Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant
Abstract
1. Introduction
2. Materials, Methods, and Influencing Process Parameters
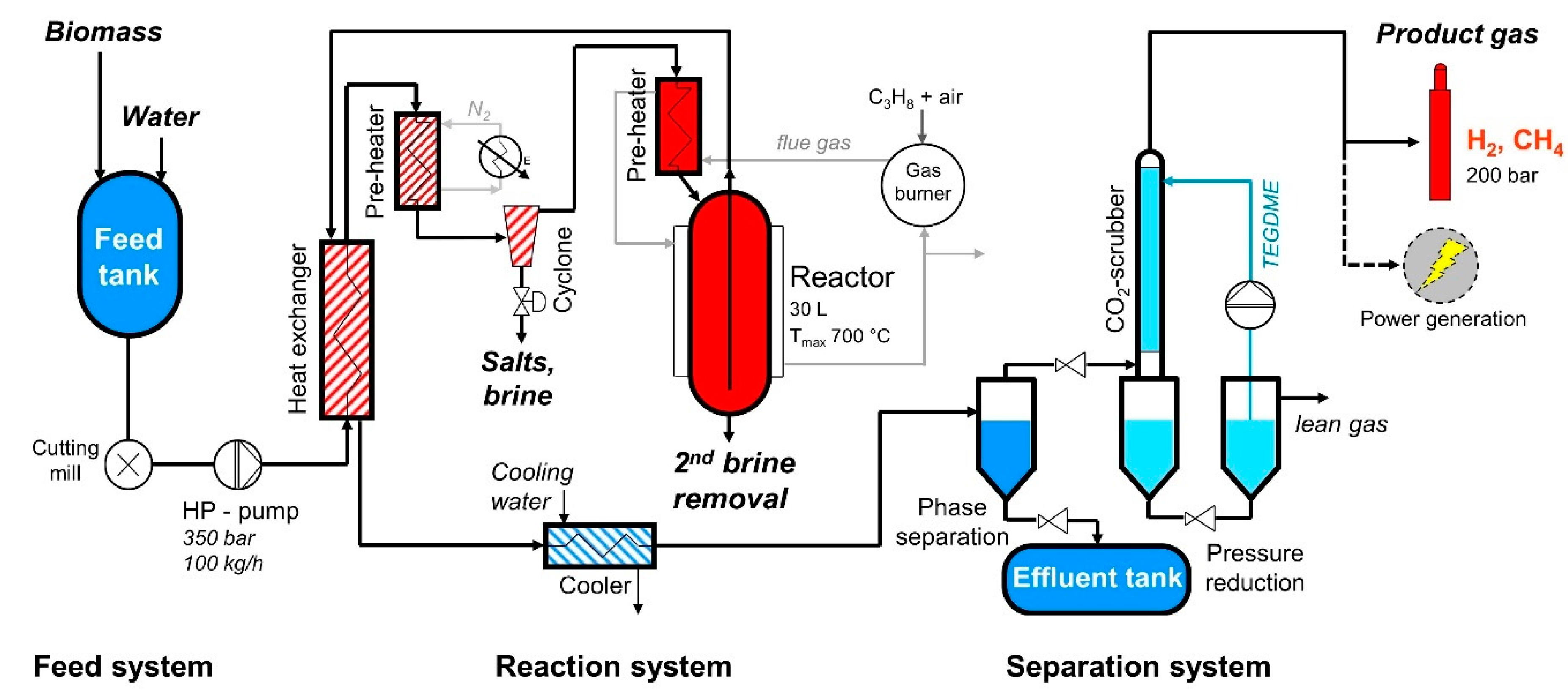
2.1. Description of the SCWG Process as Realized in the VERENA Pilot Plant
2.2. Typical Pretreatment of the Feed Material
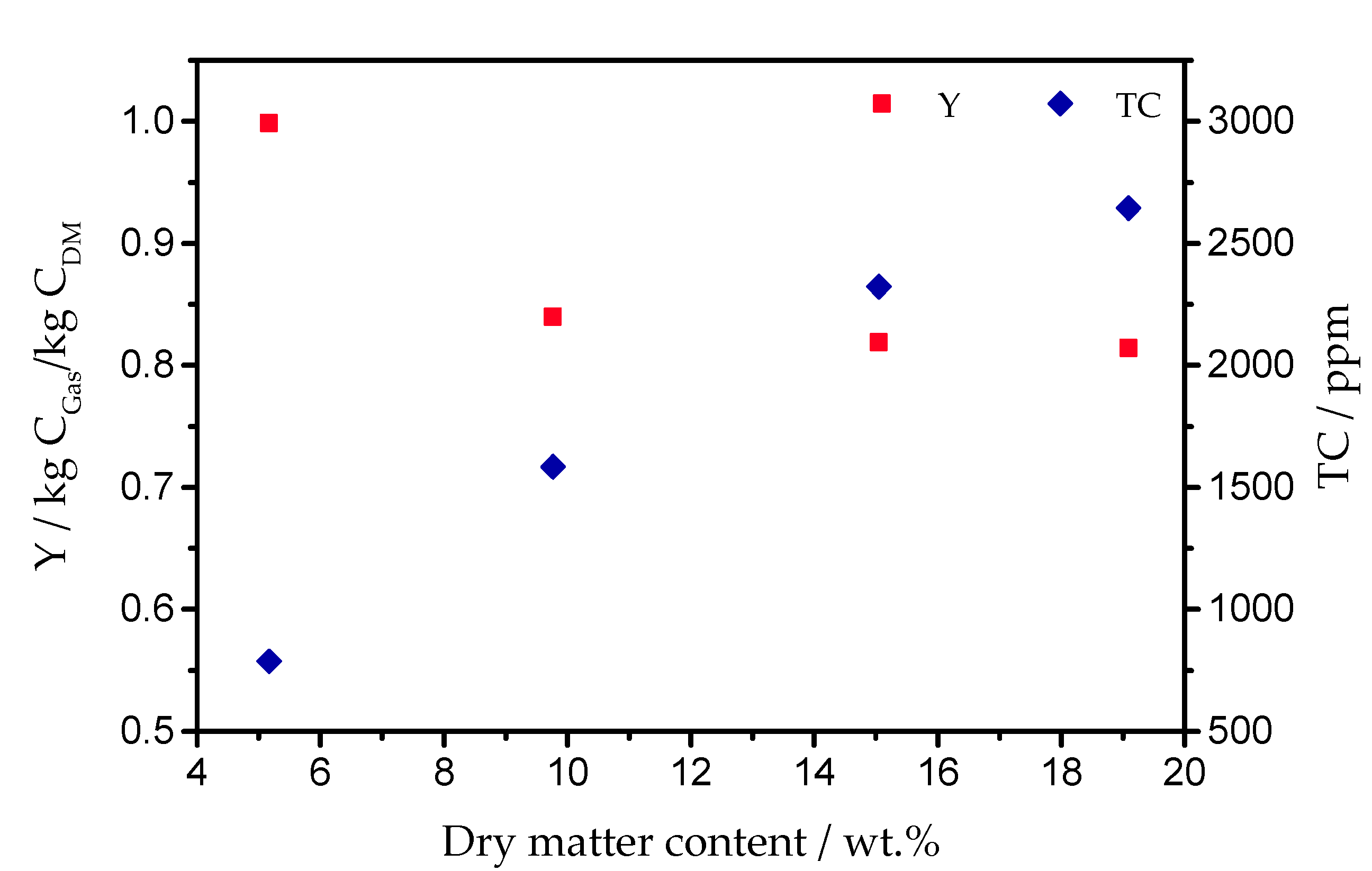
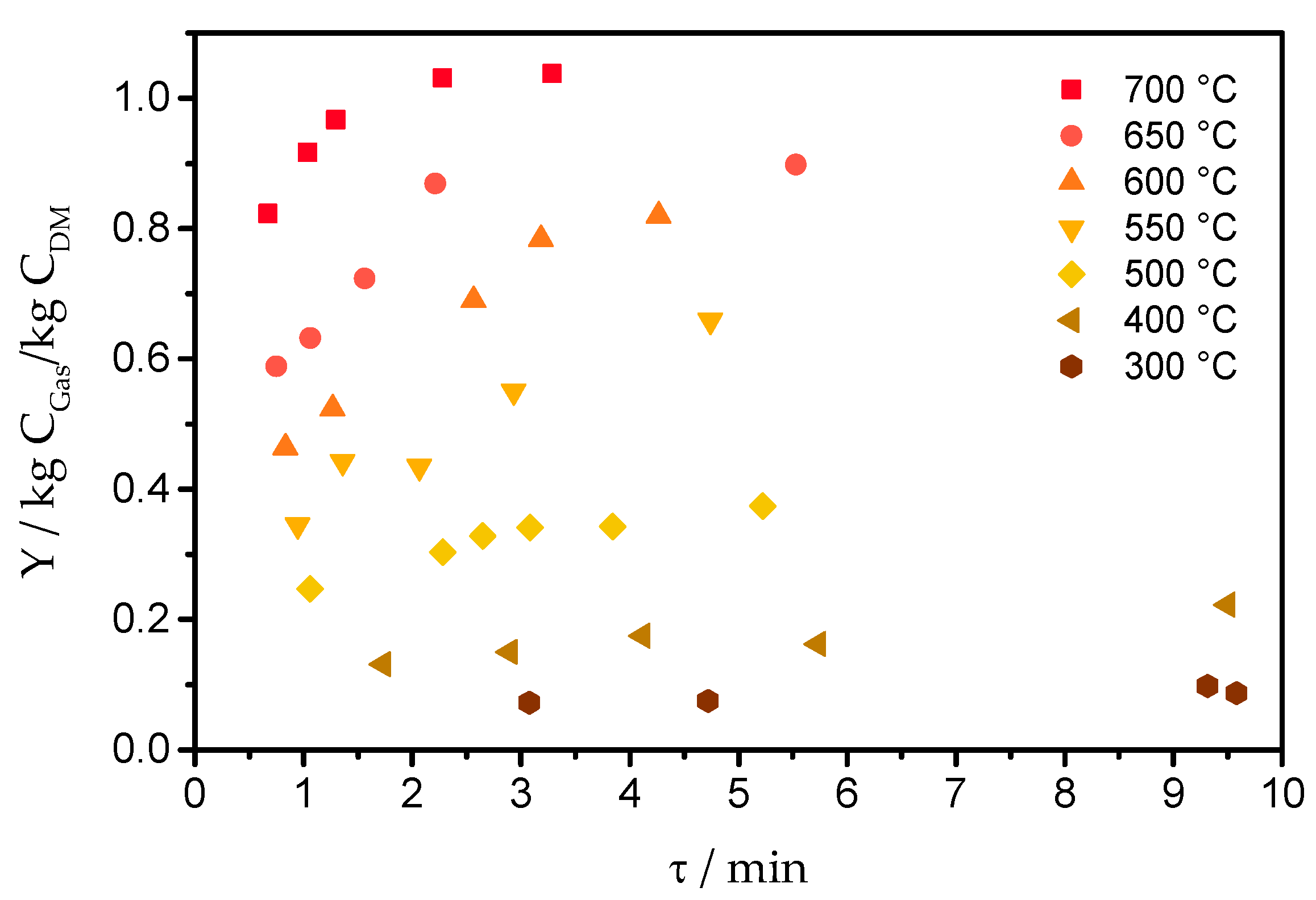
2.3. Main Influence Parameters of the Gasification Process
3. Selected Gasification Results of the VERENA Pilot Plant
| Experimental Conditions | Product Gas Composition (vol.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feed Material & Reference | T (°C) | p (bar) | Feed Conc.* (wt.%) | Flow Rate (kg/h) | Catalyst | H2 | CH4 | CO2 | C2+ | CO |
| Methanol [37] | 550 | 250 | 5 | 100 | n.a. | 80.4 | 4.8 | 15.5 | - | 1.3 |
| Ethanol [59] | 545 | 280 | 52.2 | 20 † + 80 H2O | 285 ppm K+ | 60.8 | 23.7 | 10.9 | 3.4 | 1.3 |
| Ethanol [39] | 625 | 280 | 14.4 | 100 | 100 ppm K+ | 46.2 | 24.8 | 20.2 | 6.1 | 0.8 |
| Glycerol [38] | 594 | 280 | 60 | 9.8 † + 40 H2O | 285 ppm K+ | 56.6 | 13.5 | 26.4 | 4.0 | 1.2 |
| Crude glycerol [38] | 584 | 280 | 50 | 16.2 † + 64 H2O | 11,020 ppm K+ | 34.3 | 13.4 | 30.1 | 6.1 | 12.4 |
| Pyroligneous acid [37] | 620 | 270 | TOC:3.73 | 50 | 111 ppm K+ | 36.4 | 31.0 | 27.4 | 3.2 | 0.5 |
| Corn silage [39] | 610 | 280 | 9.2 | 50 | n.a. | 31.6 | 28.0 | 27.8 | 9.9 | 0.5 |
| Green waste [unpublished data] | 620 | 280 | 9.6 | 50 | n.a. | 18.7 | 20.1 | 52.5 | 7.5 | 1.1 |
| Brewer’s spent grain [unpublished data] | 608 | 250 | 17.8 | 25 †+ 51 H2O | 2500 ppm K+ | 35.0 | 23.0 | 26.8 | 14.5 | 0.7 |
| Sewage sludge [63] | 654 | 280 | 11.8 | 45.3 | 2500 ppm K+ | 18.8 | 39.7 | 23.9 | 14.6 | 2.1 |
| Sewage sludge [63] | 640 | 270 | 8.5 | 50 | 2500 ppm K+ | 29.6 | 33.6 | 18.3 | 16.6 | 1.9 |
| Digestated sludge [24] | 423 | 280 | 6.7 | 25 | ZnO Ru/C | 16 | 60 | 21 | - | 0.1 |
4. Challenges for Process Design
4.1. Reactor Materials
4.1.1. Mechanical Strength
4.1.2. Corrosion Resistance
4.1.3. Erosion
4.1.4. Mechanical Connections: Welding vs. Adaptors, Dimensions of Tubes
4.2. Design of High-Pressure Components
4.2.1. Heat Exchanger and Preheater Design
4.2.2. Reactor Design and Volume
4.2.3. Phase Separation and Pressure Regulation
4.3. Challenges Related to the Reaction Conditions and Economic Aspects
4.3.1. Separation of Solids
4.3.2. Formation of Tars and Wastewater Quality
4.3.3. Recycling of Wastewater
4.3.4. Product Gas Upgrading
5. Conclusions
6. Patents
- Schmieder, H.; Boukis, N.; Dinjus, E.; Penninger, J. Verfahren zur Erzeugung von Wasserstoff. DE 19955150A1, 13 June 2001.
- Galla, U.; Boukis, N. Anlage zur Behandlung von fließfähigen Stoffen in überkritischem Wasser. Gebrauchsmuster DE 20220307.7, 30 April 2003.
- Boukis, N.; Galla, U. Verfahren zur Behandlung von fließfähigen Stoffen in überkritischem Wasser, DE 10210178, 22 January 2003.
- Boukis, N. Verfahren und Vorrichtung zur Behandlung von organischen Stoffen. DE 10217165, 26 March 2004.
- Boukis, N. Verfahren zur Vorbehandlung von Reaktoren zur Wasserstofferzeugung und Reaktor. DE 10135431, 20 February 2003.
- Boukis, N.; Galla, U. Dinjus, E. Verfahren zur Umwandlung von organischen Edukten in ölartige Produkte. DE 102004031023, 21 May 2007
- Boukis, N.; Galla, U. Vorrichtung und Verfahren zur Abscheidung von anorganischen Feststoffen aus einer wässrigen Lösung. DE 102005037469, 20 October 2007.
- Boukis, N.; Galla, U. Verfahren zur hydrothermalen Vergasung von Biomasse in überkritischem Wasser DE 102006044116, 20 September 2006.
- Boukis, N.; Galla, U. Reaktor für Reaktionen bei hohem Druck und hoher Temperatur und dessen Verwendung. DE 102010009514 und EP 2361675, 26 February 2010.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carey, D.E.; Yang, Y.; McNamara, P.J.; Mayer, B.K. Recovery of Agricultural Nutrients from Biorefineries. Bioresour. Technol. 2016, 215, 186–198. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Boukis, N.; Korving, L.; Hauer, E.; Herbig, S.; Sauer, J. Gasification of Sewage Sludge in Supercritical Water, Experimental Results from the Gasification of Dutch Sewage Sludge. In Proceedings of the 23rd European Biomass Conference and Exhibition, Vienna, Austria, 1–4 June 2015; ETA-Florence Renewable Energies: Florence, Italy, 2015. 5p. [Google Scholar]
- McKendry, P. Energy Production from Biomass (Part 2): Conversion Technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food Waste-to-Energy Conversion Technologies: Current Status and Future Directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the Gasification Performance of Biomass: A Review on Biomass Gasification Process Conditions, Optimization and Economic Evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sheeba, K.N. Combined Slow Pyrolysis and Steam Gasification of Biomass for Hydrogen Generation-a Review: Combined Slow Pyrolysis and Steam Gasification of Biomass. Int. J. Energy Res. 2015, 39, 147–164. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A Critical Review on Biomass Gasification, Co-Gasification, and Their Environmental Assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Zevaco, T.; Dinjus, E.; Hitzmann, B. Influence of the Heating Rate and the Potassium Concentration of the Feed Solution on the Hydrothermal Liquefaction of Used Yeast and Apple Pomace under Reducing Conditions. Biomass Conv. Bioref. 2015, 5, 125–139. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Hauer, E.; Galla, U.; Dinjus, E.; Hitzmann, B.; Larsen, T.; Nygaard, S.D. Catalytic Conversion of Waste Biomass by Hydrothermal Treatment. Fuel 2011, 90, 555–562. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Dinjus, E.; Hitzmann, B. Conversion of Yeast by Hydrothermal Treatment under Reducing Conditions. Fuel 2011, 90, 3424–3432. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal Conversion of Biomass to Fuels and Energetic Materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.; Xu, Z.; Jiaqiang, E. Assessment of Supercritical Water Gasification Process for Combustible Gas Production from Thermodynamic, Environmental and Techno-Economic Perspectives: A Review. Energy Convers. Manag. 2020, 226, 113497. [Google Scholar] [CrossRef]
- Antal, M.J.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass Gasification in Supercritical Water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- Anaerobic Biotechnology for Bioenergy Production; Khanal, S.K., Ed.; Wiley-Blackwell: Oxford, UK, 2008; ISBN 978-0-8138-0454-5. [Google Scholar]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic Digestion of Lignocellulosic Biomass: Challenges and Opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Galkin, A.A.; Lunin, V.V. Subcritical and Supercritical Water: A Universal Medium for Chemical Reactions. Russ. Chem. Rev. 2005, 74, 21. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot Compressed Water as Reaction Medium and Reactant. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Waldner, M.H.; Vogel, F. Renewable Production of Methane from Woody Biomass by Catalytic Hydrothermal Gasification. Ind. Eng. Chem. Res. 2005, 44, 4543–4551. [Google Scholar] [CrossRef]
- Azadi, P.; Afif, E.; Azadi, F.; Farnood, R. Screening of Nickel Catalysts for Selective Hydrogen Production Using Supercritical Water Gasification of Glucose. Green Chem. 2012, 14, 1766. [Google Scholar] [CrossRef]
- Elliott, D.C. Catalytic Hydrothermal Gasification of Biomass. Biofuels Bioprod. Biorefining 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Boukis, N.; Hauer, E.; Herbig, S.; Sauer, J.; Vogel, F. Catalytic Gasification of Digestate Sludge in Supercritical Water on the Pilot Plant Scale. Biomass Conv. Bioref. 2017, 7, 415–424. [Google Scholar] [CrossRef]
- Kruse, A.; Meier, D.; Rimbrecht, P.; Schacht, M. Gasification of Pyrocatechol in Supercritical Water in the Presence of Potassium Hydroxide. Ind. Eng. Chem. Res. 2000, 39, 4842–4848. [Google Scholar] [CrossRef]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Alkali-Promoted Hydrothermal Gasification of Biomass Food Processing Waste: A Parametric Study. Int. J. Hydrogen Energy 2010, 35, 7405–7415. [Google Scholar] [CrossRef]
- Onwudili, J.A. Catalytic Hydrothermal Gasification of Algae for Hydrogen Production: Composition of Reaction Products and Potential for Nutrient Recycling. Bioresour. Technol. 2013, 127, 72–80. [Google Scholar] [CrossRef]
- Kritzer, P.; Boukis, N.; Dinjus, E. Factors Controlling Corrosion in High-Temperature Aqueous Solutions: A Contribution to the Dissociation and Solubility Data Influencing Corrosion Processes. J. Supercrit. Fluids 1999, 15, 205–227. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.; Prins, W.; Vanswaaij, W.; Vandebeld, B.; Elliott, D.; Neuenschwander, G.; Kruse, A. Biomass Gasification in Near- and Super-Critical Water: Status and Prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical Water Gasification. Biofuels Bioprod. Bioref. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Acelas, N.Y.; López, D.P.; Brilman, D.W.F.; Kersten, S.R.A.; Kootstra, A.M.J. Supercritical Water Gasification of Sewage Sludge: Gas Production and Phosphorus Recovery. Bioresour. Technol. 2014, 174, 167–175. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass for Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Vogel, F. Pilot-Scale Demonstration of the Hydrothermal Gasification of Wet Biomass. Available online: https://docplayer.org/70285637-Pilot-scale-demonstration-of-the-hydrothermal-gasification-of-wet-biomass-f-vogel-paul-scherrer-institut-and-fachhochschule-nordwestschweiz.html (accessed on 25 January 2021).
- Möbius, A.; Boukis, N.; Galla, U.; Dinjus, E. Gasification of Pyroligneous Acid in Supercritical Water. Fuel 2012, 94, 395–400. [Google Scholar] [CrossRef]
- Möbius, A.; Boukis, N.; Sauer, J. Gasification of Biomass in Supercritical Water (SCWG). Mater. Process. Energy Commun. Curr. Res. Technol. Dev. 2013, 264–268. [Google Scholar] [CrossRef]
- Boukis, N.; Neumann, M.; Galla, U.; Dinjus, E. Gasification of Herbage in Supercritical Water, Experimental Results. In Proceedings of the 18th European Biomass Conference and Exhibition, Lyon, France, 3–7 May 2010; ETA-Florence Renewable Energies: Florence, Italy, 2010; pp. 562–566. [Google Scholar]
- Boukis, N.; Galla, U.; Diem, V.; Dinjus, E. Biomass Gasification in Supercritical Water: First Results of the Pilot Plant. In Science in Thermal and Chemical Biomass Conversion: Contributions based on papers delivered to the Sixth International Conference on Science in Thermal and Chemical Biomass Conversion, Victoria, BC, Canada, 30 August–2 September 2004; Bridgwater, A.V., Boocock, D.G.B., Eds.; CPL Press: Newbury, UK, 2006; Volume 2, pp. 975–990. [Google Scholar]
- Boukis, N.; Galla, U.; Müller, H.; Dinjus, E. Hydrothermal Gasification of Glycerol on the Pilot Plant Scale. In Proceedings of the 16th European Biomass Conference & Exhibition, Valencia, Spain, 2 June 2008; pp. 1898–1901. [Google Scholar]
- Boukis, N.; Galla, U.; D’Jesús, P.; Müller, H.; Dinjus, E. Gasification of Wet Biomass in Supercritical Water. Results of Pilot Plant Experiments. In Proceedings of the 14th European Conference on Biomass for Energy, Industrie and Climate protection, Paris, France, 17 October 2005; pp. 964–967. [Google Scholar]
- D’Jesús, P.; Boukis, N.; Kraushaar-Czarnetzki, B.; Dinjus, E. Influence of Process Variables on Gasification of Corn Silage in Supercritical Water. Ind. Eng. Chem. Res. 2006, 45, 1622–1630. [Google Scholar] [CrossRef]
- D’Jesús, P.; Artiel, C.; Boukis, N.; Kraushaar-Czarnetzki, B.; Dinjus, E. Influence of Educt Preparation on Gasification of Corn Silage in Supercritical Water. Ind. Eng. Chem. Res. 2005, 44, 9071–9077. [Google Scholar] [CrossRef]
- Boukis, N.; Stoll, I.K.; Sauer, J.; Fischer, J.; Kansy, R. Separation of Salts During the Gasification of Spent Grain in Supercritical Water. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden, 12–15 June 2017; ETA-Florence Renewable Energies: Florence, Italy, 2017; pp. 338–343. [Google Scholar]
- D’Jesús Montilva, P.M. Die Vergasung von realer Biomasse in überkritischem Wasser: Untersuchung des Einflusses von Prozessvariablen und Edukteigenschaften; Universität Karlsruhe (TH): Karlsruhe, Germany, 2007. [Google Scholar]
- Sınaǧ, A.; Kruse, A.; Schwarzkopf, V. Key Compounds of the Hydropyrolysis of Glucose in Supercritical Water in the Presence of K2CO3. Ind. Eng. Chem. Res. 2003, 42, 3516–3521. [Google Scholar] [CrossRef]
- Kruse, A.; Faquir, M. Hydrothermal Biomass Gasification—Effects of Salts, Backmixing and Their Interaction. Chem. Eng. Technol. 2007, 30, 749–754. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhu, W.; Gong, M.; Zhang, H.W. Direct Gasification of Dewatered Sewage Sludge in Supercritical Water. Part 1: Effects of Alkali Salts. Int. J. Hydrogen Energy 2013, 38, 3963–3972. [Google Scholar] [CrossRef]
- Onsager, O. Hydrogen Production from Water and CO via Alkali Metal Formate Salts. Int. J. Hydrogen Energy 1996, 21, 883–885. [Google Scholar] [CrossRef]
- Yanik, J.; Ebale, S.; Kruse, A.; Saglam, M.; Yüksel, M. Biomass Gasification in Supercritical Water: II. Effect of Catalyst. Int. J. Hydrogen Energy 2008, 33, 4520–4526. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Influence of Salts During Hydrothermal Biomass Gasification: The Role of the Catalysed Water-Gas Shift Reaction. Z. Für Phys. Chem. 2005, 219, 341–366. [Google Scholar] [CrossRef]
- Minowa, T.; Ogi, T. Hydrogen Production from Cellulose Using a Reduced Nickel Catalyst. Catal. Today 1998, 45, 411–416. [Google Scholar] [CrossRef]
- Byrd, A.; Pant, K.; Gupta, R. Hydrogen Production from Glycerol by Reforming in Supercritical Water over Ru/Al2O3 Catalyst. Fuel 2008, 87, 2956–2960. [Google Scholar] [CrossRef]
- Frusteri, F.; Frusteri, L.; Costa, F.; Mezzapica, A.; Cannilla, C.; Bonura, G. Methane Production by Sequential Supercritical Gasification of Aqueous Organic Compounds and Selective CO2 Methanation. Appl. Catal. A Gen. 2017, 545, 24–32. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizzone, E.; Giordano, G. Municipal Waste Leachate Conversion via Catalytic Supercritical Water Gasification Process. Fuel 2017, 206, 155–161. [Google Scholar] [CrossRef]
- Behnia, I.; Yuan, Z.; Charpentier, P.; Xu, C. (Charles) Production of Methane and Hydrogen via Supercritical Water Gasification of Renewable Glucose at a Relatively Low Temperature: Effects of Metal Catalysts and Supports. Fuel Process. Technol. 2016, 143, 27–34. [Google Scholar] [CrossRef]
- Minowa, T.; Inoue, S. Hydrogen Production from Biomass by Catalytic Gasification in Hot Compressed Water. Renew. Energy 1999, 16, 1114–1117. [Google Scholar] [CrossRef]
- Azadi, P.; Farnood, R. Review of Heterogeneous Catalysts for Sub- and Supercritical Water Gasification of Biomass and Wastes. Int. J. Hydrogen Energy 2011, 36, 9529–9541. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of Catalytic Supercritical Water Gasification for Hydrogen Production from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Boukis, N.; Galla, U.; Diem, V.; D’Jesús, P.; Dinjus, E. Hydrogen Generation from Wet Biomass in Supercritical Water. In Proceedings of the 2nd World Conference and Technology Exhibition on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10 May 2004; pp. 738–741. [Google Scholar]
- Boukis, N.; Galla, U.; Müller, H.; Dinjus, E. Biomass Gasification in Supercritical Water. Experimental Progress Achieved with the VERENA Pilot-Plant. In Proceedings of the 15th European Biomass Conference & Exhibition, Berlin, Germany, 7 May 2007; pp. 1013–1016. [Google Scholar]
- Boukis, N.; Galla, U.; Müller, H.; Dinjus, E. Gasification of Corn Silage in Supercritical Water on the Pilot—Plant Scale. In Proceedings of the Success and Visions for Bioenergy, Salzburg, Austria, 21 March 2007. [Google Scholar]
- Boukis, N.; Galla, U.; Müller, H.; Dinjus, E. Die VERENA-Anlage—Erzeugung von Wasserstoff Aus Biomasse. In Proceedings of the Gülzower Fachgespräche, Fachagentur Nachwachsende Rohstoffe, Gülzow, Germany, 7 October 2006. [Google Scholar]
- Boukis, N.; Galla, U.; Müller, H.; Dinjus, E. Behaviour of Inorganic Salts during Hydrothermal Gasification of Biomass. In Proceedings of the 17th European Biomass Conference & Exhibition: From research to Industry and Markets, Hamburg, Germany, 29 July 2009. [Google Scholar]
- Boukis, N.; Herbig, S.; Hauer, E. Gasification of Dutch Sewage Sludge in Supercritical Water; Karlsruhe Institute of Technology (KIT): Karlsruhe, Germany, 2016; p. 168. [Google Scholar]
- Abolghasemi, S.; Williamson, J.; Lindley, T.C.; Lee, P.D. Embrittlement of Alloy 625 and Effect of Remedial Treatments. Proc. Imeche 2016, 230, 328–331. [Google Scholar] [CrossRef]
- Boukis, N.; Habicht, W.; Franz, G.; Dinjus, E. Behavior of Ni-Base Alloy 625 in Methanol-Supercritical Water Systems. Mater. Corros. 2003, 54, 326–330. [Google Scholar] [CrossRef]
- Fujisawa, R.; Sakaihara, M.; Kurata, Y.; Watanabe, Y. Corrosion Behaviour of Nickel Base Alloys and 316 Stainless Steel in Supercritical Water under Alkaline Conditions. Corros. Eng. Sci. Technol. 2005, 40, 244–248. [Google Scholar] [CrossRef]
- Boukis, N.; Habicht, W.; Hauer, E.; Weiss, K.; Dinjus, E. Corrosion Behavior of Ni-Base Alloy 625 in Supercritical Water Containing Alcohols and Potassium Hydrogen Carbonate. In Proceedings of the EUROCORR 2007—The European Corrosion Congress, Freiburg, Germany, 9–13 September 2007. [Google Scholar]
- Boukis, N.; Habicht, W.; Hauer, E.; Weiss, K.; Dinjus, E. Corrosion Behavior of Ni-Base Alloys and Stainless Steels in Supercritical Water Containing Potassium Hydrogen Carbonate. In Proceedings of the EUROCORR 2008: The European Corrosion Congress, Edinburgh, UK, 7 September 2008. [Google Scholar]
- Habicht, W.; Boukis, N.; Hauer, E.; Dinjus, E. Analysis of Hydrothermally Formed Corrosion Layers in Ni-Base Alloy 625 by Combined FE-SEM and EDXS. X-Ray Spectrom. 2011, 40, 69–73. [Google Scholar] [CrossRef]
- Boukis, N.; Habicht, W.; Hauer, E.; Dinjus, E. Challenges of Selecting Materials for the Process of Biomass Gasification in Supercritical Water. In Proceedings of the First International Conference on Materials for Energy, Karlsruhe, Germany, 4 July 2010; pp. 348–350. [Google Scholar]
- Boukis, N.; Hauer, E.; Habicht, W. Corrosion Behaviour of Ni-Base Alloys in Supercritical Water Containing Alkali Chlorides. In Proceedings of the EUROCORR 2008: The European Corrosion Congress, Estoril, Portugal, 2 September 2013. [Google Scholar]
- Elsayed, S.; Boukis, N.; Patzelt, D.; Hindersin, S.; Kerner, M.; Sauer, J. Gasification of Microalgae Using Supercritical Water and the Potential of Effluent Recycling. Chem. Eng. Technol. 2016, 39, 335–342. [Google Scholar] [CrossRef]
- Guo, L.; Lu, Y.; Zhang, X.; Ji, C.; Guan, Y.; Pei, A. Hydrogen Production by Biomass Gasification in Supercritical Water: A Systematic Experimental and Analytical Study. Catal. Today 2007, 129, 275–286. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, L.; Zhang, X.; Ji, C. Hydrogen Production by Supercritical Water Gasification of Biomass: Explore the Way to Maximum Hydrogen Yield and High Carbon Gasification Efficiency. Int. J. Hydrogen Energy 2012, 37, 3177–3185. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, L.; Ji, C.; Zhang, X.; Hao, X.; Yan, Q. Hydrogen Production by Biomass Gasification in Supercritical Water: A Parametric Study. Int. J. Hydrogen Energy 2006, 31, 822–831. [Google Scholar] [CrossRef]

| Particle Size (mm) | Total Mass Fraction (%) | ||
|---|---|---|---|
| After 1. Treatment (Meat Grinder) | After 2. Treatment (Meat Grinder) | After 3. Treatment (Macerator) | |
| >3.35 | 1.7 | - | 0 |
| >2.0 | 5.4 | 2.5 | 0.9 |
| >1.0 | 13.5 | 11.8 | 3.3 |
| >0.5 | 12.0 | 15.2 | 8.9 |
| <0.5 | 67.4 | 70.5 | 86.9 |
| Element | Concentration in Different Output Streams (ppm) | ||
|---|---|---|---|
| Separation 1: Cyclone | Separation 2: Reactor Bottom | Reactor Effluent | |
| Sodium | 41.6 | 4.3 | 4.6 |
| Potassium | 6860 | 377 | n.a. |
| Magnesium | 82.3 | 0.5 | 0.05 |
| Calcium | 1385 | 14.5 | 0.75 |
| Silicium | 266 | 31.8 | 10.5 |
| Iron | 5.2 | <0.05 | <0.05 |
| Phosphorus | 132 | 0.9 | <0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukis, N.; Stoll, I.K. Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant. Processes 2021, 9, 455. https://doi.org/10.3390/pr9030455
Boukis N, Stoll IK. Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant. Processes. 2021; 9(3):455. https://doi.org/10.3390/pr9030455
Chicago/Turabian StyleBoukis, Nikolaos, and I. Katharina Stoll. 2021. "Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant" Processes 9, no. 3: 455. https://doi.org/10.3390/pr9030455
APA StyleBoukis, N., & Stoll, I. K. (2021). Gasification of Biomass in Supercritical Water, Challenges for the Process Design—Lessons Learned from the Operation Experience of the First Dedicated Pilot Plant. Processes, 9(3), 455. https://doi.org/10.3390/pr9030455






