsorn, and Patcharin Worathanakul. 2021. "Static and Dynamic Simulation of Single and Binary Component Adsorption of CO2 and CH4 on Fixed Bed Using Molecular Sieve of Zeolite 4A" Processes 9, no. 7: 1250. https://doi.org/10.3390/pr9071250
APA StyleParinyakit, S., & Worathanakul, P. (2021). Static and Dynamic Simulation of Single and Binary Component Adsorption of CO2 and CH4 on Fixed Bed Using Molecular Sieve of Zeolite 4A. Processes, 9(7), 1250. https://doi.org/10.3390/pr9071250