Thermal Treatment under Vacuum for Obtaining a Quenchant from Rapeseed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Analysis Methods
2.2. Pyrolysis under Vacuum
2.3. Quenching Tests
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troell, E.; Kristoffersen, H.; Bodin, J.; Segerberg, S. Chapter 12.04. Controlling the Cooling Process Measurement, Analysis, and Quality Assurance. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 12, pp. 99–120. ISBN 9780080965338. [Google Scholar]
- Best Types Quenching Oils for Blacksmithing 2021 [Updated], Working the Flame. Available online: https://workingtheflame.com/quenching-oils-for-blacksmithing/ (accessed on 15 October 2021).
- Kobasko, N.I.; de Souza, E.C.; Canale, L.C.F.; Totten, G.E. Vegetable Oil Quenchants: Calculation and Comparison of The Cooling Properties of a Series of Vegetable Oils. J. Mech. Eng. 2010, 56, 131–142. [Google Scholar] [CrossRef]
- Fujimura, Y.; Sato, T. The composition of quenching oil and quenching effects. Iron Steel Inst. Jpn. (ISIJ) 1963, 49, 1008–1015. [Google Scholar] [CrossRef][Green Version]
- Farah, A.F.; Crnkovic, O.R.; Canale, L.C.F. Castor oil: Studies for heat treatment applications. In Proceedings of the 19th Heat Treating; Including Steel Heat Treating in the New Millennium Conference Proceedings, ASM International, Cincinnati, OH, USA, 1–4 November 1999; pp. 251–254, ISBN 0871706814. [Google Scholar]
- Totten, G.E.; de Souza, E.C.; Canale, L.C.F. Quenchants Derived from Vegetable Oils as Alternatives to Petroleum Oil, Industrial Heating 2011. Available online: https://www.industrialheating.com/articles/89954-quenchants-derived-from-vegetable-oils-as-alternatives-to-petroleum-oil (accessed on 15 October 2021).
- Canale, L.C.F.; Totten, G.E. Hardening of Steel. In Quenching Theory and Technology, 2nd ed.; Liscic, B., Tensi, H.M., Canale, L.C.F., Totten, G.E., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–42. ISBN 9780429129650. [Google Scholar]
- Civera, C.; Rivolta, B.; Simencio-Otero, R.L.; Lúcio, J.G.; Totten, G.E.; Canale, L.C.F. Vegetable Oils as Quenchants for Steels: Residual Stresses and Dimensional Changes. ASTM Int. Mater. Perform. Charact. 2014, 3, 20140039. [Google Scholar] [CrossRef]
- Fernandes, P.; Prabhu, K.N. Comparative study of heat transfer and wetting behaviour of conventional and bioquenchants for industrial heat treatment. Int. J. Heat Mass Transf. 2008, 51, 526–538. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.; Liu, Z.; Perez, J. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim. Acta 2000, 364, 87–97. [Google Scholar] [CrossRef]
- Said, D.; Belinato, G.; Sarmiento, G.S.; Otero, R.L.S.; Totten, G.E.; Gastón, A.; Canale, L.C.F. Comparison of Oxidation Stability and Quenchant Cooling Curve Performance of Soybean Oil and Palm Oil. J. Mater. Eng. Perform. 2013, 22, 1929–1936. [Google Scholar] [CrossRef]
- Otero, R.L.S.; Canale, L.C.F.; Schicchi, D.S.; Agaliotis, E.; Totten, G.E.; Sarmiento, G.S. Epoxidized Soybean Oil: Evaluation of Oxidative Stabilization and Metal Quenching/Heat Transfer Performance. J. Mater. Eng. Perform. 2013, 22, 1937–1944. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An Overview on Catalytic Hydrodeoxygenation of Pyrolysis Oil and Its Model Compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Suota, M.J.; Simionatto, E.L.; Scharf, D.R.; Meier, H.F.; Wiggers, V.R. Esterification, Distillation, and Chemical Characterization of Bio-Oil and Its Fractions. Energy Fuels 2019, 33, 9886–9894. [Google Scholar] [CrossRef]
- Prabhu, K.N.; Fernandes, P. Determination of Wetting Behavior, Spread Activation Energy, and Quench Severity of Bioquenchants. Met. Mater. Trans. B 2007, 38, 631–640. [Google Scholar] [CrossRef]
- Hasan, H.S.; Peet, M.J.; Jalil, J.M.; Bhadeshia, H.K.D.H. Heat transfer coefficients during quenching of steels. Heat Mass Transf. 2010, 47, 315–321. [Google Scholar] [CrossRef]
- Hernandez-Morales, B. Cooling: Curve Analysis. In Encyclopedia of Iron, Steel, and Their Alloys, 1st ed.; Taylor& Francis Encyclopedia; Colás, R., Totten, G.E., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 854–874. ISBN 9781351254502. [Google Scholar] [CrossRef]
- de Souza, E.C.; Bronzini, C.A.; Gaston, A.; Canale, L.C.F.; Sanchez Sarmiento, G.; Totten, G.E. Temperature dependence of the quenching properties of vegetable oils compared to petroleum oil quenchants. In Proceedings of the International Metallurgy and Materials Congress, IMMC’15, Istanbul, Turkey, 11–13 November 2010; UCTEA Union of Chambers of Engineers and Architectsb of Turkey: Istanbul, Turkey, 2010; pp. 1694–1707. [Google Scholar]
- Totten, G.E.; Tensi, H.M.; Lainer, K. Performance of Vegetable Oils as a Cooling Medium in Comparison to a Standard Mineral Oil. J. Mater. Eng. Perform. 1999, 8, 409–416. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Mofijur, M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Ok, Y.S.; Peng, W.; Chong, C.T.; Ma, N.L.; Chase, H.A.; Liew, Z.; Yusup, S.; Kwon, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Sivriu, A.M.; Koncsag, C.-I.; Mares, A.-M.; Tirpan, R.; Sapunaru, O.; Jinescu, G. Olefins and Fuels from Frying Palm Oil through Pyrolysis. Environ. Eng. Manag. J. 2020, 19, 345–352. [Google Scholar] [CrossRef]
- Sivriu, A.M.; Jinescu, G.; Sapunaru, O.; Tîrpan, D.R.; Koncsag, C.I. Pyrolysis of Waste Palm Oil in the Presence of Steam. Rev. Chim. 2019, 70, 1992–1995. [Google Scholar] [CrossRef]
- Idem, R.O.; Katikaneni, S.P.R.; Bakhshi, N.N. Thermal Cracking of Canola Oil: Reaction Products in the Presence and Absence of Steam. Energy Fuels 1996, 10, 1150–1162. [Google Scholar] [CrossRef]
- Li, L.; Quan, K.; Xu, J.; Liu, F.; Liu, S.; Yu, S.; Xie, C.; Zhang, B.; Ge, X. Liquid hydrocarbon fuels from catalytic cracking of rubber seed oil using USY as catalyst. Fuel 2014, 123, 189–193. [Google Scholar] [CrossRef]
- Mota, S.; Mancio, A.D.A.; Lhamas, D.; de Abreu, D.; da Silva, M.; dos Santos, W.; de Castro, D.; de Oliveira, R.; Araújo, M.; Borges, L.E.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]

 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.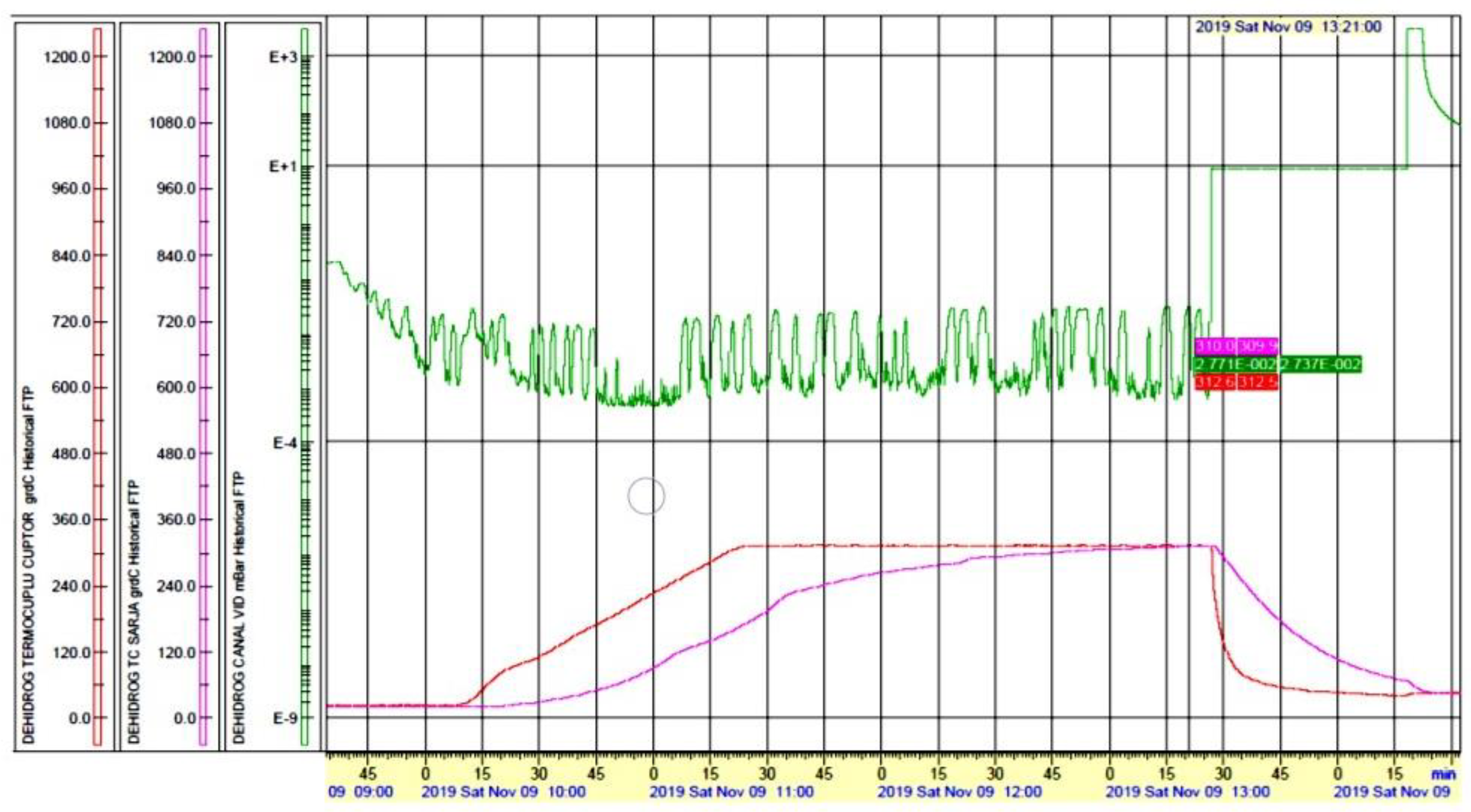
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
 pressure;
pressure;  batch temperature;
batch temperature;  oven temperature.
oven temperature.
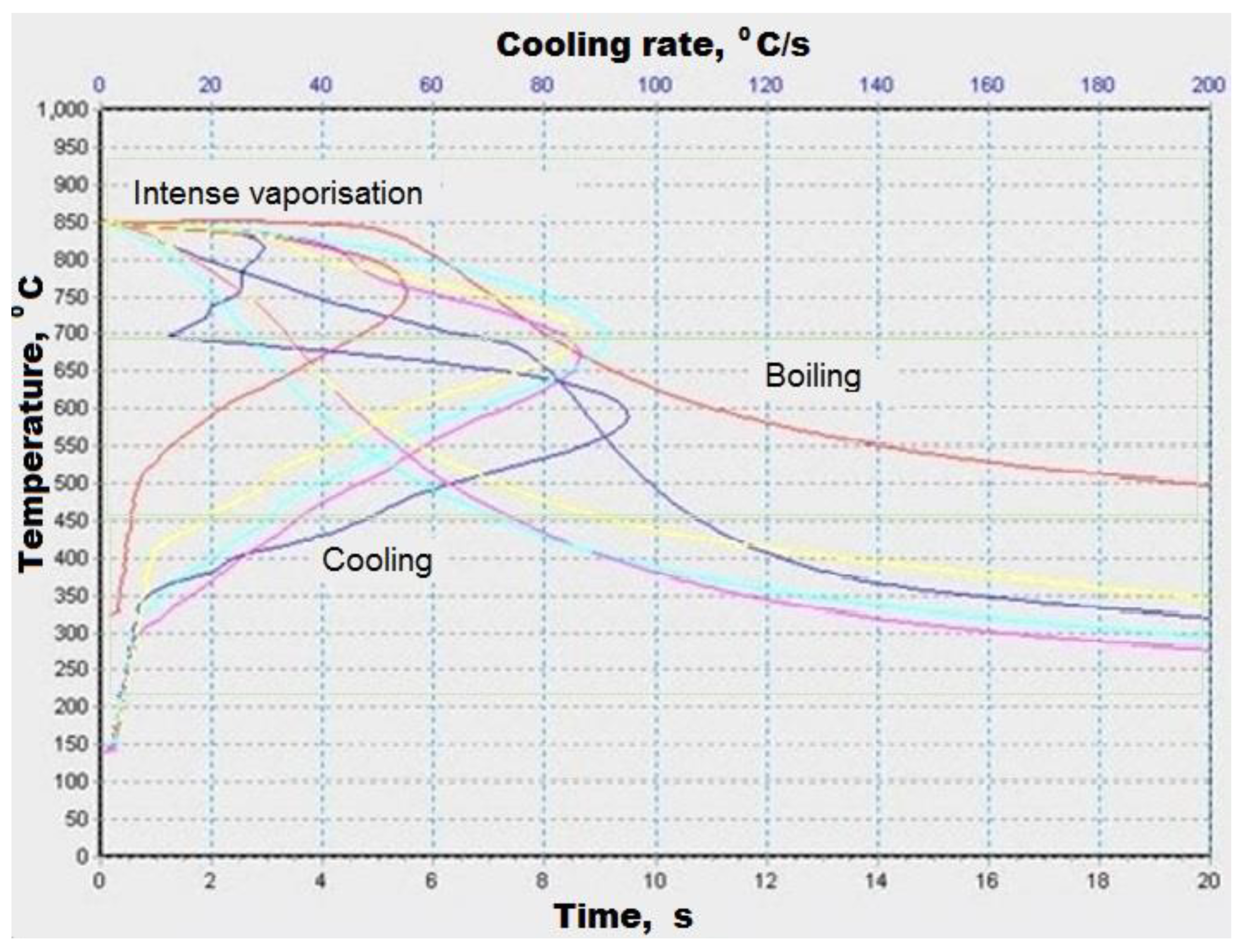
 rapeseed oil
rapeseed oil  bio oil at 300 °C
bio oil at 300 °C  bio-oil at 310 °C
bio-oil at 310 °C  bio oil at 320 °C
bio oil at 320 °C  .
.
 rapeseed oil
rapeseed oil  bio oil at 300 °C
bio oil at 300 °C  bio-oil at 310 °C
bio-oil at 310 °C  bio oil at 320 °C
bio oil at 320 °C  .
.
| Characteristic | Unit | Rapeseed Oil | IloquenchTM 1 |
|---|---|---|---|
| Density at 15 °C | g/cm3 | 0.910 | 0.870 |
| Flash point (Marcusson) | °C | 239 | >190 |
| Iodine number | g I2/100 g sample | 5.8 | - |
| Kinematic visccosity at 40 °C | mm2/s | 43 | 20 |
| Kinematic visccosity at 100 °C | mm2/s | 12.8 | 5 |
| Cooling rate at 300 °C | °C/s | 6.13 | 6.18 |
| Process Temperature, °C | Rapeseed Oil Processed by Pyrolysis, g | Gases Obtained, g | Pyrolysis Oil Obtained, g | Liquid Product Yield, % |
|---|---|---|---|---|
| 300 | 910.43 | 120.44 | 789.99 | 87% |
| 310 | 872.72 | 337.97 | 534.75 | 61% |
| 320 | 946.23 | 520.43 | 425.80 | 45% |
| 375 | 920.42 | 920.42 | 0 | 0% |
| Oil Type | Kinematic Viscosity at 40 °C, mm2/s | Kinematic Viscosity at 100 °C, mm2/s | Density at 20 °C, g/cm3 | Flash Point, °C | Iodine Number, g I2/100 g Sample |
|---|---|---|---|---|---|
| Rapeseed waste oil | 43 | 12.8 | 0.9180 | 202 | 5.8 |
| Bio-oil resulted at 300 °C | 42.9 | 10.9 | 0.9174 | 200 | 5.9 |
| Bio-oil resulted at 310 °C | 42.3 | 9.5 | 0.9162 | 200 | 5.9 |
| Bio-oil resulted at 320 °C | 38.7 | 9.3 | 0.9150 | 198 | 6.0 |
| Mineral oil IloquenchTM 1 | 20 | 5 | 0.8712 | 192 | - |
| Oil/Hardness | Before Quenching | After Quenching | ||||
|---|---|---|---|---|---|---|
| - | Mineral | Rapeseed Oil | Bio-Oil Obtained at 300 °C | Bio-Oil Obtained at 310 °C | Bio-Oil Obtained at 320 °C | |
| HRC units | 29–30 | 45 | 46 | 44–45 | 43 | 43–44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivriu, A.M.; Sapunaru, O.V.; Sterpu, A.E.; Cioroiu Tirpan, D.-R.; Chis, T.V.; Dobre, T. Thermal Treatment under Vacuum for Obtaining a Quenchant from Rapeseed Oil. Processes 2021, 9, 2189. https://doi.org/10.3390/pr9122189
Sivriu AM, Sapunaru OV, Sterpu AE, Cioroiu Tirpan D-R, Chis TV, Dobre T. Thermal Treatment under Vacuum for Obtaining a Quenchant from Rapeseed Oil. Processes. 2021; 9(12):2189. https://doi.org/10.3390/pr9122189
Chicago/Turabian StyleSivriu, Ana Maria, Olga Valerica Sapunaru, Ancaelena Eliza Sterpu, Doinita-Roxana Cioroiu Tirpan, Timur Vasile Chis, and Tanase Dobre. 2021. "Thermal Treatment under Vacuum for Obtaining a Quenchant from Rapeseed Oil" Processes 9, no. 12: 2189. https://doi.org/10.3390/pr9122189
APA StyleSivriu, A. M., Sapunaru, O. V., Sterpu, A. E., Cioroiu Tirpan, D.-R., Chis, T. V., & Dobre, T. (2021). Thermal Treatment under Vacuum for Obtaining a Quenchant from Rapeseed Oil. Processes, 9(12), 2189. https://doi.org/10.3390/pr9122189








