Role of Activated Carbon Precursor for Mercury Oxidation and Removal: Oxidized Surface and Carbene Site Interaction
Abstract
1. Introduction
1.1. Physisorption
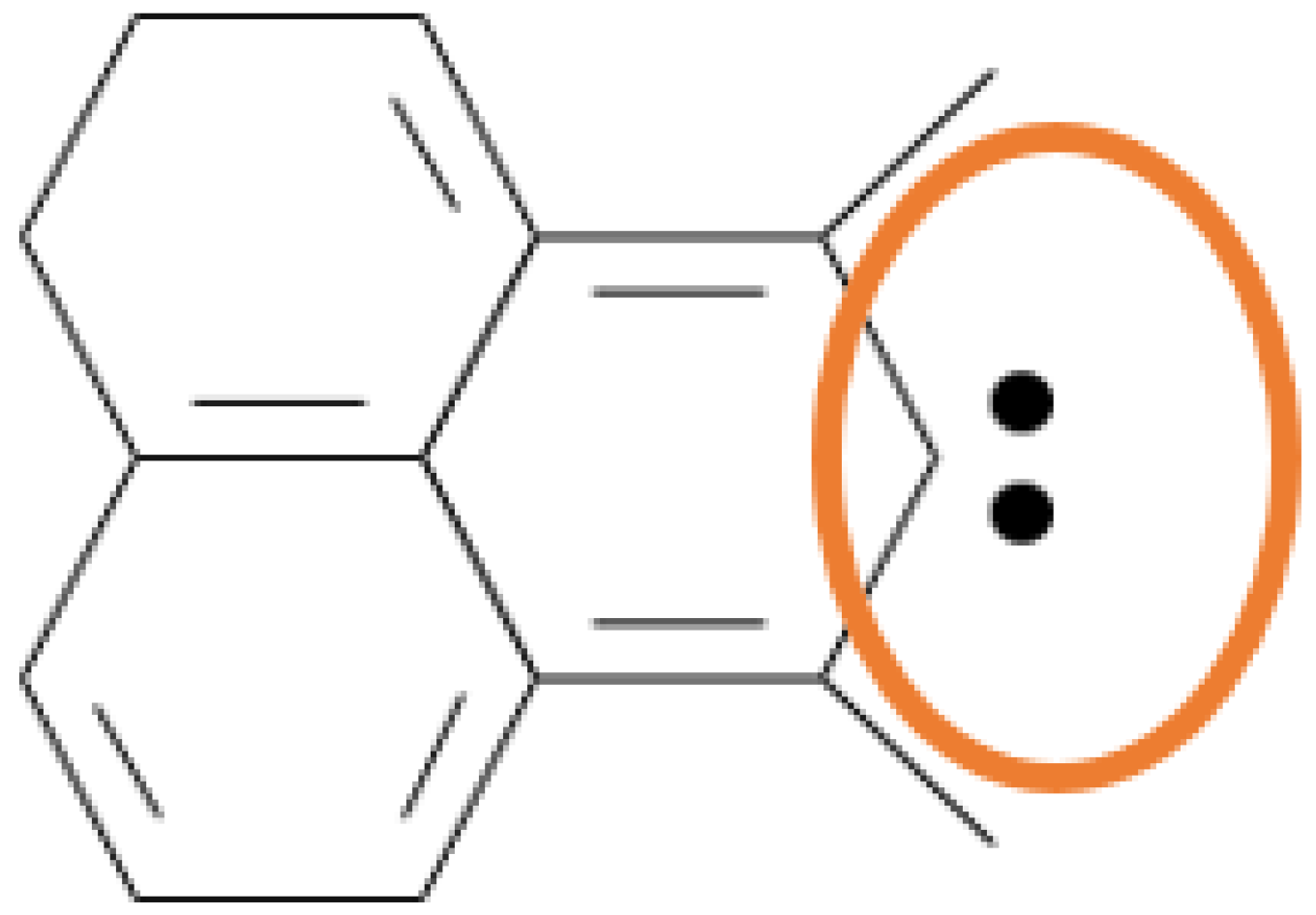
1.2. Chemisorption at a Carbene Site
1.3. Oxygen Functional Groups
2. Materials and Methods
2.1. Physical and Chemical Characterization of Activated Carbons
2.1.1. Activated Carbon Raw Material
2.1.2. Surface Area, Pore Volume, and Pore Size Characterization
2.1.3. Point of Zero Charge (pHpzc)
2.1.4. Fourier Transformed Infrared Spectroscopy
2.1.5. Boehm Titrations
2.2. Surface Chemistry Modifications of Sorbents
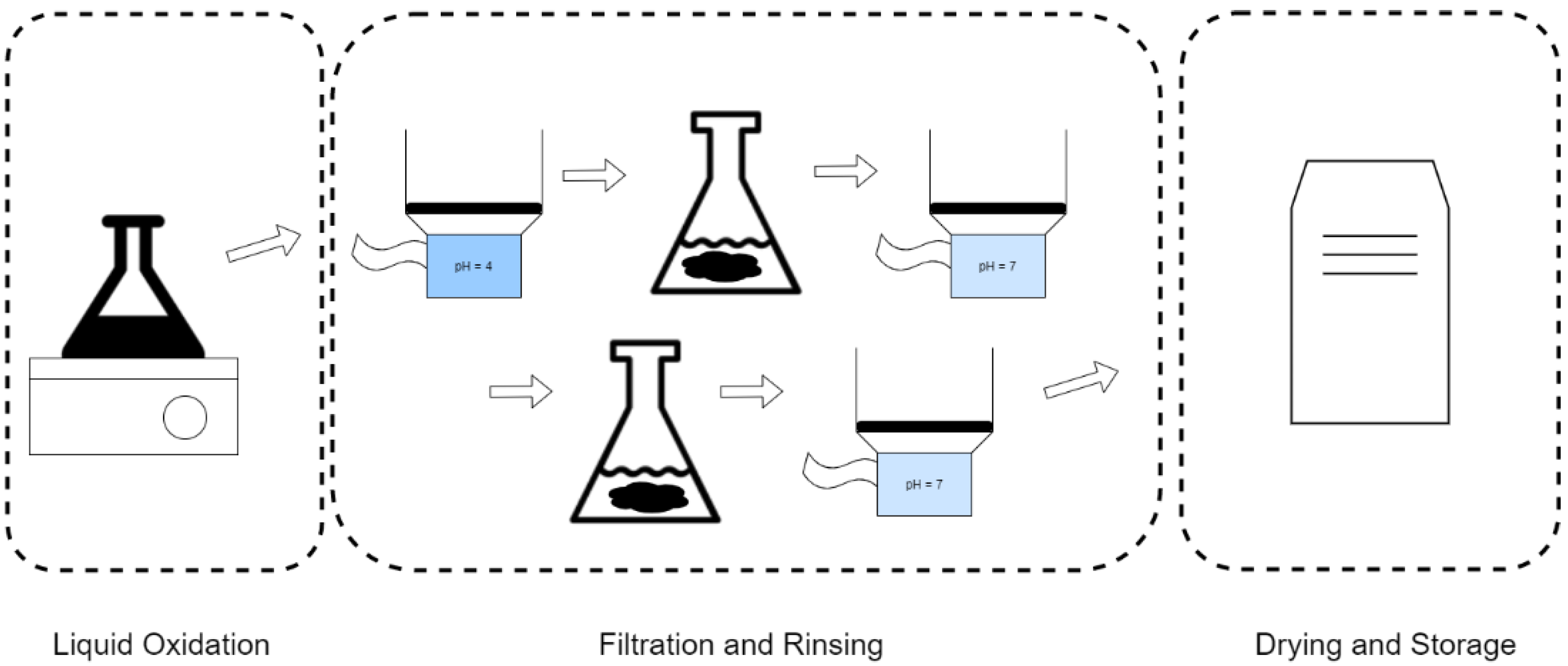
2.2.1. Nitric Acid Treatment of Activated Carbon
2.2.2. Peroxide Treatment of Activated Carbon
2.2.3. Nitrogen Gas Treatment of Activated Carbon
2.3. Gas Phase Mercury Removal
3. Results & Discussion
3.1. Physical Properties
3.2. Surface Chemistry Developed on Activated Carbons
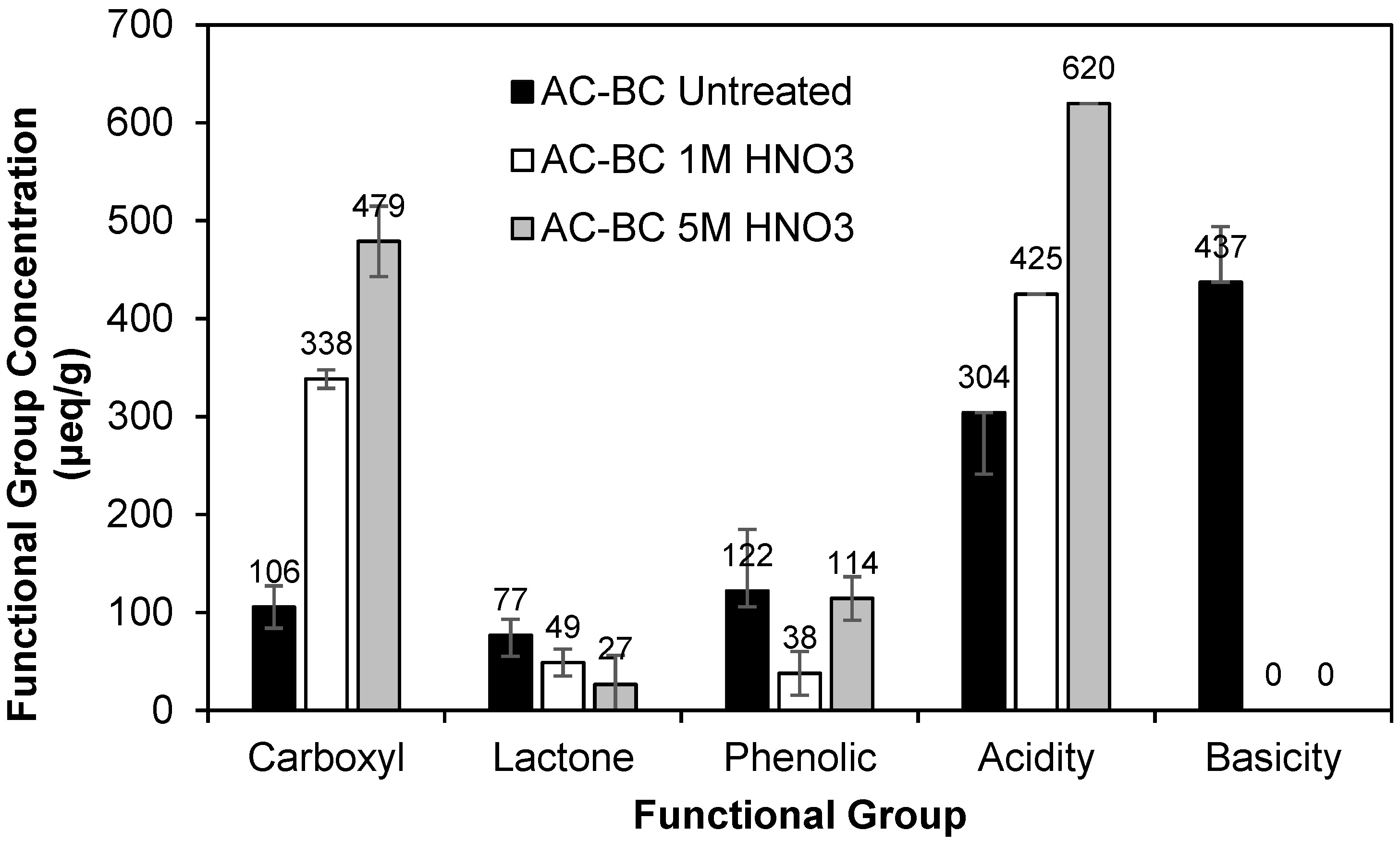
3.2.1. Boehm Results
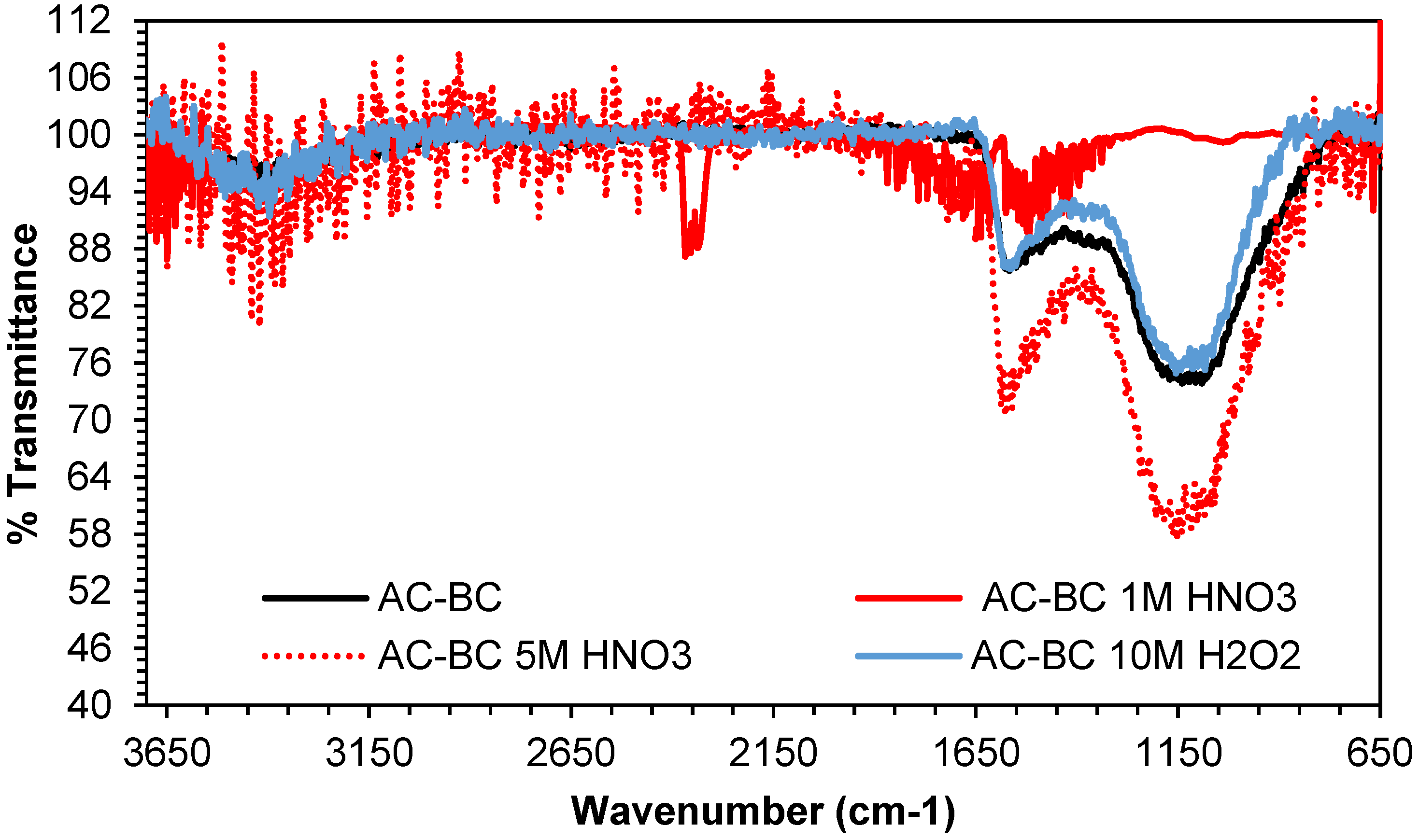
3.2.2. FTIR Analysis
3.3. Oxidation Impacts on Mercury Removal
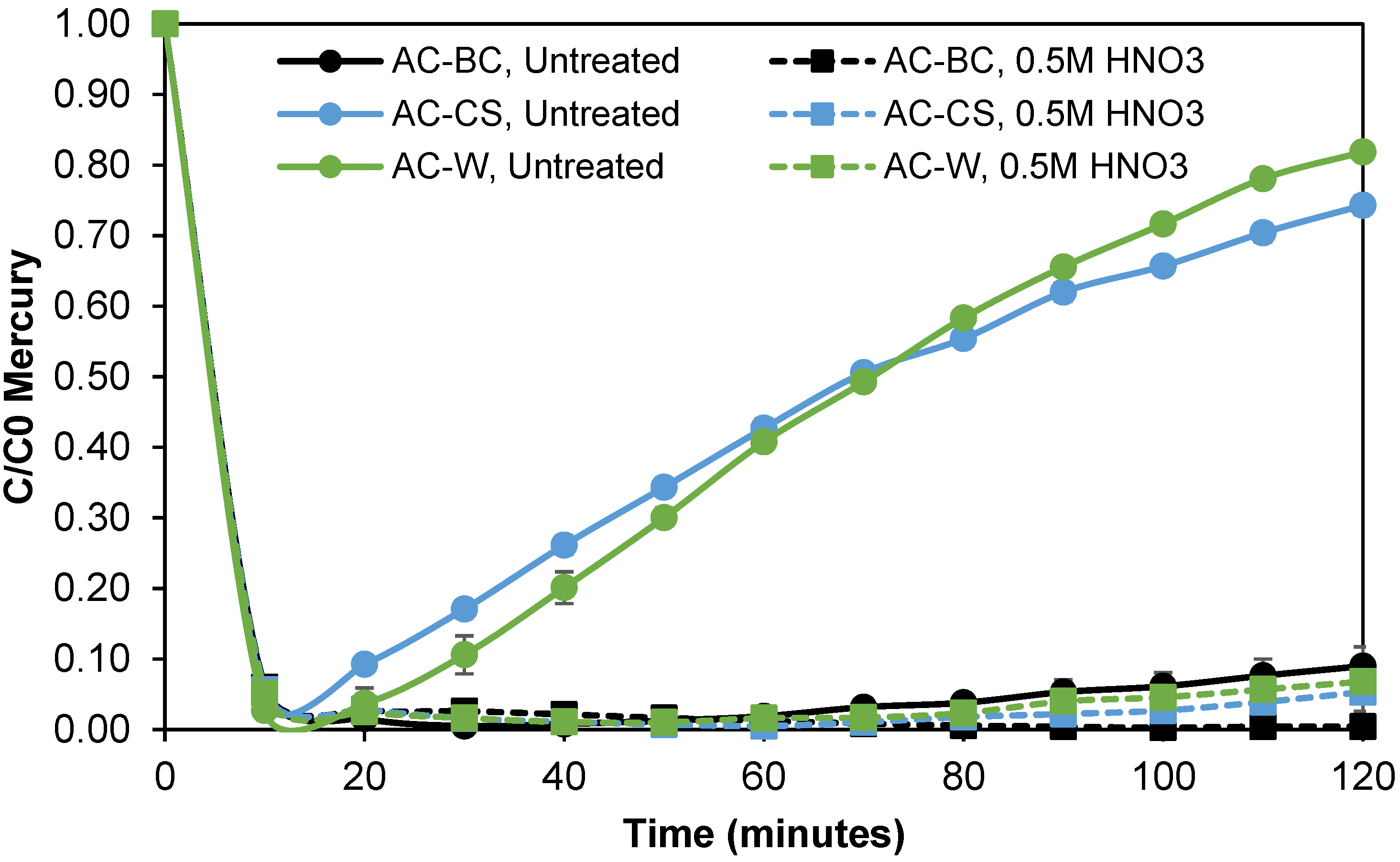
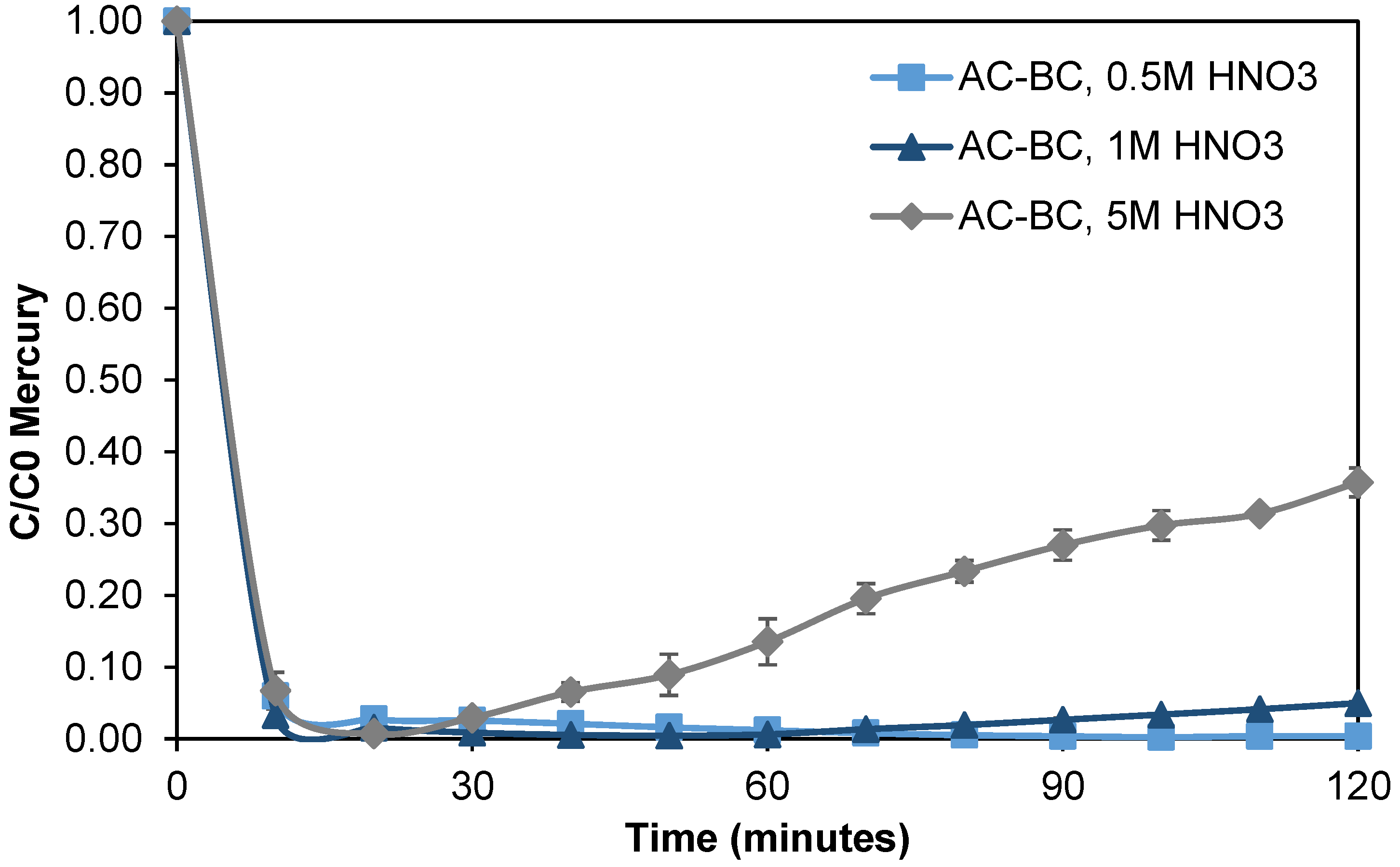
3.3.1. Nitric Acid Oxidation Treatments
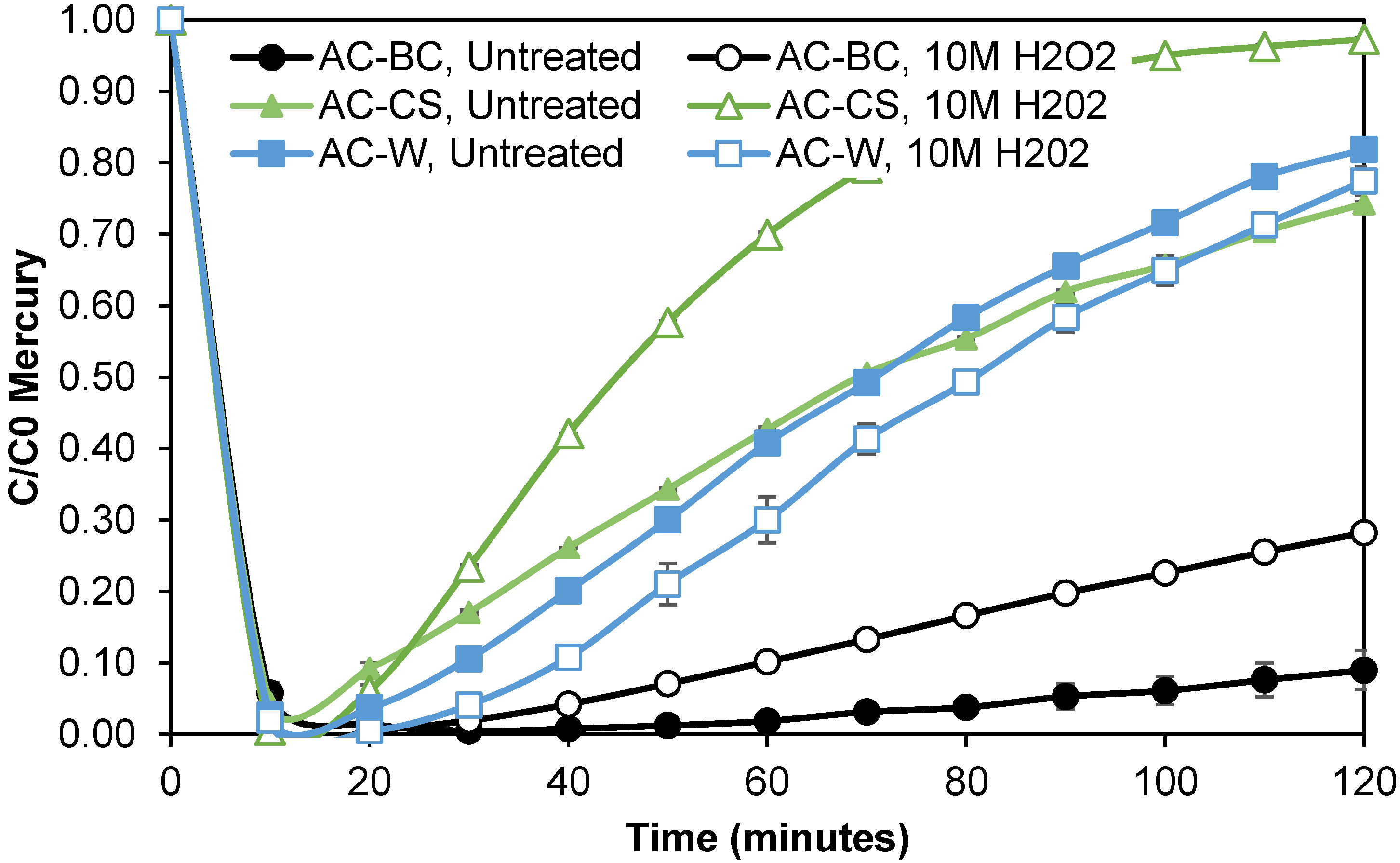
3.3.2. Hydrogen Peroxide Oxidation
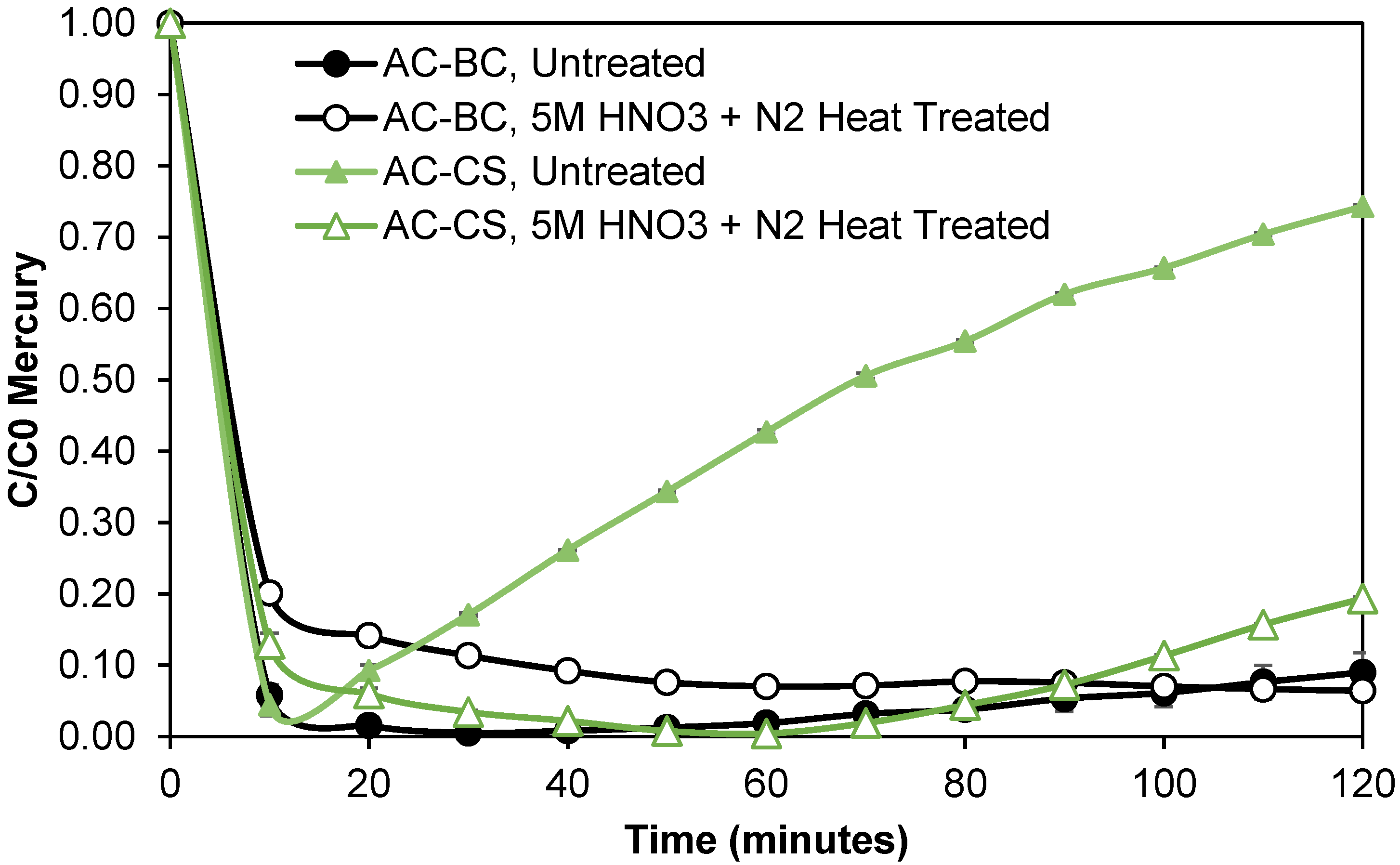
3.3.3. Creation of Carbene sites through Surface Etching
3.4. Graphite as a Comparison for Aromatic Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloss, L.L. Economics of Mercury Control; IEA Clean Coal Centre: London, UK, 2008. [Google Scholar]
- Auzmendi-Murua, I.; Castillo, A.; Bozzelli, J.W. Mercury oxidation via chlorine, bromine, and iodine under atmospheric conditions: Thermochemistry and kinetics. J. Phys. Chem. A 2014, 118, 2959–2975. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.S. Full Scale Calcium Bromide Injection with Subsequent Mercury Oxidation and Removal within Wet Flue Gas Desulphurization System: Experience at a 700 MW Coal-Fired Power Facility; The University of Alabama at Birmingham: Birmingham, AL, USA, 2012. [Google Scholar]
- Dranga, B.A.; Lazar, L.; Koeser, H. Oxidation Catalysts for Elemental Mercury in Flue Gases—A Review atalysts. Catalysts 2012, 2, 139. [Google Scholar] [CrossRef]
- Qu, Z.; Yan, N.; Liu, P.; Chi, Y.; Jia, J. Bromine chloride as an oxidant to improve elemental mercury removal from coal-fired flue gas. Environ. Sci. Technol. 2009, 43, 8610–8615. [Google Scholar] [CrossRef]
- Pavlish, H.J.; Hamre, L.L.; Zhuang, Y. Mercury control technologies for coal combustion and gasification systems. Fuel 2010, 89, 838. [Google Scholar] [CrossRef]
- Shewchuk, S.; Azargohar, R.; Dalaia, K. Elemental mercury capture using activated carbon: A review. J. Environ. Anal. Toxicol. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Reddy, B.M.; Durgasri, N.; Kumar, T.V.; Bhargava, S.K. Abatement of gas-phase mercury—Recent developments. Catal. Rev. 2012, 54, 344–398. [Google Scholar] [CrossRef]
- Skodras, G.; Diamantopoulou, I.; Zabaniotou, A.; Stavropoulos, G.; Sakellaropoulos, G. Enhanced mercury adsorption in activated carbons from biomass materials and waste tires. Fuel Process. Technol. 2007, 88, 749–758. [Google Scholar] [CrossRef]
- Skodras, G.; Diamantopoulou, I.; Pantoleontos, G.; Sakellaropoulos, G. Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor. J. Hazard. Mater. 2008, 158, 1–13. [Google Scholar] [CrossRef]
- Diamantopoulou, I.; Skodras, G.; Sakellaropoulos, G. Sorption of mercury by activated carbon in the presence of flue gas components. Fuel Process. Technol. 2010, 91, 158–163. [Google Scholar] [CrossRef]
- Vidic, R.D.; Siler, D.P. Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon 2001, 31, 3–14. [Google Scholar] [CrossRef]
- Rupp, E.C.; Wilcox, J. Mercury chemistry of brominated activated carbons–packed-bed breakthrough experiments. Fuel 2014, 117, 351–353. [Google Scholar] [CrossRef]
- Saha, A.; Abram, D.N.; Kuhl, K.P.; Paradis, J.; Crawford, J.L.; Sasmaz, E.; Chang, R.; Jaramillo, T.F.; Wilcox, J. An X-ray photoelectron spectroscopy study of surface changes on brominated and sulfur-treated activated carbon sorbents during mercury capture: Performance of pellet versus fiber sorbents. Environ. Sci. Technol. 2013, 47, 13695–13701. [Google Scholar] [CrossRef]
- Sasmaz, E.; Kirchofer, A.; Jew, A.D.; Saha, A.; Abram, D.; Jaramillo, T.F.; Wilcox, J. Mercury chemistry on brominated activated carbon. Fuel 2012, 99, 188–196. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Sorbents for Mercury Removal from Flue Gas; U.S. Department of Energy: Pittsburgh, PA, USA, 1998.
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Novel sorbents for mercury removal from flue gas. Ind. Eng. Chem. Res. 2000, 39, 1020–1029. [Google Scholar] [CrossRef]
- Li, Y.H.; Lee, C.W.; Gullett, B.K. Importance of activated carbon’s oxygen surface functional groups on elemental mercury adsorption. Fuel 2003, 82, 451. [Google Scholar] [CrossRef]
- Faulconer, E.K.; Mazyck, D.W. Influence of activated carbon surface oxygen functionality on elemental mercury adsorption from aqueous solution. J. Environ. Chem. Eng. 2017, 7, 2879–2885. [Google Scholar] [CrossRef]
- Li, N.; Ma, X.; Zha, Q.; Kim, K.; Chen, Y.; Song, C. Maximizing the number of oxygen-containing functional groups on activated carbon by using ammonium persulfate and improving the temperature-programmed desorption characterization of carbon surface chemistry. Carbon 2011, 49, 5002–5013. [Google Scholar] [CrossRef]
- Karatza, D.; Lancia, A.; Prisciandaro, M.; Musmarra, D.; Di Celso, G.M. Influence of oxygen on adsorption of elemental mercury vapors onto activated carbon. Fuel 2013, 111, 485–491. [Google Scholar] [CrossRef]
- Leon y Leon, C.A.; Radovic, L.R. Influence of Oxygen Functional Groups on the Performance of Carbon-Supported Catalysts. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1991; p. 1007. [Google Scholar]
- Jaramillo, J.; Álvarez, P.M.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 256, 5232. [Google Scholar] [CrossRef]
- Walker, P., Jr.; Austin, L.; Tietjen, J. Chemisorption of oxygen on graphite as followed by thermoelectric power measurements. Carbon 1965, 2, 434–436. [Google Scholar] [CrossRef]
- Olson, E.S.; Laumb, J.D.; Benson, S.A.; Dunham, G.E.; Sharma, R.K.; Mibeck, B.A.; Miller, S.J.; Holmes, M.J.; Pavlish, J.H. The Mechanism for Chemisorption of Elemental Mercury on Activated Carbons in Flue Gas. 2004. Available online: https://www.researchgate.net/profile/Blaise-Mibeck/publication/267382254_THE_MECHANISM_FOR_CHEMISORPTION_OF_ELEMENTAL_MERCURY_ON_ACTIVATED_CARBONS_IN_FLUE_GAS/links/546e1a330cf2bc99c2151f2f/THE-MECHANISM-FOR-CHEMISORPTION-OF-ELEMENTAL-MERCURY-ON-ACTIVATED-CARBONS-IN-FLUE-GAS.pdf (accessed on 7 July 2021).
- Rodriguez, R.; Contrino, D.; Mazyck, D.W. Role of Activated Carbon Precursor in Mercury Removal. Ind. Eng. Chem. Res. 2020, 59, 17740–17747. [Google Scholar] [CrossRef]
- Hsi, H.; Rood, M.J.; Rostam-Abadi, M.; Chang, Y. Effects of sulfur, nitric acid, and thermal treatments on the properties and mercury adsorption of activated carbons from bituminous coals. Aerosol Air Qual. Res. 2013, 13, 730. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Zhang, Y.; Granite, E.J.; Tang, Z.; Pennline, H.W. Effect of porous structure and surface functionality on the mercury capacity of a fly ash carbon and its activated sample. Fuel 2005, 84, 105–108. [Google Scholar] [CrossRef]
- Radovic, L.R. The mechanism of CO2 chemisorption on zigzag carbon active sites: A computational chemistry study. Carbon 2005, 43, 907–915. [Google Scholar] [CrossRef]
- Radovic, L.R.; Bockrath, B. What exactly is on the edges of graphene layers in carbon: The unfolding story. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2002, 47, 428–431. [Google Scholar]
- Olson, E.S.; Miller, S.J.; Sharma, R.K.; Dunham, G.E.; Benson, S.A.; Olson, E.S. Catalytic effects of carbon sorbents for mercury capture. J. Hazard. Mater. 2000, 74, 61. [Google Scholar] [CrossRef]
- Olson, E.S.; Laumb, J.D.; Benson, S.A.; Dunham, G.E.; Sharma, R.K.; Miller, S.J.; Pavlish, J.H. The multiple site model for flue gas Mercury interactions on activated carbons: The Basic Site. Fuel Chem. Div. Prepr. 2003, 48, 1. [Google Scholar]
- Olson, E.S.; Mibeck, B.A.; Benson, S.A.; Laumb, J.D.; Crocker, C.R.; Dunham, G.E.; Sharma, R.K.; Miller, S.J.; Pavlish, J.H. The mechanistic model for flue gas-mercury interactions on activated carbons: The oxidation site. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2004, 49, 279. [Google Scholar]
- Olson, E.S.; Azenkeng, A.; Laumb, J.D.; Jensen, R.R.; Benson, S.A.; Hoffmann, M.R. New developments in the theory and modeling of mercury oxidation and binding on activated carbons in flue gas. Fuel Process. Technol. 2009, 90, 1360. [Google Scholar] [CrossRef]
- Wilcox, J.; Sasmaz, E.; Kirchofer, A.; Lee, S. Heterogeneous mercury reaction chemistry on activated carbon. J. Air Waste Manag. Assoc. 2011, 61, 418–426. [Google Scholar] [CrossRef]
- Padak, B.; Brunetti, M.; Lewis, A.; Wilcox, J. Mercury binding on activated carbon. Environ. Prog. 2006, 25, 319–326. [Google Scholar] [CrossRef]
- Padak, B.; Wilcox, J. Understanding mercury binding on activated carbon. Carbon 2009, 47, 2855–2864. [Google Scholar] [CrossRef]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.H.; Negreira, A.S.; Kirchofer, A.; Feng, F.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90, 4–20. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Phillips, J.; Xia, B.; Radovic, L.R. On the modification and characterization of chemical surface properties of activated carbon: In the search of carbons with stable basic properties. Langmuir 1996, 12, 4404–4410. [Google Scholar] [CrossRef]
- Li, Y.H.; Lee, C.W.; Gullett, B.K. The effect of activated carbon surface moisture on low temperature mercury adsorption. Carbon 2002, 40, 65. [Google Scholar] [CrossRef]
- Hall, B.; Schager, P.; Weesmaa, J. The homogeneous gas phase reaction of mercury with oxygen, and the corresponding heterogeneous reactions in the presence of activated carbon and fly ash. Chemosphere 1995, 30, 611–627. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y. Gas-phase mercury removal by carbon-based sorbents. Fuel Process. Technol. 2003, 84, 197–206. [Google Scholar] [CrossRef]
- Kwon, S.; Borguet, E.; Vidic, R. Impact of surface heterogeneity on mercury uptake by carbonaceous sorbents under UHV and atmospheric pressure. Environ. Sci. Technol. 2002, 36, 4162–4169. [Google Scholar] [CrossRef]
- Lizzio, A.A.; DeBarr, J.A. Mechanism of SO2 removal by carbon. Energy Fuels 1997, 11, 284–291. [Google Scholar] [CrossRef]
- University of Colorado Boulder. Table of Characteristic IR Absorptions. Available online: https://www.orgchemboulder.com/Spectroscopy/irtutor/tutorial.shtml (accessed on 7 July 2021).
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Boehm, H.P.; Diehl, E.; Heck, W.; Sappok, R. Surface oxides of carbon. Angew. Chem. Int. Ed. Engl. 1964, 3, 669. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L. Evaluation of Modified Boehm Titration Methods for Use with Biochars. J. Environ. Qual. 2013, 42, 1771–1778. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm Titration. Part I. CO2 Expulsion and Endpoint Determination. Carbon 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Oickle, A.M.; Goertzen, S.L.; Hopper, K.R.; Abdalla, Y.O.; Andreas, H.A. Standardization of the Boehm Titration: Part II. Method of Agitation, Effect of Filtering and Dilute Titrant. Carbon 2010, 48, 3313–3322. [Google Scholar] [CrossRef]
- Barkauskas, J.; Dervinyte, M. An investigation of the functional groups on the surface of activated carbons. J. Serb. Chem. Soc. 2004, 69, 363. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Piriz-Almeida, F.; Durán-Valle, C.J.; Pastor-Villegas, J. Formation of oxygen structures by air activation. A study by FT-IR spectroscopy. Carbon 1999, 37, 1517–1528. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Lee, J.; Keener, T.C.; Liu, Z.; Yao, X. The effect of surface properties in activated carbon on mercury adsorption. Ind. Eng. Chem. Res. 2012, 51, 9136–9144. [Google Scholar] [CrossRef]
- Kim, B.; Bae, K.; An, K.; Park, S. Elemental mercury adsorption behaviors of chemically modified activated carbons. Bull. Korean Chem. Soc. 2011, 32, 1321–1326. [Google Scholar] [CrossRef][Green Version]
- Salame, I.I.; Bandosz, T.J. Surface Chemistry of Activated Carbons Combining the Results of Temperature Programmed Desorption, Boehm, And Potentiometric Titrations. J. Colloid Interface Sci. 2001, 240, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Ferro-Garcia, M.A.; Joly, J.P.; Bautista-Toledo, I.; Carrasco-Marin, F.; Rivera-Utrilla, J. Activated carbon surface modifications by nitric acid, hydrogen peroxide, and ammonium peroxydisulfate treatments. Langmuir 1995, 11, 4386–4392. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marın, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Molina-Sabio, M.; Munecas, M.A. Effect of microporosity and oxygen surface groups of activated carbon in the adsorption of molecules of different polarity. J. Phys. Chem. 1992, 96, 2707–2713. [Google Scholar] [CrossRef]
- Stokke, J.M.; Mazyck, D.W. Development of a regenerable system employing silica-titania composites for the recovery of mercury from end-box exhaust at a chlor-alkali facility. J. Air Waste Manag. Assoc. 2008, 58, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.P.; Walker, P., Jr. Hydrogen Sorption on Graphite at Elevated Temperatures1, 2. J. Phys. Chem. 1960, 64, 1093–1099. [Google Scholar] [CrossRef]





| Sample | Raw Material |
|---|---|
| AC—CS | Coconut Shell |
| AC—W | Wood |
| AC—BC | Bituminous Coal |
| GH | Graphite |
| Frequency, cm−1 | Bond | Oxygen Containing Functional Groups |
|---|---|---|
| 3640–3610 (s, sh) | O–H stretch, free hydroxyl | alcohols, phenols |
| 3500–3200 (s,b) | O–H stretch, H–bonded | alcohols, phenols |
| 3300–2500 (m) | O–H stretch | carboxylic acids |
| 1760–1665 (s) | C=O stretch | carbonyls (general) |
| 1760–1690 (s) | C=O stretch | carboxylic acids |
| 1320–1000 (s) | C–O stretch | alcohols, carboxylic acids, esters, ethers |
| 950–910 (m) | O–H bend | carboxylic acids |
| Carbon Raw Material | Carbon Sample | BET Surface Area (m2/g) | Average Pore Size (Å) | Total Pore Volume (cc/g) | BJH Pore Volume (cc/g) | pHPZC |
|---|---|---|---|---|---|---|
| Bituminous Coal | AC—BC | 889 | 21.1 | 0.47 | 0.09 | 8.1 |
| AC-BC 0.5 M HNO3 | 1027 | 22.3 | 0.57 | 0.11 | 4.9 | |
| AC-BC 1 M HNO3 | 889 | 21.2 | 0.47 | 0.10 | 3.7 | |
| AC-BC 5 M HNO3 | 948 | 22.0 | 0.52 | 0.10 | 3.2 | |
| AC-BC 10 M H2O2 | 890 | 20.8 | 0.46 | 0.07 | 6.8 | |
| AC-BC 5 M HNO3 NT | 949 | 22.8 | 0.54 | 0.11 | 9.8 | |
| Coconut Shell | AC—CS | 1125 | 17.5 | 0.49 | 0.03 | 8.8 |
| AC-CS 0.5 M HNO3 | 1079 | 17.1 | 0.46 | 0.02 | 4.2 | |
| AC-CS 1 M HNO3 | 1007 | 17.3 | 0.44 | 0.02 | 4.0 | |
| AC-CS 5 M HNO3 | 1042 | 17.0 | 0.44 | 0.01 | 3.7 | |
| AC-CS 5 M HNO3 NT | 1219 | 17.3 | 0.53 | 0.02 | 9.0 | |
| Wood | AC—W | 423 | 22.3 | 0.24 | 0.05 | 8.4 |
| AC-W 0.5 M HNO3 | 405 | 23.8 | 0.23 | 0.06 | 4.4 | |
| AC-W 1 M HNO3 | 390 | 23.8 | 0.23 | 0.06 | 4.6 | |
| AC-W 10 M H2O2 | 475 | 22.3 | 0.26 | 0.04 | 7.9 |
| Sample | BET Surface Area | Average Pore Size | Total Pore Volume |
|---|---|---|---|
| (m2/g) | (Å) | (cc/g) | |
| GH | 7 | 159.4 | 0.03 |
| GH—HT | 39 | 95.9 | 0.09 |
| GH—NT (1 M HNO3) | 11 | 185.4 | 0.05 |
| Sample | Carbon Raw Material | Hg0 Loading @ 120 min | Hg0 Loading @ 120 min per Surface Area |
|---|---|---|---|
| (ng/g) | (ng/m2) | ||
| AC—BC | Bituminous Coal | 2949 | 3 |
| AC—BC HT | 1641 | 2 | |
| AC—BC NT (1 M HNO3) | 3014 | 3 | |
| GH | Graphite | 776 | 111 |
| GH—HT | 1005 | 26 | |
| GH—NT (1 M HNO3) | 926 | 82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, R.; Contrino, D.; Mazyck, D. Role of Activated Carbon Precursor for Mercury Oxidation and Removal: Oxidized Surface and Carbene Site Interaction. Processes 2021, 9, 1190. https://doi.org/10.3390/pr9071190
Rodriguez R, Contrino D, Mazyck D. Role of Activated Carbon Precursor for Mercury Oxidation and Removal: Oxidized Surface and Carbene Site Interaction. Processes. 2021; 9(7):1190. https://doi.org/10.3390/pr9071190
Chicago/Turabian StyleRodriguez, Regina, Domenic Contrino, and David Mazyck. 2021. "Role of Activated Carbon Precursor for Mercury Oxidation and Removal: Oxidized Surface and Carbene Site Interaction" Processes 9, no. 7: 1190. https://doi.org/10.3390/pr9071190
APA StyleRodriguez, R., Contrino, D., & Mazyck, D. (2021). Role of Activated Carbon Precursor for Mercury Oxidation and Removal: Oxidized Surface and Carbene Site Interaction. Processes, 9(7), 1190. https://doi.org/10.3390/pr9071190






