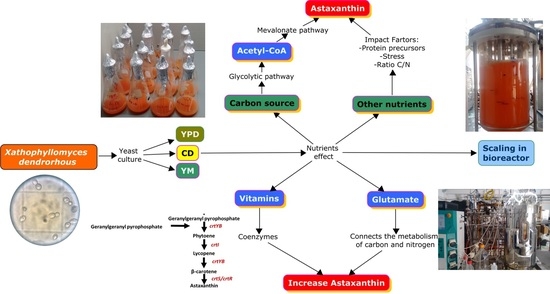
Improvement of a Specific Culture Medium Based on Industrial Glucose for Carotenoid Production by Xanthophyllomyces dendrorhous
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum Activation
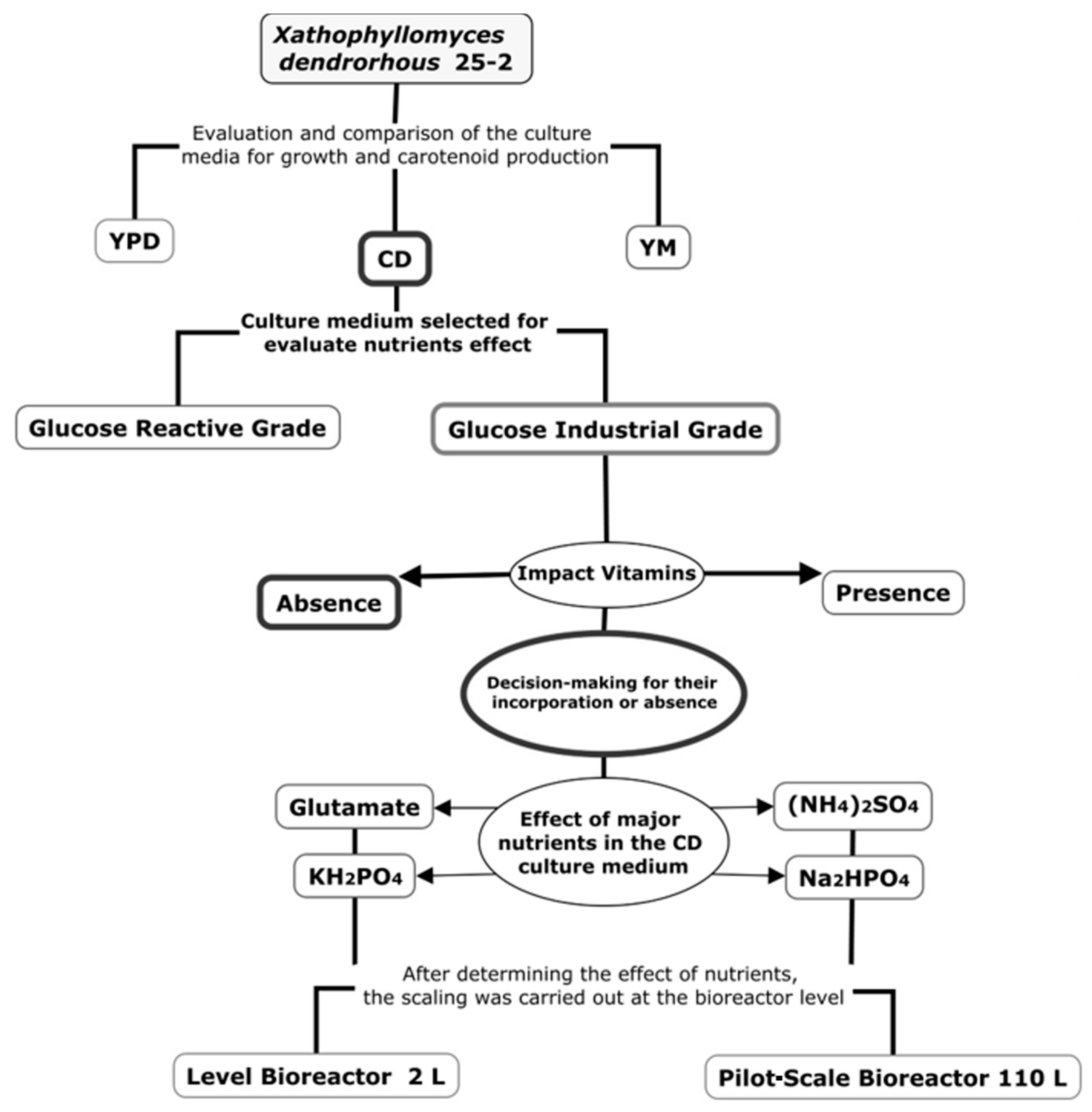
2.2. Evaluation of the Low-Complexity CD Culture Medium at Shake Flask Level
2.3. Impact of Vitamins on Carotenoid Production and Evaluation of an Industrial Carbon Source
2.4. Effect of the CD Culture Medium Macronutrients on Carotenoid and Biomass Production
2.5. Carotenoid Production at the Bioreactor Level
2.6. Analytical Techniques
2.7. Statistical Analysis
3. Results and Discussion
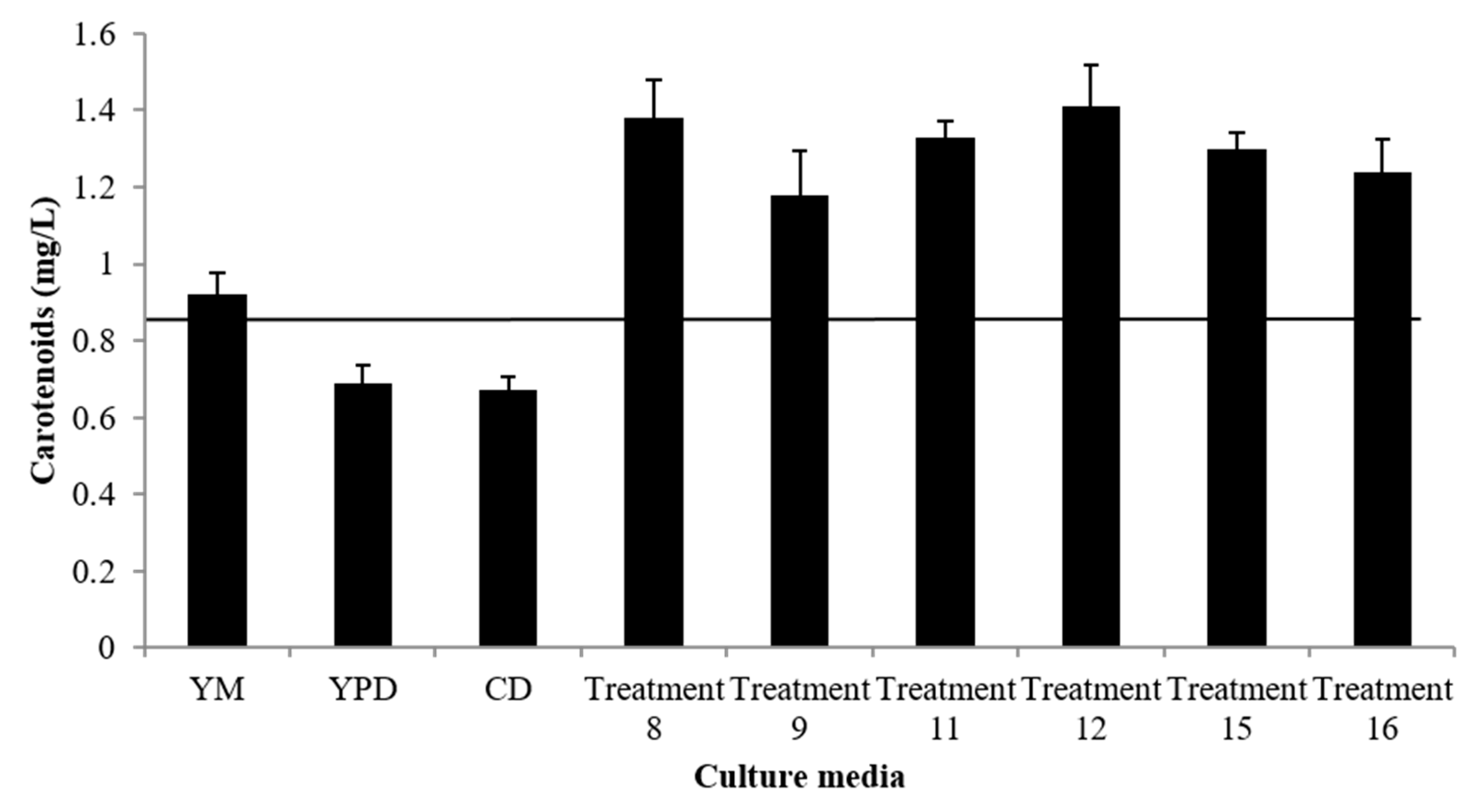
3.1. Evaluation of the Culture Media for Growth and Carotenoid Production
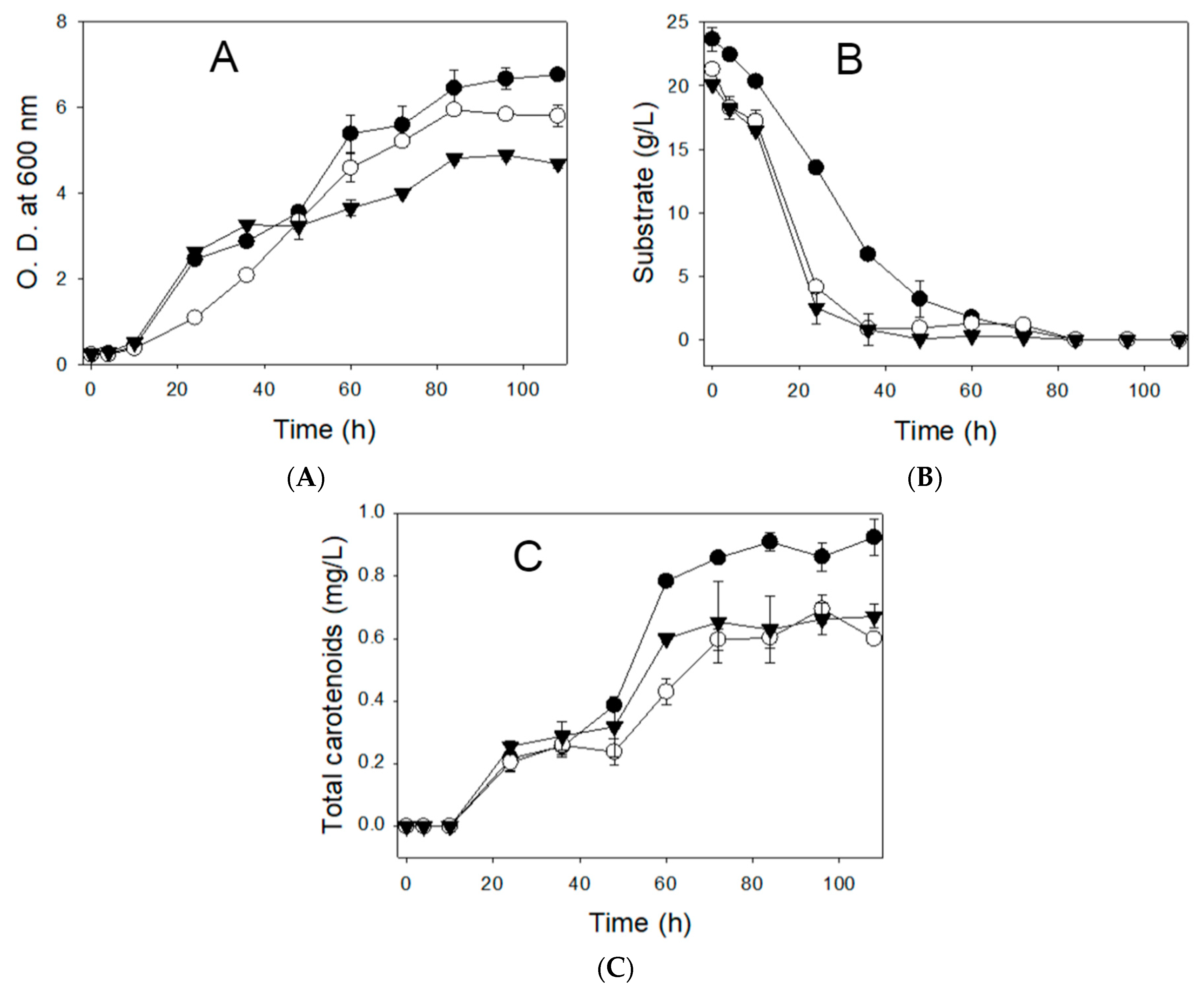
3.2. Impact of Vitamins on Growth and Carotenoid Production Using the CD Medium with Regent-Grade Glucose
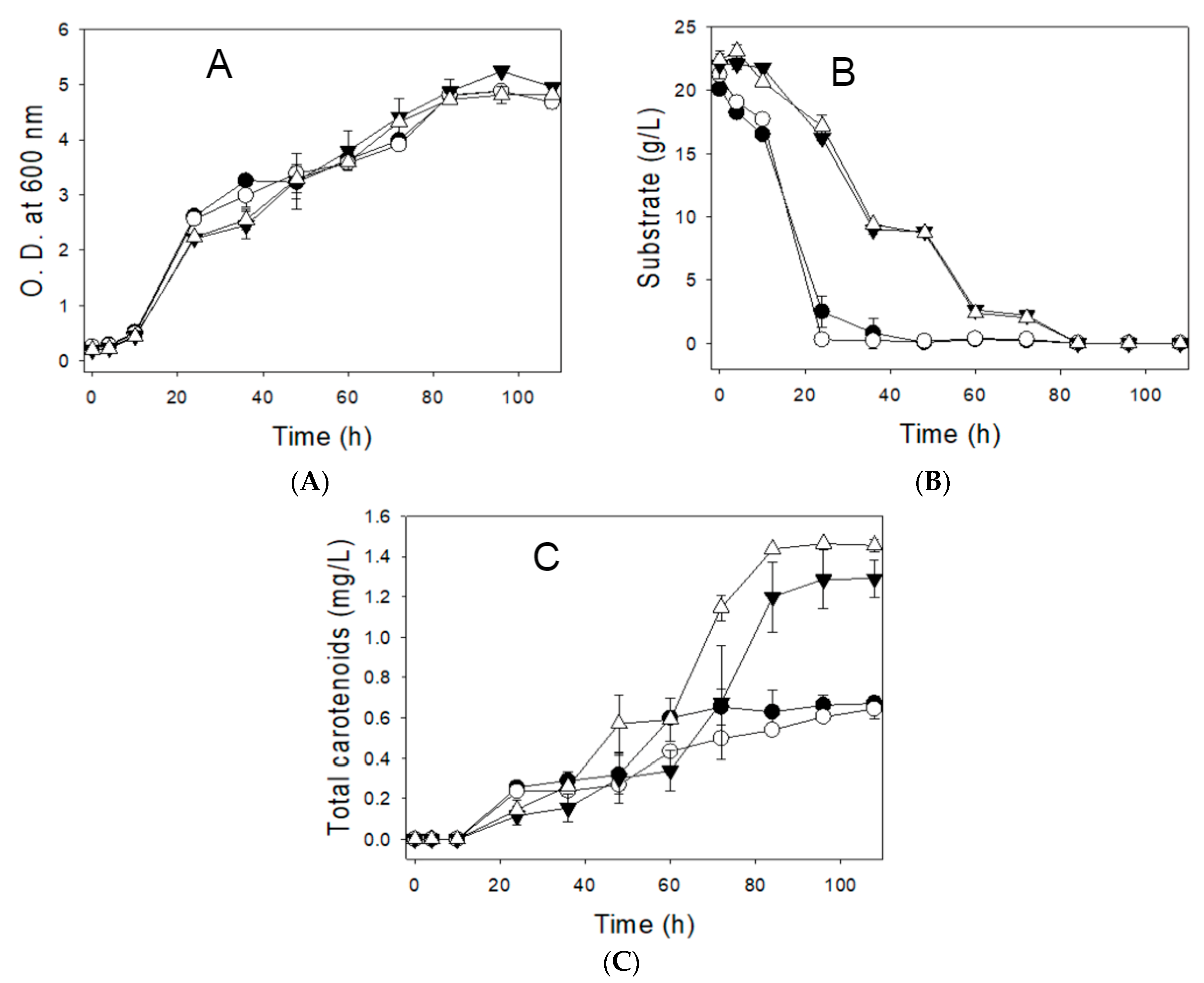
3.3. Impact of Vitamins on Growth and Carotenoid Production Using the CD Medium with Industrial-Grade Glucose
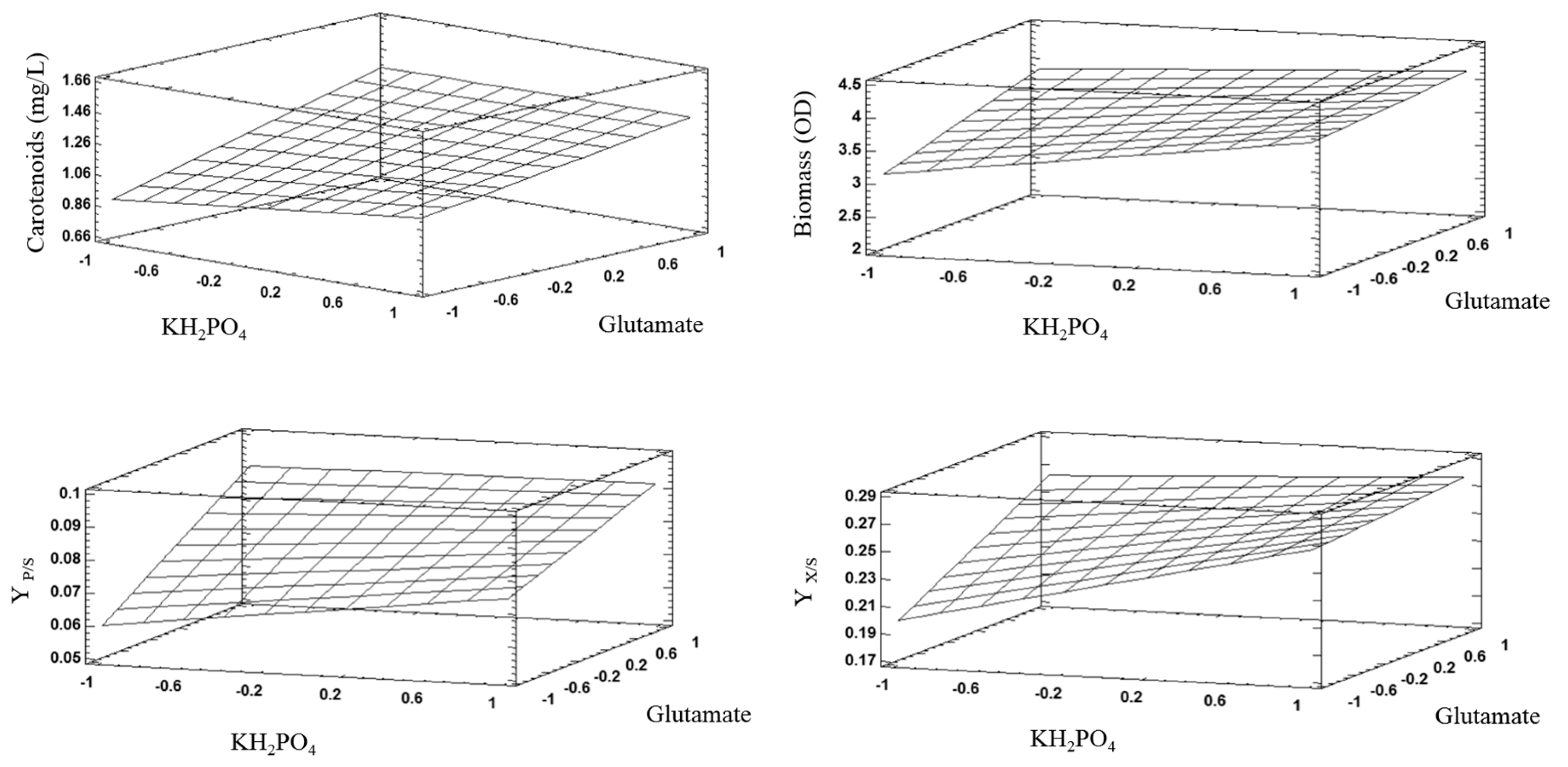
3.4. Effect of Major Nutrients Contained in the CD Culture Medium on Growth (Kinetic Parameters) and Carotenoid Production
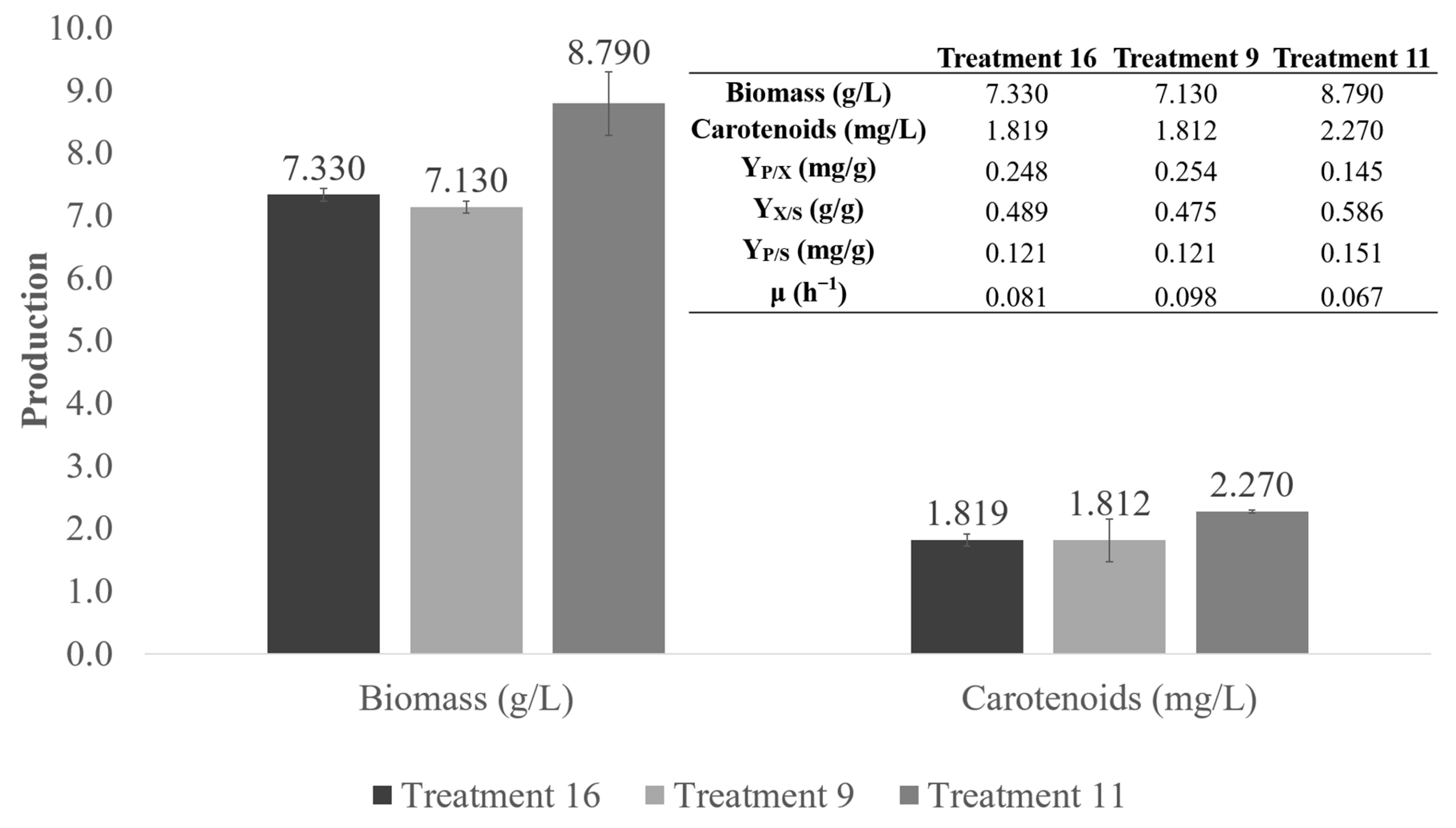
3.5. Bioreactor Scale-Up of Carotenoid Production Using a Specific CD Culture Medium Based on Industrial Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avalos, J.; Limón, M.C. Biological roles of fungal carotenoids. Curr Genet. 2015, 61, 309–324. [Google Scholar] [CrossRef]
- Ytrestoyl, T.; Bjerkeng, B. Dose response in uptake and deposition of intraperitoneally administered astaxanthin in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.). Aquaculture 2007, 263, 179–191. [Google Scholar] [CrossRef]
- Miao, F.; Lu, D.; Li, Y.; Zeng, M. Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Anal. Biochem. 2006, 352, 176–181. [Google Scholar] [CrossRef]
- Chávez-Cabrera, C.; Flores-Bustamante, Z.R.; Flores-Cotera, L.B. Una Vista Integral de la Síntesis de Astaxantina en Phaffia Rhodozyma. World J. Microbiol. Biotechnol. 2010, 14, 24–38. [Google Scholar]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Xin-Yi, C.; Yi-Jiao, X.; Miao-Miao, Y.; Ming-Jun, Z. Preparation of astaxanthin mask from Phaffia rhodozyma and its evaluation. Process. Biochem. 2019, 79, 195–202. [Google Scholar] [CrossRef]
- Domínguez-Bocanegra, A.R.; Ponce-Noyola, T.; Torres-Muñoz, J.A. Astaxanthin production by Phaffia rhodozyma and Haematococcus pluvialis: A comparative study. Appl. Microbiol. Biotechnol. 2007, 75, 783–791. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A.; Cohen, Z.; Richmond, A. Procedure for large-scale production of astaxanthin from Haematococcus. U.S. Patent No. 6,022,701, 8 February 2000. Available online: https://patents.google.com/patent/US6022701A/en (accessed on 12 January 2020).
- Johnson, E.A. Phaffia rhodozyma: Colorful odyssey. Int. Microbiol. 2003, 6, 169–174. [Google Scholar] [CrossRef]
- Amado, I.; Vázquez, J. Mussel processing wastewater: A low-cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous. Microb. Cell Fact. 2015, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 1–11. [Google Scholar] [CrossRef]
- Gio-Bin, L.; Sang-Yun, L.; Eun-Kyu, L.; Seung-Joo, H.; Woo-Sick, K. Separation of astaxanthin from red yeast Phaffia rhodozyma by supercritical carbon dioxide extraction. Biochem. Eng. J. 2002, 11, 181–187. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Zhang, L.; Gross, M.; Tomita, Y. Immunomodulating actions of carotenoids: Enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr. Cancer 1994, 21, 47–58. [Google Scholar] [CrossRef]
- Gui-Li, J.; Ling-Yan, Z.; Yu-Tao, W.; Ming-Jun, Z. Astaxanthin from Jerusalem artichoke: Production by fed-batch fermentation using Phaffia rhodozyma and application in cosmetics. Process. Biochem. 2017, 63, 16–25. [Google Scholar] [CrossRef]
- Wang, W.; Yu, L. Effects of oxygen supply on growth and carotenoids accumulation by Xanthophyllomyces dendrorhous. Z. Nat. C 2009, 64, 853–858. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wu, J.Y. Optimization of cell growth and carotenoid production of Xanthophyllomyces dendrorhous through statistical experiment design. Biochem. Eng. J. 2007, 36, 182–189. [Google Scholar] [CrossRef]
- Ramírez, J.; Gutiérrez, H.; Gschaedler, A. Optimization of astaxanthin production by Phaffia rhodozyma through factorial design and response surface methodology. J. Biotechnol. 2001, 88, 259–268. [Google Scholar] [CrossRef]
- Meyer, P.S.; du Preez, J.C. Effect of culture conditions on astaxanthin production by a mutant of Phaffia rhodozyma in batch and chemostat culture. Appl. Microbiol. Biotechnol. 1994, 40, 780–785. [Google Scholar] [CrossRef]
- Wang, B.; Panc, X.; Jiae, J.; Xiongc, W.; Manirafashac, E.; Lingc, X.; Lu, Y. Strategy and regulatory mechanisms of glutamate feeding to enhance astaxanthin yield in Xanthophyllomyces dendrorhous. Enzym. Microb. Technol. 2019, 125, 45–52. [Google Scholar] [CrossRef]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Humbelin, M.; Sandmann, G.; Schrader, J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrohous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Luna-Flores, C.H.; Ramírez-Cordova, J.J.; Pelayo-Ortiz, C.; Femat, R.; Herrera-López, E.J. Batch and fed-batch modeling of carotenoids production by Xanthophyllomyces dendrorhous using Yucca fillifera date juice as substrate. Biochem. Eng. J. 2010, 53, 131–136. [Google Scholar] [CrossRef]
- Fang, T.J.; Wang, J.M. Extractability of astaxanthin in a mixed culture of a carotenoid over-producing mutant of Xanthophyllomyces dendrorhous and Bacillus circulans in two-stage batch fermentation. Process. Biochem. 2002, 37, 1235–1245. [Google Scholar] [CrossRef]
- Tibayrenc, P.; Preziosi-Belloy, L.; Roger, J.M.; Ghommidh, C. Assessing yeast viability from cell-size measurements? J. Biotechnol. 2010, 149, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Máquina, M.; González, N.; Castro, Y. Caracterización fenotípica y genotípica de Rizobios autóctonos provenientes de diversas regiones de Venezuela. Rev. Biol. Trop. 2011, 59, 64–72. Available online: http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S003477442011000300005&lng=en&nr=iso (accessed on 21 September 2019).
- De la Fuente, J.C.; Oyarzún, B.; Quezada, N.; del Valle, J.M. Solubility of carotenoid pigments (lycopene and astaxanthin) in supercritical carbon dioxide. Fluid Phase Equilib. 2006, 247, 90–95. [Google Scholar] [CrossRef]
- Sánchez, A. Monitoreo del estado fisiológico de la levadura Xanthophyllomyces dendrorhous durante la producción de astaxantina por medio de técnicas espectroscópicas y análisis de imágenes. In Tesis de Maestría en Biotecnología Productiva; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco: Jalisco, Mexico, 2015; pp. 4–96. Available online: https://ciatej.repositorioinstitucional.mx/jspui/handle/1023/54 (accessed on 30 August 2018).
- Pan, X.; Ling, X.; Ye, C.; Wu, Y.; Lu, Y. Nitrogen feeding strategies on astaxanthin production by Xanthophyllomyces dendrorhous. J. Xiamen Univ. 2013, 52, 545–552. [Google Scholar] [CrossRef]
- Madigan, M.; Martinko, J.; Parker, J. Biología de los Microorganismos, 10th ed.; Editorial Pearson Educación: Madrid, España, 2003; pp. 102–108. [Google Scholar]
- Kim, J.H.; Kim, C.W.; Chang, H.I. Screening and characterization of red yeast Xanthophyllomyces dendrorhous mutants. J. Microbiol. Biotechnol. 2004, 14, 570–575. Available online: https://koreauniv.pure.elsevier.com/en/publications/screening-and-characterization-of-red-yeast-xanthophyllomyces-den (accessed on 5 March 2017).
- Farías, L.J.; Gschaedler, A.; Sanchez, A.; Cervantes, J.; Arellano, M.; Zamora, C.; Amillastre, E.; Herrera, E.J. Xanthophyllomyces dendrorhous physiological stages determination using combined measurements from dielectric and Raman spectroscopies, a cell counter system and fluorescence flow cytometry. Biochem. Eng. J. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sedmak, J.; Weerasinghe, D.; Setsuko, J.S. Extraction and quantification of astaxanthin from Phaffia rhodozyma. Biotechnol. Technol. 1990, 4, 107–112. [Google Scholar] [CrossRef]
- Bozzo, A.P. Optimización de proceso en Planta Productora de Extracto de Malta, Memoria Para Tener el Título de Ingeniero Civil. Químico; Universidad de Chile Facultad de Ciencias Físicas y Matemáticas, Departamento de Ingeniería Química y Biotecnología: Santiago, Chile, 2012; pp. 4–15. Available online: http://repositorio.uchile.cl/handle/2250/112048 (accessed on 24 October 2018).
- Phaff, H.J.; Miller, M.W.; Yoneyama, M.; Soneda, M. A comparative study of the yeast florae associated with trees on the Japanese Islands and on the west coast of North America. Fourth Int. Ferment. Symp. 1972, 759–774. [Google Scholar]
- Zheng, Y.G.; Hu, Z.C.; Wang, Z.; Shen, Y.C. Large-scale production of astaxanthin by Xanthophyllomyces dendrorhous. Food Bioprod. Process. 2006, 84, 164–166. [Google Scholar] [CrossRef]
- Jeong-Hwang, K.; Seong-Woo, K.; Seung-Wook, K.; Hyo-Ihl, C. High-Level Production of Astaxanthin by Xanthophyllomyces dendrorhous Mutant JH1 Using Statistical Experimental Designs. Biosci. Biotech. Bioch. 2005, 69, 1743–1748. [Google Scholar] [CrossRef]
- Reynders, M.; Rawlings, D.; Harrison, S. Demonstration of the Crabtree effect in Phaffia rhodozyma during continuous and fed-batch cultivation. Biotechnol. Lett. 1977, 19, 549–552. [Google Scholar] [CrossRef]
- Barbachano-Torres, A.; Castelblanco-Matiz, L.M.; Ramos-Valdivia, A.C.; Cerda-García-Rojas, C.M.; Salgado, L.M.; Flores-Ortiz, C.M.; Ponce-Noyola, T. Analysis of proteomic changes in colored mutants of Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Archiv. Microbiol. 2014, 196, 411–421. [Google Scholar] [CrossRef]
- Martinez-Moya, P.; Watt, S.A.; Niehaus, K.; Alcaíno, J.; Baeza, M.; Cifuentes, V. Proteomic analysis of the carotenogenic yeast Xanthophyllomyces dendrorhous. BMC Microbiol. 2011, 11, 131. [Google Scholar] [CrossRef]
- Clarke, M.A. Chemical and Physical Properties of Corn Syrups. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic: Cambridge, MA, USA, 2003; Available online: https://www.sciencedirect.com/referencework/9780122270550/encyclopedia-of-food-sciences-and-nutrition (accessed on 3 December 2019).
- Nangia, H.; Hasan, M.; Mohd, A.; Bhatt, P.C.; Panda, B.P. Strain improvement of Phaffia rhodozyma for astaxanthin production. Drug Des. Dev. Ther. 2016, 7, 63–68. [Google Scholar] [CrossRef]
- Ramírez, J.; Obledo, N.; Arellano, M.; Herrera, E. Astaxanthin production by Phaffia rhodozyma in a fed-batch culture using a low cost medium feeding. e-Gnosis 2006, 4, 1–9. Available online: http://www.redalyc.org/articulo.oa?id=73000405 (accessed on 15 February 2016).
- An, G.H. Improved growth of the red yeast, Phaffia rhodozyma (Xanthophyllomyces dendrorhous), in the presence of tricarboxylic acid cycle intermediates. Biotechnol. Lett. 2001, 23, 1005–1009. [Google Scholar] [CrossRef]
- Yamane, Y.; Higashida, K.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Influence of oxygen and glucose on primary metabolism and astaxanthin production by Phaffia rhodozyma in batch and fed-batch cultures: Kinetic and stoichiometric analysis. Appl. Environ. Microbiol. 1997, 63, 4471–4478. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1389289/pdf/hw4471.pdf (accessed on 30 May 2016). [CrossRef]
- Gill-Hwan, A.; Schuman, D.B.; Johnson, E.A. Isolation of Phaffia rhodozyma mutants with increased astaxanthin content. Appl. Environ. Microbiol. 1989, 55, 116–121. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC184064/ (accessed on 5 November 2019).
- Stoklosa, R.G.; Johnston, D.B.; Nghiem, N.P. Phaffia rhodozyma cultivation on structural and non-structural sugars from sweet sorghum for astaxanthin generation. Process. Biochem. 2019, 83, 9–17. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Patil, K.R.; Schalk, M.; Clark, A.; Nielsen, J. Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab. Eng. 2009, 11, 328–334. [Google Scholar] [CrossRef]
- Rodríguez-Sáiz, M.; Godio, R.P.; Alvarez, V.; de la Fuente, V.L.; Martín, J.F.; Barredo, J.L. The NADP-dependent glutamate dehydrogenase gene from the astaxanthin producer Xanthophyllomyces dendrorhous: Use of Its promoter for controlled gene expression. Mol. Biotechnol. 2009, 41, 165–172. [Google Scholar] [CrossRef]
- Newsholme, P.; Procopio, J.; Ramos, M.; Pithon-Curi, T.; Curi, R. Glutamine and glutamate-their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Lewis, M.J. Astaxanthin formation by the yeast Phaffia rhodozyma. J. Gen. Microbiol. 1979, 115, 173–183. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhang, J.F.; Zheng, Y.G.; Shen, Y.C. Improvement of astaxanthin production by a newly isolated Phaffia rhodozyma mutant with low-energy ion beam implantation. Appl. Microbiol. 2008, 104, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.C.; Zheng, Y.G.; Wang, Z.; Shen, Y.C. Production of astaxanthin by Xanthophyllomyces dendrorhous ZJUT46 with fed-batch fermentation in 2.0 m3 fermentation. Food Technol. Biotech. 2007, 45, 209–212. Available online: https://hrcak.srce.hr/file/43787 (accessed on 18 April 2018).
- Reyes, L.H.; Gomez, J.M.; Kao, K.C. Improving carotenoids production in yeast via adaptive laboratory evolution. Metab. Eng. 2014, 21, 26–33. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Li, F.; Wang, S. Selectable marker recycling in the nonconventional yeast Xanthophyllomyces dendrorhous by transient expression of Cre on a genetically unstable vector. Appl. Microbiol. Biotechnol. 2014, 103, 963–971. [Google Scholar] [CrossRef]
- Gassel, S.; Schewe, H.; Schmidt, I.; Schrader, J.; Sandmann, G. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol. Lett. 2013, 35, 565–569. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Li, A.; Wang, F.; Yao, Z.; Biang, Q.; Zhu, Y.; Yu, H.; Ye, L. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzym. Microb. Technol. 2017, 100, 28–36. [Google Scholar] [CrossRef] [PubMed]
| Treatment | KH2PO4 (g/L) | Na2HPO4 (g/L) | (NH4)2SO4 (g/L) | Glutamic Acid (g/L) |
|---|---|---|---|---|
| 1 | 0.50 | 0.50 | 0.50 | 0.20 |
| 2 | 3.00 | 0.50 | 0.50 | 0.20 |
| 3 | 0.50 | 1.49 | 0.50 | 0.20 |
| 4 | 3.00 | 1.49 | 0.50 | 0.20 |
| 5 | 0.50 | 0.50 | 3.00 | 0.20 |
| 6 | 3.00 | 0.50 | 3.00 | 0.20 |
| 7 | 0.50 | 1.49 | 3.00 | 0.20 |
| 8 | 3.00 | 1.49 | 3.00 | 0.20 |
| 9 | 0.50 | 0.50 | 0.50 | 1.00 |
| 10 | 3.00 | 0.50 | 0.50 | 1.00 |
| 11 | 0.50 | 1.49 | 0.50 | 1.00 |
| 12 | 3.00 | 1.49 | 0.50 | 1.00 |
| 13 | 0.50 | 0.50 | 3.00 | 1.00 |
| 14 | 3.00 | 0.50 | 3.00 | 1.00 |
| 15 | 0.50 | 1.49 | 3.00 | 1.00 |
| 16 | 3.00 | 1.49 | 3.00 | 1.00 |
| 17 | 2.20 | 1.16 | 2.20 | 0.73 |
| 18 | 1.35 | 0.83 | 1.35 | 0.47 |
| Carbon Source | Vitamins p-Value > 0.05 | pH 6.0 | |
|---|---|---|---|
| OD600 nm | Carotenoids (mg/L) | ||
| Glucose, reagent-grade | Present | 4.88 ± 0.102 | 0.67 ± 0.038 |
| Absent | 4.88 ± 0.127 | 0.64 ± 0.050 | |
| Glucose, industrial-grade | Present | 5.24 ± 0.032 | 1.29 ± 0.092 |
| Absent | 4.82 ± 0.021 | 1.45 ± 0.029 | |
| Treatment | Cell Density (OD600 nm) | Carotenoids (mg/L) | YP/X (mg/DO) | YP/S (mg/g) | YX/S (DO/g) | * Productivity (mg/L·h) | C/N |
|---|---|---|---|---|---|---|---|
| 1 | 2.54 ± 0.75 | 0.75 ± 0.11 | 0.293 ± 0.13 | 0.050 ± 0.00 | 0.170 ± 0.05 | 0.008 | 64.34 |
| 2 | 3.77 ± 0.43 | 1.09 ± 0.00 | 0.288 ± 0.03 | 0.072 ± 0.00 | 0.251 ± 0.03 | 0.011 | 64.34 |
| 3 | 3.09 ± 0.94 | 0.94 ± 0.22 | 0.303 ± 0.02 | 0.062 ± 0.02 | 0.206 ± 0.06 | 0.010 | 64.34 |
| 4 | 3.94 ± 0.03 | 1.08 ± 0.10 | 0.274 ± 0.02 | 0.072 ± 0.01 | 0.262 ± 0.00 | 0.011 | 64.34 |
| 5 | 2.95 ± 0.75 | 0.80 ± 0.15 | 0.269 ± 0.02 | 0.053 ± 0.01 | 0.197 ± 0.05 | 0.008 | 12.45 |
| 6 | 3.51 ± 0.62 | 1.13 ± 0.05 | 0.321 ± 0.04 | 0.075 ± 0.00 | 0.234 ± 0.04 | 0.012 | 12.45 |
| 7 | 3.26 ± 0.68 | 0.91 ± 0.35 | 0.278 ± 0.05 | 0.060 ± 0.02 | 0.217 ± 0.04 | 0.009 | 12.45 |
| 8 | 3.76 ± 0.22 | 1.38 ± 0.10 | 0.368 ± 0.05 | 0.092 ± 0.01 | 0.250 ± 0.01 | 0.014 | 12.45 |
| 9 | 3.73 ± 0.18 | 1.18 ± 0.08 | 0.316 ± 0.00 | 0.079 ± 0.01 | 0.249 ± 0.01 | 0.012 | 38.60 |
| 10 | 4.14 ± 0.29 | 1.33 ± 0.01 | 0.320 ± 0.01 | 0.088 ± 0.01 | 0.276 ± 0.02 | 0.014 | 38.60 |
| 11 | 3.90 ± 0.11 | 1.33 ± 0.03 | 0.341 ± 0.02 | 0.089 ± 0.00 | 0.260 ± 0.01 | 0.014 | 38.60 |
| 12 | 4.26 ± 0.28 | 1.41 ± 0.11 | 0.331 ± 0.01 | 0.094 ± 0.01 | 0.284 ± 0.02 | 0.015 | 38.60 |
| 13 | 3.31 ± 0.99 | 1.06 ± 0.25 | 0.321 ± 0.02 | 0.071 ± 0.02 | 0.220 ± 0.07 | 0.011 | 11.03 |
| 14 | 3.96 ± 0.32 | 1.22 ± 0.20 | 0.307 ± 0.03 | 0.081 ± 0.01 | 0.264 ± 0.02 | 0.013 | 11.03 |
| 15 | 3.52 ± 0.69 | 1.33 ± 0.08 | 0.378 ± 0.10 | 0.089 ± 0.01 | 0.235 ± 0.04 | 0.014 | 11.03 |
| 16 | 3.20 ± 0.64 | 1.24 ± 0.08 | 0.388 ± 0.05 | 0.083 ± 0.01 | 0.213 ± 0.04 | 0.013 | 11.03 |
| 17 | 3.72 ± 0.82 | 1.20 ± 0.10 | 0.323 ± 0.05 | 0.080 ± 0.01 | 0.248 ± 0.05 | 0.013 | 15.05 |
| 18 | 2.95 ± 1.20 | 1.09 ± 0.02 | 0.368 ± 0.18 | 0.072 ± 0.00 | 0.196 ± 0.08 | 0.011 | 24.36 |
| Factor | ||||
|---|---|---|---|---|
| KH2PO4 | Na2HPO4 | (NH4)2SO4 | Glutamic Acid | |
| Carotenoids | 0.0002 | 0.0076 | 0.9161 | 0.0000 |
| Biomass | 0.0164 | 0.5470 | 0.2630 | 0.0644 |
| *YX/S | 0.0000 | 0.1286 | 0.0053 | 0.0000 |
| *YP/S | 0.0000 | 0.0000 | 0.8670 | 0.0000 |
| Bioreactor 2 L | Pilot Scale 110 L | |
|---|---|---|
| Biomass (g/L) | 8.79 ± 0.50 | 8.96 ± 0.10 |
| Carotenoids (mg/L) | 2.270 ± 0.03 | 1.928 ± 0.13 |
| YP/X (mg/g) | 0.145 ± 0.02 | 0.215 ± 0.02 |
| YX/S (g/g) | 0.585 ± 0.03 | 0.448 ± 0.01 |
| YP/S (mg/g) | 0.151 ± 0.01 | 0.095 ± 0.01 |
| μ (h−1) | 0.067 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Haro, A.; Gschaedler, A.; Mateos-Díaz, J.C.; Herrera-López, E.J.; Camacho-Ruíz, R.M.; Arellano-Plaza, M. Improvement of a Specific Culture Medium Based on Industrial Glucose for Carotenoid Production by Xanthophyllomyces dendrorhous. Processes 2021, 9, 429. https://doi.org/10.3390/pr9030429
Torres-Haro A, Gschaedler A, Mateos-Díaz JC, Herrera-López EJ, Camacho-Ruíz RM, Arellano-Plaza M. Improvement of a Specific Culture Medium Based on Industrial Glucose for Carotenoid Production by Xanthophyllomyces dendrorhous. Processes. 2021; 9(3):429. https://doi.org/10.3390/pr9030429
Chicago/Turabian StyleTorres-Haro, Alejandro, Anne Gschaedler, Juan C. Mateos-Díaz, Enrique J. Herrera-López, Rosa M. Camacho-Ruíz, and Melchor Arellano-Plaza. 2021. "Improvement of a Specific Culture Medium Based on Industrial Glucose for Carotenoid Production by Xanthophyllomyces dendrorhous" Processes 9, no. 3: 429. https://doi.org/10.3390/pr9030429
APA StyleTorres-Haro, A., Gschaedler, A., Mateos-Díaz, J. C., Herrera-López, E. J., Camacho-Ruíz, R. M., & Arellano-Plaza, M. (2021). Improvement of a Specific Culture Medium Based on Industrial Glucose for Carotenoid Production by Xanthophyllomyces dendrorhous. Processes, 9(3), 429. https://doi.org/10.3390/pr9030429