Abstract
Tertiary amines have been used as alternative absorbents for traditional primary and secondary amines in the process of carbon capture. However, the carbon dioxide (CO2) absorption rates in these kinds of amine are relatively slow, which implies greater investment and construction costs and limits the large-scale application of carbon capture. Carbonic anhydrase (CA) is considered to be an ideal homogeneous catalyst for accelerating the rate of CO2 into aqueous amine solution. In this work, CO2 absorption combining CA with two single aqueous tertiary amines, namely triethanolamine (TEA) and 2-(diethylamino)ethanol (DEEA), was studied by use of the stopped-flow apparatus over temperature ranging from 293 to 313 K. The concentrations of selected aqueous amine solution and CA used in the experiment were ranging among 0.1–0.5 kmol/m and 0–50 g/m, respectively. Compared to the solution without the addition of CA, the pseudo first-order reaction rate in the presence of CA () is significantly increased. The values of have been calculated by a new kinetics model. The results of experimental and calculated and in CO2-amine-H2O solutions were also investigated, respectively.
1. Introduction
Carbon dioxide (CO2) absorption using chemical absorption with traditional aqueous amine solution, such as monoethanolamine (MEA) and diethanolamine (DEA), is a terminal processing technology that can be deployed on a large scale in coal-fired power plants [1]. However, there are a number of existing drawbacks, such as very high energy consumption during CO2 desorption process [2]. These energy costs account for approximately 80% of the operating costs [3]. In addition, in the presence of oxygen the amine solution is easily to degrade and produce harmful volatile organic compounds resulting in the loss of solvent and even corrosion problems [4]. With the deepening of research, tertiary amines such as methyldiethanolamine (MDEA), triethanolamine (TEA) and 2-(diethylamino)ethanol (DEEA) were proposed as alternative solvents for MEA due to their lower energy consumption for carbon capture [5]. Though there is lower energy consumption for the desorption process in these kinds of tertiary amine solvents, the relatively slow absorption rate of CO2 in the absorber means that the better absorber is needed to meet the demand for carbon capture. Carbonic anhydrase (CA) can very easily accelerate the reaction of CO2 hydration and is considered to be a promising homogeneous catalyst for CO2 absorption in carbon capture process [6,7,8].
In this work, the kinetics of CO2 reacting with TEA and DEEA solution was studied with the use of stopped-flow apparatus. The experimental kinetics data of CO2 with aqueous amine solution catalyzed by CA over temperature ranging from 293 to 313 K were collected. The concentration of CA in the aqueous amine solution with its concentration ranging among 0.1–0.5 kmol/m varies in 0–50 g/m. The base-catalyzed hydration mechanism is selected to interpret the reaction mechanism of CO2 reaction with aqueous amine solutions. The collected experimental data is fitted by the empirical power law equation to determine the possible reaction order in each reaction system. The kinetic constants under the catalysis of CA were also investigated and the results showed that the pseudo first-order reaction rate () catalyzed by CA is significantly increased as compared to the absence of CA. A new kinetics model of CA-catalyzed absorption of CO2 in aqueous tertiary amine solutions was established and used to compare with experimental date.
2. Materials and Methods
2.1. Chemicals
TEA and DEEA are tertiary amines ordered from aladdin Industrial Corporation (Shanghai, China). Their molecular structures are listed in Figure 1. Bovine carbonic anhydrase (BCA) was obtained from Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). The basic information for the chemical reagents are given in Table 1. The desired solution concentration is obtained by confirming the dilution of the chemical reagents with DI-water.
Figure 1.
Molecular structure of: (a) triethanolamine (TEA) and (b) 2-(diethylamino)ethanol (DEEA).

Table 1.
Chemicals used.
2.2. Experimental Procedure
In an experimental case, the sample of desired CO2 absorbent was prepared by dissolving pre-weighed amine solvent into deionized water with a certain amount of CA. The pure CO2 gas was bubbled into DI-water for a certain period of time to obtain the saturated aqueous solution of CO2. The of CO2 absorption into the aqueous amine solution with or without CA was obtained by the stopped-flow apparatus. The schematic diagram of the stopped-flow apparatus is shown in Figure 2 and its detailed operational description can be found in our previous work [6,9].
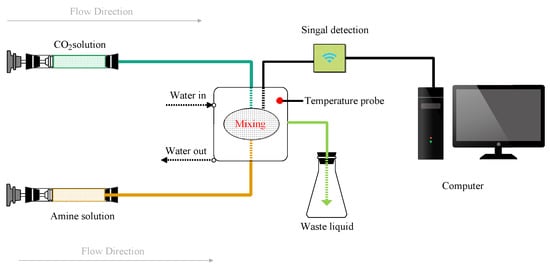
Figure 2.
Schematic diagram of the experimental device.
In a typical experimental operation, equal volumes of fresh CO2 and amine solutions are pushed into the conductance cell by a gas-driven source. A change in the conductivity signal is observed in the cell since the reaction of CO2 with aqueous amine forms new ionic products. Then the detected signal tends to stabilize before the absorption reaction is complete and reaches equilibrium. The kinetics constant can be obtained by the fitting curve of the conductivity changing with time at different temperatures as listed in our previous work [10]. Each measurement was repeated at least 7 times to ensure the accuracy of the collected data. The effectiveness of the test set-up has been demonstrated in a good consistent with that of Ali et al. [11,12] in our previous work [13].
2.3. Reaction Kinetics of CO2 Absorption
The catalyzed hydration mechanism proposed by Donaldson and Nguyen [14] is commonly used to explain the absorption mechanism of CO2 in tertiary alkanolamine solution (). In the process of gas–liquid two-phase contact, the performance of tertiary alkanolamine is like a catalyst that catalyzes the hydration of CO2. The solvent itself does not react directly with CO2. Expressions of the reaction mechanism in CO2-amine-H2O system are given in Equations (1)–(3):
Then, the overall rate constant (kmol/m·s) of CO2 absorption in aqueous amine solution can be expressed as:
In most of the published studies, the contribution of CO2 reacting with OH− and H2O shown in reactions 2–3 to the overall absorption process can be neglected [15,16]. Then the reaction kinetic constants () throughout the CO2 absorption process is completely determined by reaction 1. Subsequently, the pseudo first-order equation shown in Equation (5) can be used to describe the reaction between CO2 and tertiary amine solution.
CA was first found in red blood cells [17] and has been proved to be a very efficient catalyst for the conversion of CO2 to HCO3−. CA is a broad group of zinc metal-proteins (enzymes) existing in three genetically unrelated families of isoforms (). The mechanism of CO2 hydration catalyzed by CA was introduced in detail by Lindskog et al. [18] as shown in Equations (6)–(8):
3. Results and Discussion
All experimental data are given in Appendix A.
3.1. CO2-Amine-H2O System
The reaction kinetic data between CO2 and aqueous tertiary amine (TEA and DEEA) solution with temperatures ranging from 293 K to 313 K was listed in Table A1. In the CO2-tertiary amine-H2O system, amine itself is the major contributor to the reaction and the contributions of OH− and water can be negligible [15,16]. Then, the base-catalyzed hydration mechanism (Equations (1)–(3)) was used to interpret the possible reaction mechanism of CO2 when reacting with aqueous TEA and DEEA solutions. The collected kinetic data shown in Figure 3 (Table A1) was fitted by Equation (5) to verify the possible reaction order of CO2 absorption in amine solution at the temperatures of 293, 298, 303, 308, 313 K, in which the reaction order is approximately equal to one (1.30–1.36 for TEA and 0.83–1.0 for DEEA) with respect to amine concentration over temperature ranging from 293 to 313 K, respectively.
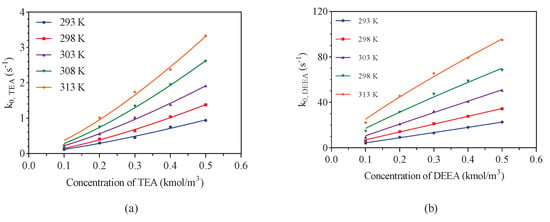
Figure 3.
The values of CO2 reacting with aqueous TEA and DEEA solution at the temperatures of 293–313 K: (a) represents TEA–CO2–H2O system and (b) represents DEEA–CO2–H2O system.
As can be observed from Figure 3, the pseudo first-order rate constants show a growing trend with increasing concentration and temperature of amine solution. Under experimental temperature, the reaction order with respect to amine concentration was approximately equal to 1 by fitting values using the power law Equation (9).
where n is the reaction order.
The above obtained values listed in Table A1 were fitted with Equation (10) in order to get values of these amines. All the fitted results of for TEA and DEEA are given in Table A2. It can be easily found that the value is just a function of temperature. It is widely accepted in published studies that can be fitted according to the Arrhenius expression:
where A is Arrhenius constant or pre-exponential constant (m/kmol·s), and represents the activation energy (kJ/mol) and R represents the universal gas constant (0.008315 kJ/mol·K).
The corresponding Arrhenius equation for with TEA was correlated according to Equation (10) and the results displayed in Figure 4. The calculated activation energy was 45.29 kJ/mol. The Ea values of these amines in the Arrhenius were replaced with calculated values in Equations (11)–(12), respectively.
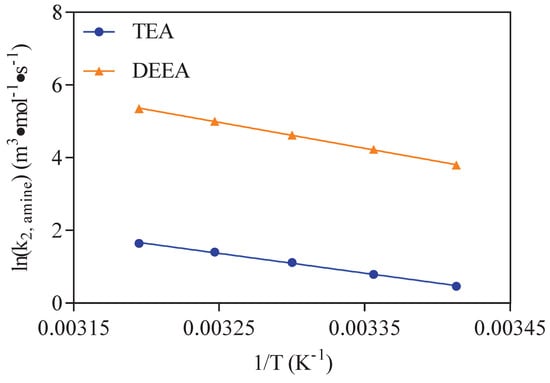
Figure 5 showed a comparison between the values obtained with base-catalyzed mechanism and the experimental data. The absolute average deviations (AADs) is calculated as shown in Equation (13). The AADs for CO2–TEA–H2O and CO2–DEEA–H2O systems were 10.8% and 4.5%, respectively.
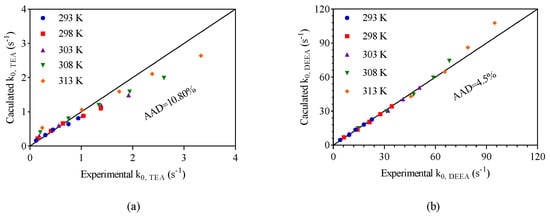
Figure 5.
A comparison between the calculated values and that of collected experimental results: (a) TEA–CO2–H2O system; (b) DEEA–CO2–H2O system.
3.2. CO2-Amine-H2O Containing CA
The collection of kinetics data was operated almost the same as Section 3.1, except that a quantitative enzyme is added to the amine solution. The concentration of CA was over a range of 0–50 g/m in 0.2 kmol/m aqueous TEA solutions. The results of collected kinetic data was listed in Table A3 and fitted by Equation (14) shown in Figure 6. Compared with the obtained results of Figure 3, it found that the value was obviously accelerated by the existing of CA in the aqueous amine solution. There is a non-linear relationship between and CA concentration, which is inconsistent with the test results provided by Alper and Deckwer [19]. The rate constants were well fitted by a linear regression at low amine concentration and reached a maximum value and not changed at higher enzyme concentration. The similar phenomenon was observed in the work of Van Elk et al. [20] on the reaction of CO2 with DMMEA catalyzed by a thermostable variant of the human carbonic anhydrase (5X CA) provided by CO2 Solutions Inc. However, the increase of overall reaction rate constants in the DEEA soulution were not significant. This is because the enzyme shows better activity in a near neutral environment and the pKa value of DEEA has a key impact on the catalytic activity of enzyme. Similar conclusions were reached by Wilk et al. [21] when using N-methyldiethanolamine (MDEA) and the activator in the form of piperazine.
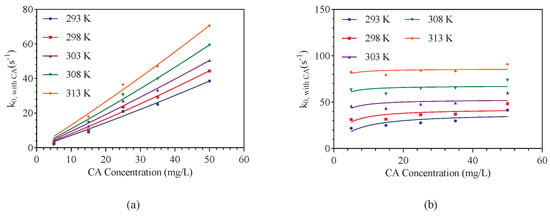
Figure 6.
The changed value of CO2 reacting with amine solutions with a certain amounts of carbonic anhydrase in the amine-CA-CO2-H2O system over temperature range of 293–313 K: (a) represents the TEA-CA-CO2-H2O system; (b) represents the DEEA-CA-CO2-H2O system.
Subsequently, the overall catalyst enhancement (CE) was defined in enzyme catalyzed CO2 absorption system, which represented the ratio of reaction rate constant as listed in Model 1 () and another correlation one marked as Model 2 (), which is used to correct the experimentally determined values in CO2-MDEA system using human carbonic anhydrase (HCA) as additive agent introduced by Penders-van Elk et al. [8] However in order to further study the effect of CA on the , the influence of amine itself needs to be removed. The termed Model 3 listed in Equation (14) was selected in this work, in which the reaction rate constant of CO2 reacting with hydroxyl ion in present of enzyme was considered to exclude the effect of amines on CA activity, which is different from Model 1 and Model 2.
It is worth noting that both above Model 1 and 2 were not selected in this work but Equation (14) was used for data fitting as listed in our previous work on MDEA and DMEA containing carbonic anhydrase [6]. Table A4 listed the kinetic data of CO2 absorption in various amine solutions at temperatures from 293 to 313 K and the obtained results as shown in Figure 7 show that there is a linear trend in both aqueous solutions of TEA and DEEA. In CO2-DEEA-H2O system, the CA shows partial activity at lower concentrations and no obvious regular catalytic activity in this amine system, which means the model of Equation (14) is not suitable for this system. This phenomenon is likely because the overall absorption rate is not limited by enzymatic turnover once the enzyme is sufficiently concentrated.
where is reaction rate constant of CA-CO2-amines-H2O system, is the reaction rate constant of CO2-amine-H2O system, and represents the reaction rate constant of CO2 reacting with OH− in the solution which can be found in the work of Pinsent et al. [22]. and are parameters of Equation (14) as a function of temperature following Arrhenius relationship. The catalyst enhancement value reflects the extent to which CA promotes the CO2 absorption into the amine solution compared to that uncatalyzed reaction.
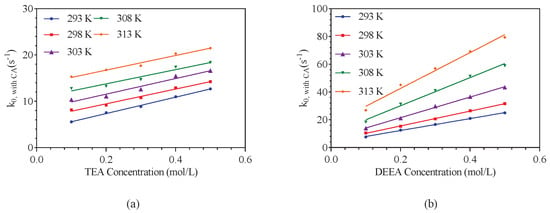
Figure 7.
The values of CO2 reacting with amine solutions under 3 g/m CA in the reaction system over temperature range of 293–313 K: (a) represents the TEA–CO2–H2O system; (b) represents the DEEA–CO2–H2O system.
Equations (15) and (16) is the results of the intercept values in Figure 8 fitted in an Arrhenius relationship. In this work, the reaction rate constant is defined as . The reaction kinetics fitting of CO2 reacting with aqueous solution of different amines including TEA were performed. Equation (20) can be obtained combing Equations (4) and (14). The can be replaced by multiplying amine concentration. The values of the calculated shown in Equations (19) and (20) are plotted versus the experimental values in Figure 9. It showed a good performance in predicting values of CO-TEA-HO solution with the AADs of 23.38%.
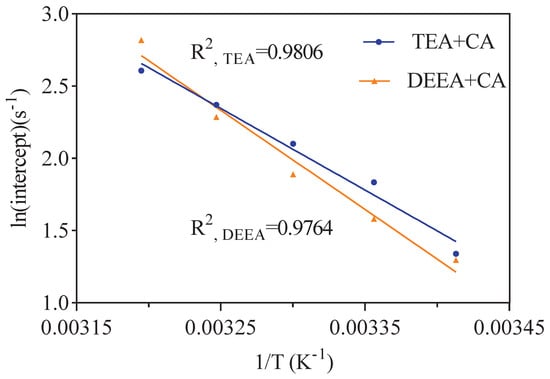
Figure 8.
Plot of the values of intercept with 3 g/m CA in CO2–TEA–H2O and CO2–DEEA–H2O system at temperatures of 293–313 K in Figure 7.
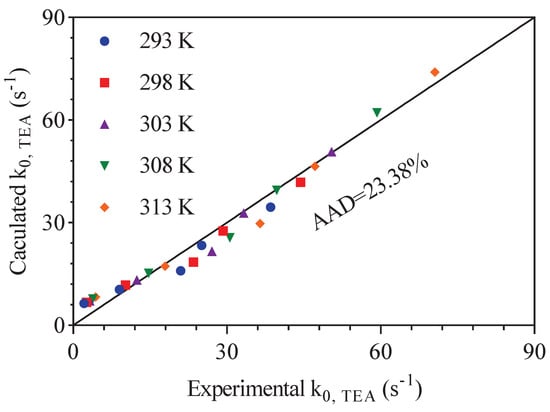
Figure 9.
The comparison of experimental and calculated pseudo first–order reaction rate constant ().
4. Conclusions
The stopped-flow apparatus was used in this work for the acquisition of the kinetic data between CO2 and aqueous TEA or DEEA solutions. With this useful kinetics data, the possible reaction mechanism of CO2 absorption in amine solutions can be inferred. By comparing the experimental values with that of the calculated results, it was found that the AADs were 10.80 and 9.95% in CO2-TEA-H2O and CO2-DEEA-H2O system, respectively. This obtained results proved that the based catalyzed mechanism was suitable to interpret the reaction pathway of CO2 absorption into aqueous TEA and DEEA solutions.
Adding a certain amount of CA to the aqueous solution of TEA and DEEA can significantly improve the absorption rate of CO2. With increasing amine concentrations, temperature and CA amounts, the reaction rate constant was obviously improved. Then, an catalyst enhancement (CE) is introduced to express the catalytic activity of CA in CO2 absorption using TEA and DEEA solutions. The results fitted by the new kinetics model showed reasonably good performance in predicting results of pseudo first-order reaction rate constant () in CO2-TEA-H2O with the AADs of 23.38%. While in CO2-DEEA-H2O system, the CE model was not applicable due to the high pKa value of DEEA.
Author Contributions
Methodology, B.L.; software, Z.C.; validation, B.L.; writing—original draft preparation, B.L.; writing—review and editing, Z.C. and W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 22178189 and Key Laboratory of Multiphase Flow Reaction and Separation Engineering of Shandong Province, grant number 2021MFRSE-D03.
Institutional Review Board Statement
The study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge that this work is supported by the National Natural Science Foundation of China (Grant No. 22178189) and Key Laboratory of Multiphase Flow Reaction and Separation Engineering of Shandong Province (Grant No. 2021MFRSE-D03). This work has also received great help and support from the Zhiwu Liang research group of Hunan University.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TEA | Triethanolamine |
| DEEA | 2-(Diethylamino)ethanol |
| CA | Carbonic Anhydrase |
| HCA | Human Carbonic Anhydrase |
| CE | Catalyst Enhancement |
| Ea | activation energy (kJ/mol) |
| R | universal gas constant (0.008315 kJ/mol·K) |
| T | temperature (K) |
| concentration (kmol·m) | |
| first order reaction rate constant (s) | |
| second order reaction rate constant (m· mol ·s) | |
| first order reaction rate constant (s) without enzyme | |
| first order reaction rate constant (s) with enzyme | |
| reaction rate constant of CO2 reacting with hydroxyl ion in absence of enzyme | |
| reaction rate constant of CO2 reacting with hydroxyl ion in present of enzyme | |
| the total CO2 reaction rate (kmol·m·s) | |
| CE | catalyst enhancement |
| reaction rate constant of CO2 reacting with hydroxyl ion determined by Pinsent et al. | |
| parameter of Equation (14) | |
| parameter of Equation (14) |
Appendix A
Appendix A.1

Table A1.
Experimental kinetics data () of TEA extracted from stopped-flow apparatus over a concentration range of 0.10–0.50 kmol/m and a temperature range of 293–313 K.
Table A1.
Experimental kinetics data () of TEA extracted from stopped-flow apparatus over a concentration range of 0.10–0.50 kmol/m and a temperature range of 293–313 K.
| Amine Concentration (kmol/m) | Temperature (K) | ||||
|---|---|---|---|---|---|
| 293 | 298 | 303 | 308 | 313 | |
| TEA | |||||
| 0.1 | 0.12 | 0.15 | 0.18 | 0.20 | 0.24 |
| 0.2 | 0.30 | 0.41 | 0.56 | 0.75 | 1.01 |
| 0.3 | 0.45 | 0.64 | 1.02 | 1.34 | 1.74 |
| 0.4 | 0.75 | 1.04 | 1.39 | 1.94 | 2.38 |
| 0.5 | 0.94 | 1.38 | 1.92 | 2.61 | 3.33 |
| Reaction order | 1.30 | 1.39 | 1.34 | 1.38 | 1.36 |
| R value | 0.9926 | 0.9971 | 0.997 | 0.9974 | 0.9943 |
| DEEA | |||||
| 0.1 | 4.12 | 6.23 | 9.33 | 14.54 | 22.12 |
| 0.2 | 9.37 | 14.29 | 21.04 | 31.38 | 45.65 |
| 0.3 | 13.07 | 21.29 | 32.23 | 47.20 | 65.60 |
| 0.4 | 18.05 | 27.71 | 40.96 | 58.74 | 79.12 |
| 0.5 | 22.67 | 34.38 | 50.61 | 68.22 | 94.81 |
| Reaction order | 1.0 | 0.99 | 0.98 | 0.89 | 0.83 |
| R value | 0.9979 | 0.998 | 0.9968 | 0.9907 | 0.9939 |

Table A2.
The values of CO2 absorption into aqueous TEA and DEEA Solutions.
Table A2.
The values of CO2 absorption into aqueous TEA and DEEA Solutions.
| T (K) | (m·mol·s) | |
|---|---|---|
| TEA | DEEA | |
| 293 | 1.59 | 44.42 |
| 298 | 2.21 | 68.55 |
| 303 | 3.06 | 101.91 |
| 308 | 4,06 | 148.58 |
| 313 | 5.17 | 211.11 |

Table A3.
Experimental kinetics data of of CO2 reacting with 0.5 kmol/m TEA and DEEA Containing various amounts of CA over a temperature range of 293–313 K.
Table A3.
Experimental kinetics data of of CO2 reacting with 0.5 kmol/m TEA and DEEA Containing various amounts of CA over a temperature range of 293–313 K.
| CA Concentration (g/m) | Temperature (K) | |||||
|---|---|---|---|---|---|---|
| 293 | 298 | 303 | 308 | 313 | ||
| CA+TEA | ||||||
| 5 | 2.12 | 2.62 | 3.20 | 3.84 | 4.36 | |
| 15 | 9.04 | 10.20 | 12.42 | 14.71 | 17.94 | |
| 25 | 20.99 | 23.50 | 27.08 | 30.56 | 36.49 | |
| 35 | 25.07 | 29.28 | 33.26 | 39.75 | 47.16 | |
| 50 | 38.55 | 44.38 | 50.45 | 59.28 | 70.57 | |
| CA+DEEA | ||||||
| 5 | 21.84 | 31.34 | 45.87 | 63.20 | 82.41 | |
| 15 | 24.97 | 31.51 | 43.34 | 58.98 | 79.34 | |
| 25 | 27.66 | 36.47 | 47.76 | 64.76 | 84.03 | |
| 35 | 29.84 | 37.16 | 49.06 | 65.36 | 83.58 | |
| 50 | 41.46 | 48.34 | 60.22 | 73.64 | 90.99 | |

Table A4.
Experimental kinetics data of of CO2 reacting with aqueous TEA and DEEA solutions containing 3 g/m CA over a temperature range of 293–313 K.
Table A4.
Experimental kinetics data of of CO2 reacting with aqueous TEA and DEEA solutions containing 3 g/m CA over a temperature range of 293–313 K.
| Amine Concentration (kmol/m) | Temperature (K) | |||||
|---|---|---|---|---|---|---|
| 293 | 298 | 303 | 308 | 313 | ||
| TEA+CA | ||||||
| 0.1 | 5.57 | 8.14 | 10.37 | 12.78 | 15.32 | |
| 0.2 | 7.54 | 9.17 | 11.09 | 13.25 | 16.80 | |
| 0.3 | 8.86 | 10.78 | 12.62 | 14.72 | 17.68 | |
| 0.4 | 10.99 | 12.92 | 15.47 | 17.41 | 20.31 | |
| 0.5 | 12.70 | 14.26 | 16.62 | 18.38 | 21.49 | |
| DEEA+CA | ||||||
| 0.1 | 7.66 | 10.44 | 13.88 | 18.38 | 26.84 | |
| 0.2 | 12.57 | 15.29 | 21.13 | 31.28 | 45.13 | |
| 0.3 | 16.59 | 20.62 | 29.68 | 41.09 | 56.92 | |
| 0.4 | 20.93 | 26.56 | 36.56 | 51.40 | 69.18 | |
| 0.5 | 24.97 | 31.51 | 43.34 | 58.98 | 79.34 | |
References
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Rees, R.J.; Conway, W.; Puxty, G.; Yang, Q.; Winkler, D.A. Computational Modeling and Simulation of CO2 Capture by Aqueous Amines. Chem. Rev. 2017, 117, 9524–9593. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.H.; Sanpasertparnich, T.; Tontiwachwuthikul, P.P.; Gelowitz, D.; Idem, R. Part 1: Design, modeling and simulation of post-combustion CO2 capture systems using reactive solvents. Carbon Manag. 2011, 2, 265–288. [Google Scholar] [CrossRef]
- Supap, T.; Saiwan, C.; Idem, R.; Tontiwachwuthikul, P.P. Part 2: Solvent management: Solvent stability and amine degradation in CO2 capture processes. Carbon Manag. 2011, 2, 551–566. [Google Scholar] [CrossRef]
- Svendsen, H.F.; Hessen, E.T.; Mejdell, T. Carbon dioxide capture by absorption, challenges and possibilities. Chem. Eng. J. 2011, 171, 718–724. [Google Scholar] [CrossRef]
- Liu, B.; Luo, X.; Liang, Z.; Olson, W.; Liu, H.; Idem, R.; Tontiwachwuthikul, P. The development of kinetics model for CO2 absorption into tertiary amines containing carbonic anhydrase. Aiche J. 2017, 63, 4933–4943. [Google Scholar] [CrossRef]
- Lindskog, S.; Silverman, D.N. The Catalytic Mechanism of Mammalian Carbonic Anhydrases. EXS; Birkhäuser: Basel, Switzerland, 2000; Volume 14, p. 175. [Google Scholar] [CrossRef]
- Penders-van Elk, N.J.; Derks, P.W.; Fradette, S.; Versteeg, G.F. Kinetics of absorption of carbon dioxide in aqueous MDEA solutions with carbonic anhydrase at 298 K. Int. J. Greenh. Gas Control. 2012, 9, 385–392. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.; Sema, T.; Rongwong, W.; Li, C.; Na, Y.; Idem, R.; Tontiwachwuthikul, P.; Idem, R.; Tontiwachwuthikul, P. Kinetics of CO2 absorption into a novel 1-diethylamino-2-propanol solvent using stopped-flow technique. AIChE J. 2014, 60, 3502–3510. [Google Scholar] [CrossRef]
- Liu, B.; Luo, X.; Gao, H.; Idem, R.; Tontiwachwuthikul, P.; Olson, W.; Liang, Z. Reaction kinetics of the absorption of carbon dioxide (CO2) in aqueous solutions of sterically hindered secondary alkanolamines using the stopped-flow technique. Chem. Eng. Sci. 2017, 170, 16–25. [Google Scholar] [CrossRef]
- Ali, S.H.; Merchant, S.Q.; Fahim, M.A. Kinetic study of reactive absorption of some primary amines with carbon dioxide in ethanol solution. Sep. Purif. Technol. 2000, 18, 163–175. [Google Scholar] [CrossRef]
- Ali, S.H.; Merchant, S.Q.; Fahim, M.A. Reaction kinetics of some secondary alkanolamines with carbon dioxide in aqueous solutions by stopped flow technique. Sep. Purif. Technol. 2002, 27, 121–136. [Google Scholar] [CrossRef]
- Liu, H.; Sema, T.; Liang, Z.; Fu, K.; Idem, R.; Na, Y.; Tontiwachwuthikul, P. CO2 absorption kinetics of 4-diethylamine-2-butanol solvent using stopped-flow technique. Sep. Purif. Technol. 2014, 136, 81–87. [Google Scholar] [CrossRef]
- Donaldson, T.L.; Nguyen, Y.N. Carbon Dioxide Reaction Kinetics and Transport in Aqueous Amine Membranes. Ind. Eng. Chem. Fundam. 1980, 19, 260–266. [Google Scholar] [CrossRef]
- Versteeg, G.; van Swaaij, W. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions—I. Primary and secondary amines. Chem. Eng. Sci. 1988, 43, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Versteeg, G.; van Swaaij, W. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions—II. Tertiary amines. Chem. Eng. Sci. 1988, 43, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Carter, N.D.; Gros, S.J. The Carbonic Anhydrases: Cellular Physiology and Molecular Genetics. Plenum Press; Springer: Boston, MA, USA, 1991. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Alper, E.; Deckwer, W.D. Kinetics of absorption of CO2 into buffer solutions containing carbonic anhydrase. Chem. Eng. Sci. 1980, 35, 549–557. [Google Scholar] [CrossRef]
- Penders-van Elk, N.J.; Fradette, S.; Versteeg, G.F. Effect of pKa on the kinetics of carbon dioxide absorption in aqueous alkanolamine solutions containing carbonic anhydrase at 298 K. Chem. Eng. J. 2015, 259, 682–691. [Google Scholar] [CrossRef]
- Wilk, A.; Wiecaw-Solny, L.; Krótki, A.; Piewak, D. Impact of the composition of absorption blend on the efficiency of CO2 removal. Chemik 2013, 67, 399–406. [Google Scholar]
- Pinsent, B.R.W.; Pearson, L.; Roughton, F.J.W. The kinetics of combination of carbon dioxide with hydroxide ions. Trans. Faraday Soc. 1956, 52, 1512–1520. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).