Molten-Salt-Assisted Synthesis of Nitrogen-Doped Carbon Nanosheets Derived from Biomass Waste of Gingko Shells as Efficient Catalyst for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
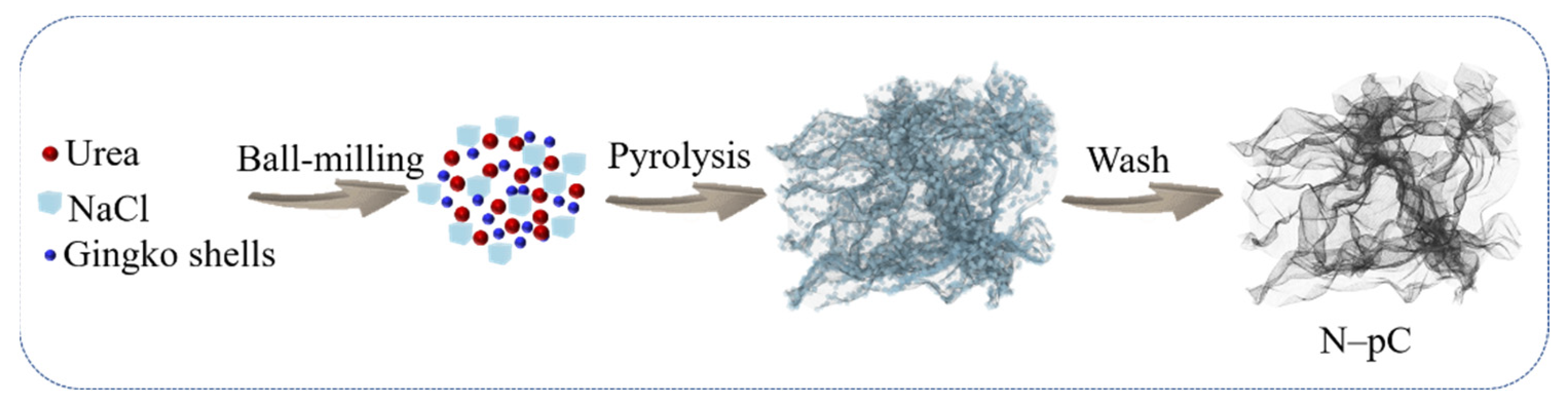
2.1. Synthesis of the Materials
2.2. Characterizations
2.3. Electrochemical Measurements
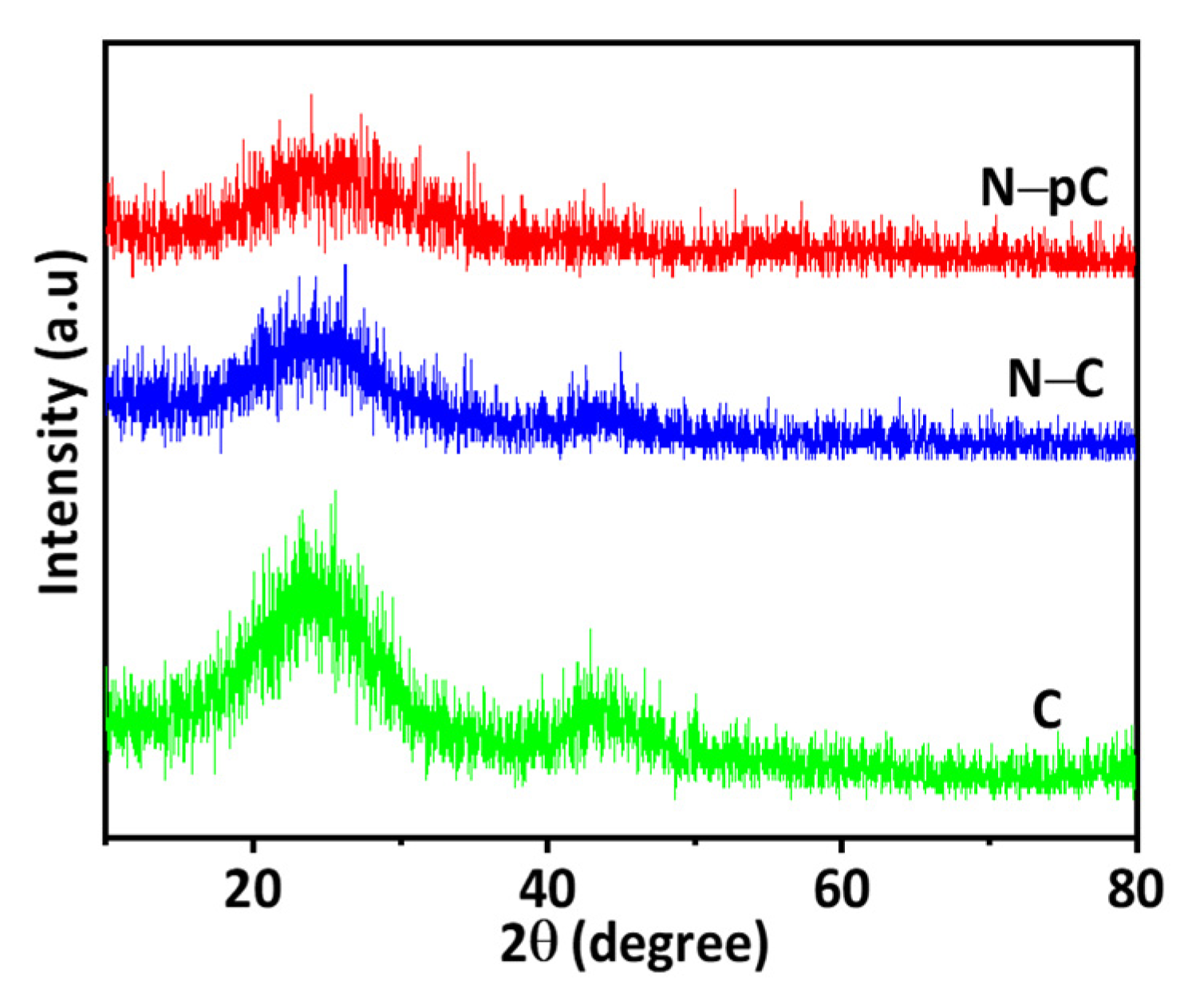
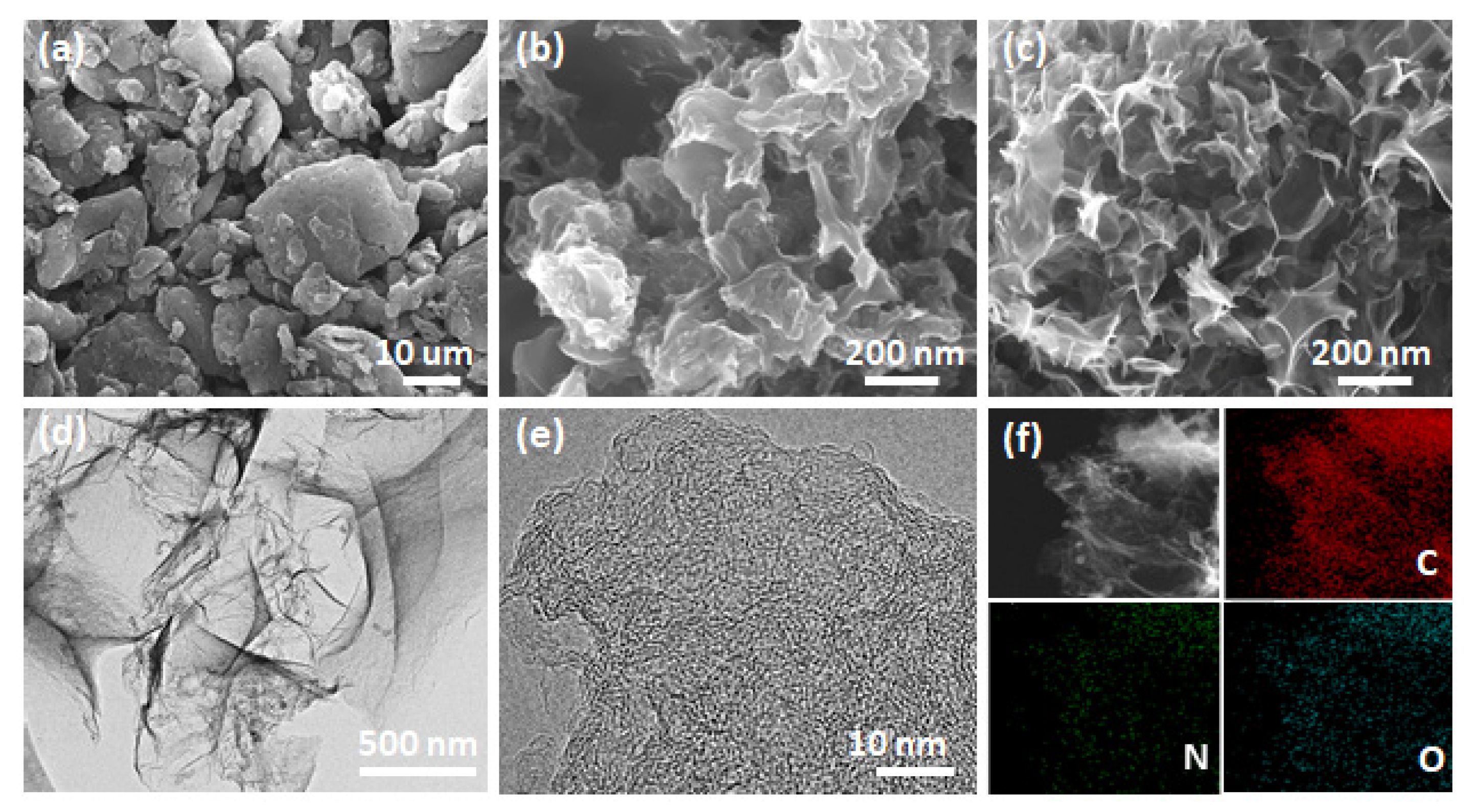
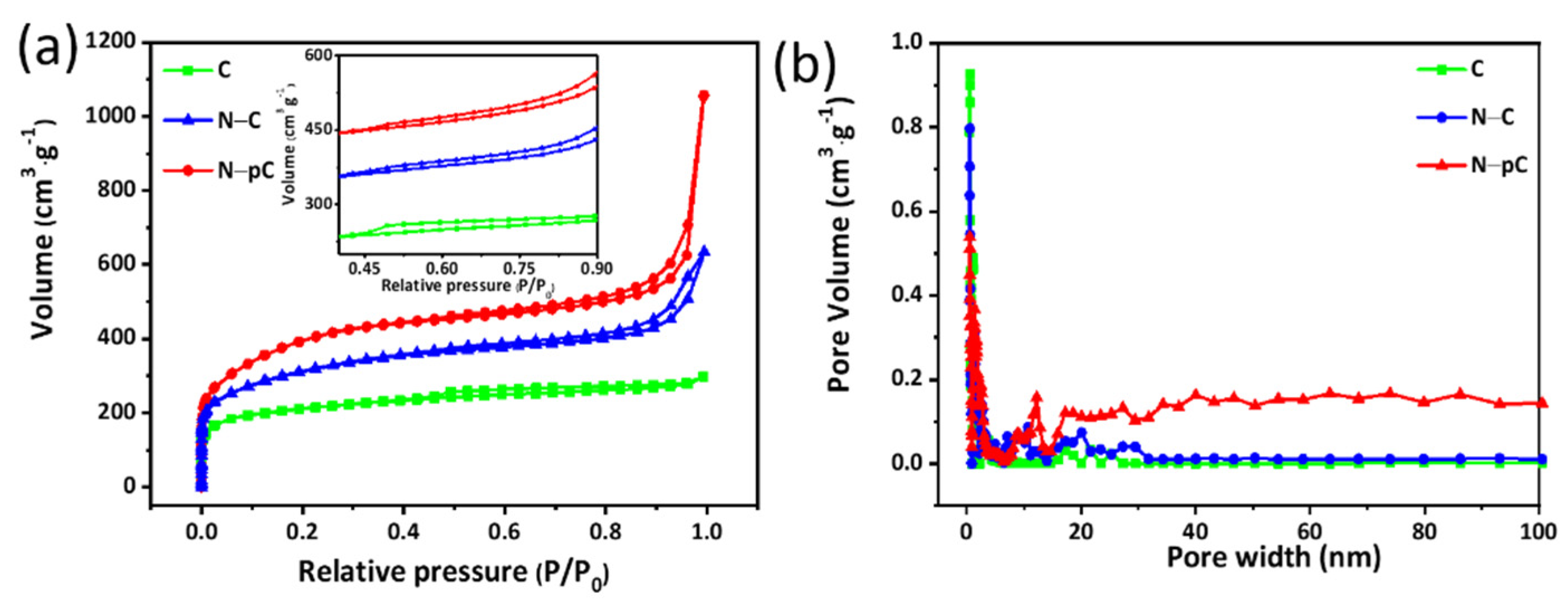
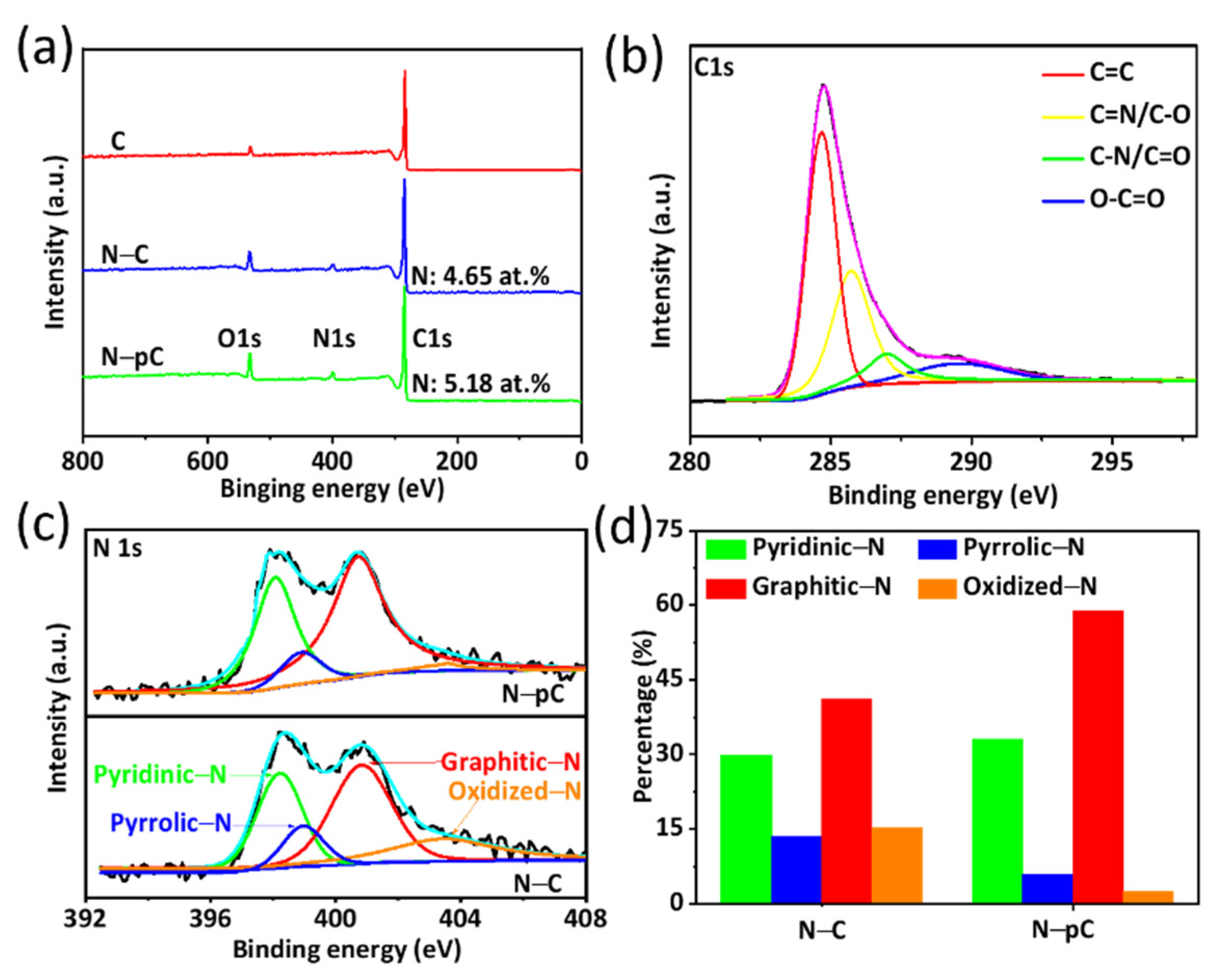
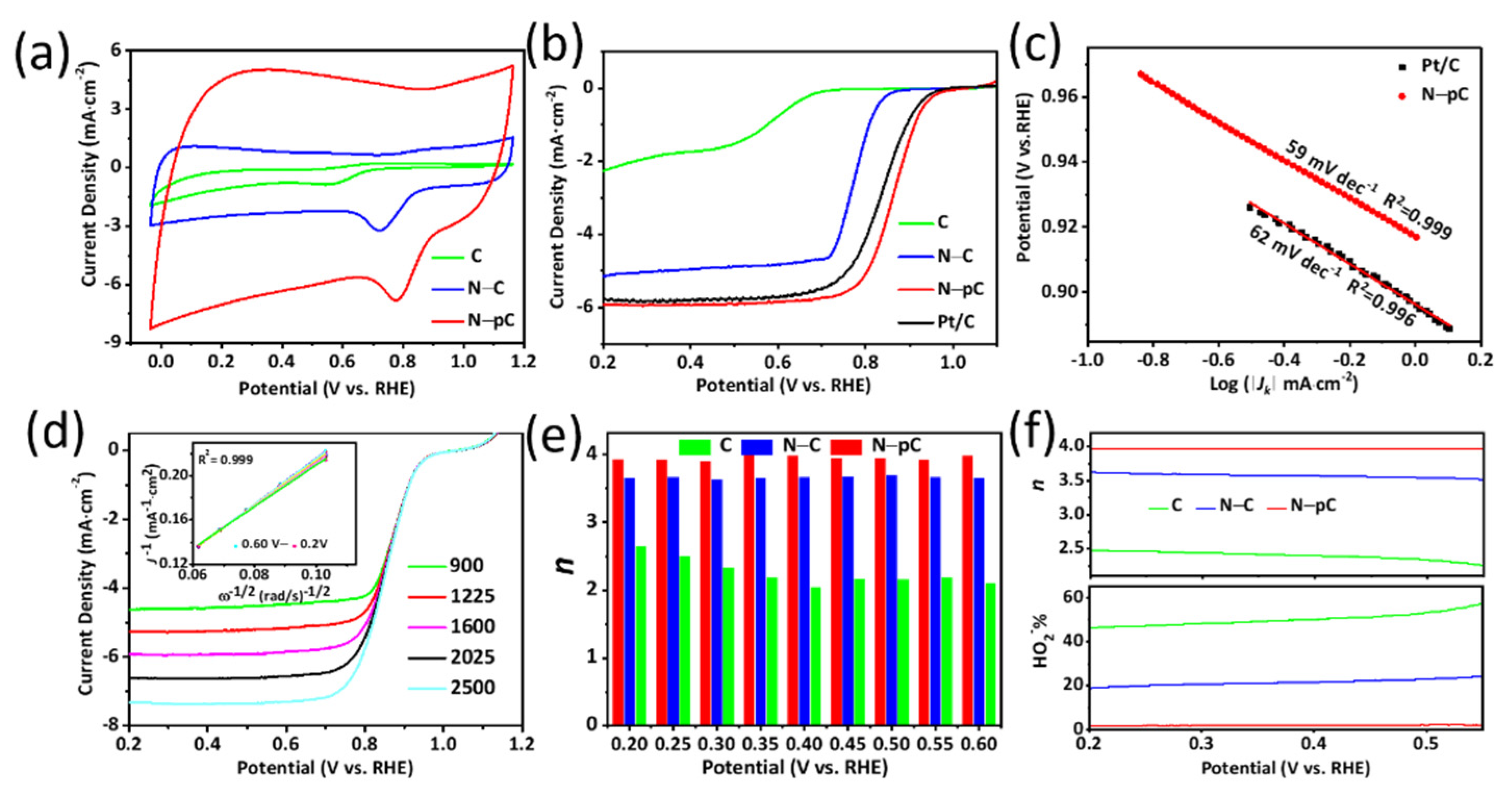
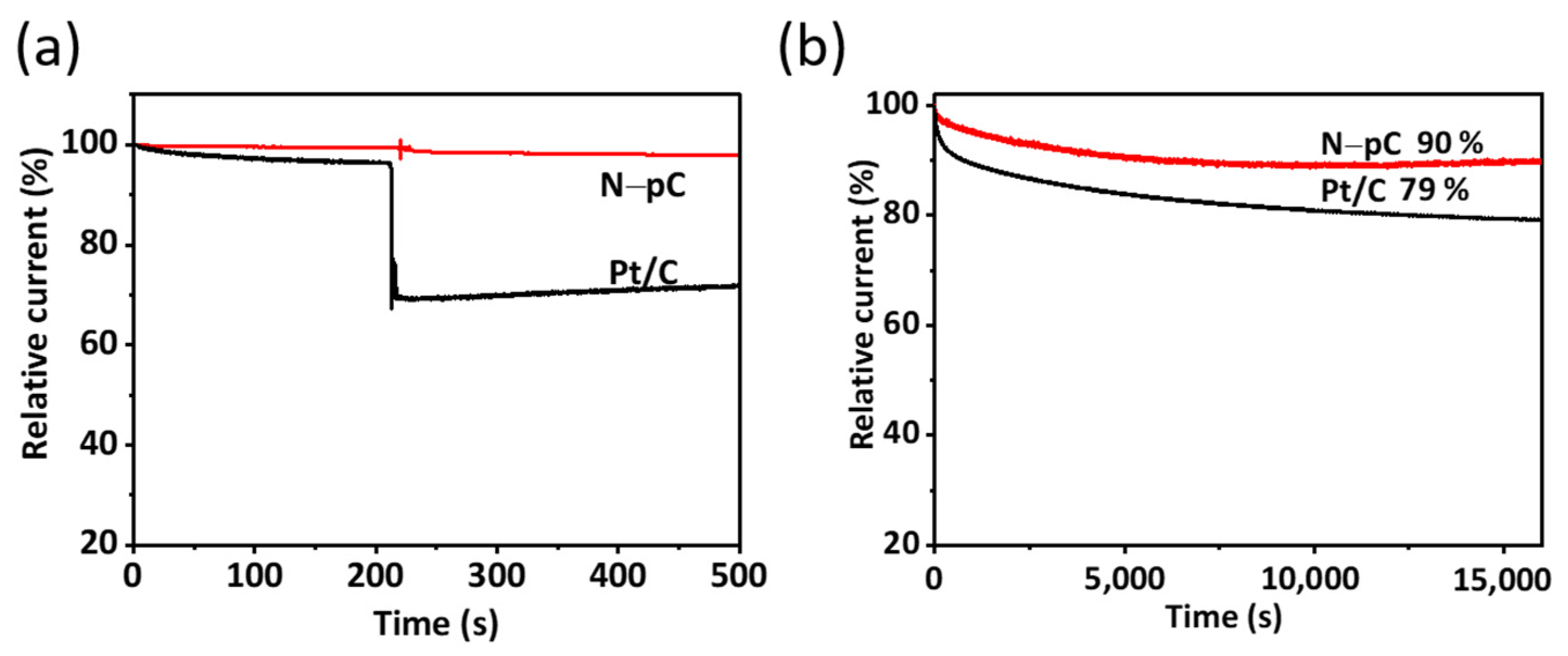
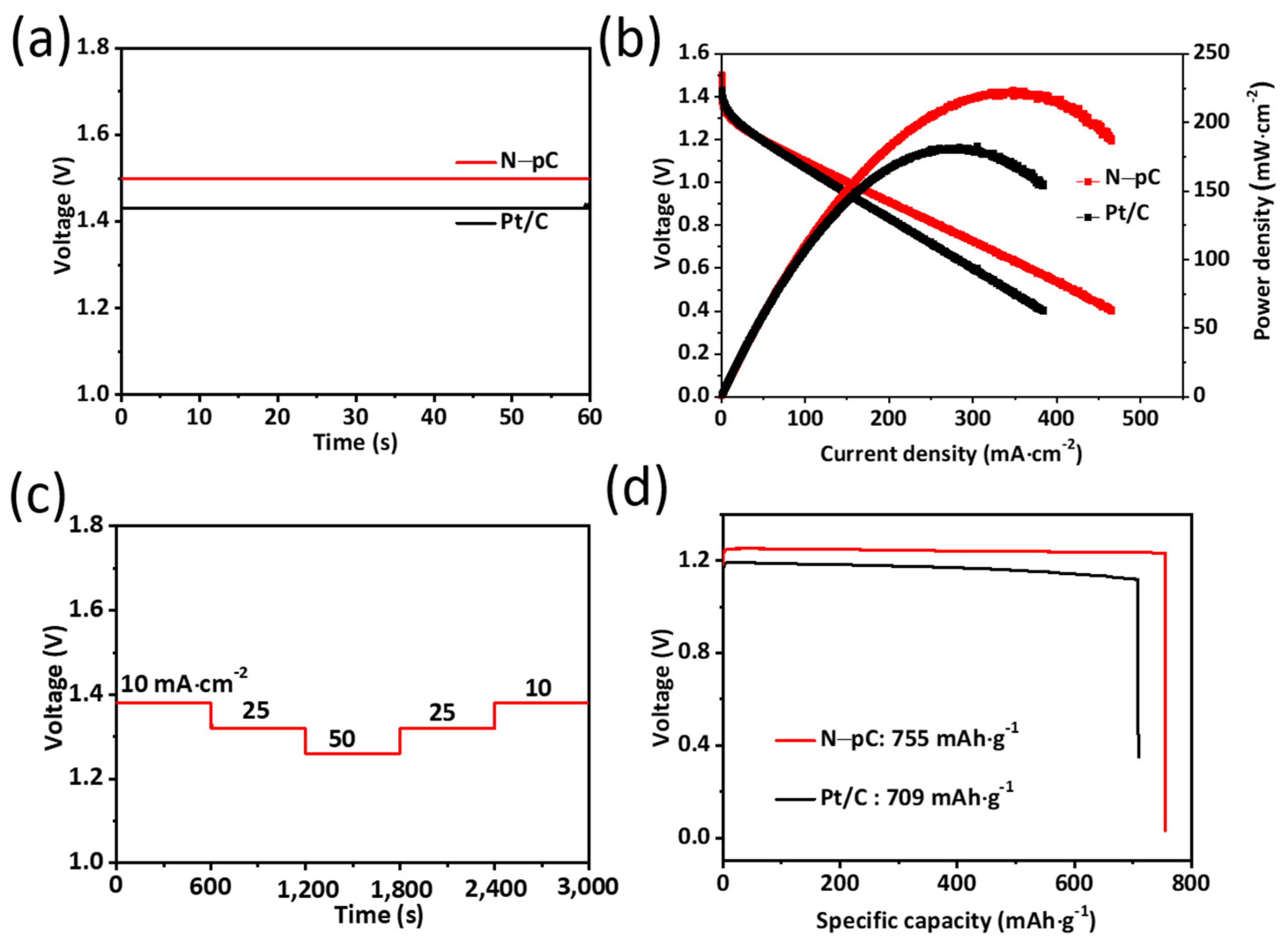
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, W.-T.; Batool, N.; Zhang, T.-H.; Liu, J.; Han, X.-F.; Tian, J.-H.; Yang, R. When MOFs meet MXenes: Superior ORR performance in both alkaline and acidic solutions. J. Mater. Chem. A 2021, 9, 3952–3960. [Google Scholar] [CrossRef]
- Cao, F.; Yang, X.; Shen, C.; Li, X.; Wang, J.; Qin, G.W.; Li, S.; Pang, X.; Li, G. Electrospinning synthesis of transition metal alloy nanoparticles encapsulated in nitrogen-doped carbon layers as an advanced bifunctional oxygen electrode. J. Mater. Chem. A 2020, 8, 7245–7252. [Google Scholar] [CrossRef]
- Pan, K.-J.; Hussain, A.M.; Huang, Y.-L.; Gong, Y.; Cohn, G.; Ding, D.; Wachsman, E.D. evolution of solid oxide fuel cells via fast interfacial oxygen crossover. ACS Appl. Energy Mater. 2019, 2, 4069–4074. [Google Scholar] [CrossRef]
- Hong, W.; Feng, X.; Tan, L.; Guo, A.; Lu, B.; Li, J.; Wei, Z. Preparation of monodisperse ferrous nanoparticles embedded in carbon aerogelsvia in situsolid phase polymerization for electrocatalytic oxygen reduction. Nanoscale 2020, 12, 15318–15324. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, R.; Wang, J.; Li, Z.; Wei, X.; Chen, J.S.; Chen, Y. Synthesis of noble metal-based intermetallic electrocatalysts by space-confined pyrolysis: Recent progress and future perspective. J. Energy Chem. 2021, 60, 61–74. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Achievements in Pt nanoalloy oxygen reduction reaction catalysts: Strain engineering, stability and atom utilization efficiency. Chem. Commun. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yanhua, L.; Tan, N.; Zhu, Y.; Huo, D.; Zhang, Y.; Sun, S.; Gao, G. Synthesis of porous N-rich carbon/MXene from MXene@Polypyrrole hybrid nanosheets as oxygen reduction reaction electrocatalysts. J. Electrochem. Soc. 2020, 167, 116503. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, H.; Liu, C.; Li, Y. The progress of metal-free catalysts for the oxygen reduction reaction based on theoretical sim-ulations. J. Mater. Chem. A 2018, 6, 13489–13508. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L. Research progress of carbon nanofiber-based precious-metal-free oxygen reaction catalysts synthesized by electrospinning for Zn-Air batteries. J. Power Sources 2021, 507, 230280. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Sun, Q.; Li, X.; Strasser, P.; Yang, R. Synthesis and electrocatalytic activity of phosphorus-doped carbon xerogel for oxygen reduction. Electrochim. Acta 2014, 127, 53–60. [Google Scholar] [CrossRef]
- Yokoyama, K.; Sato, Y.; Yamamoto, M.; Nishida, T.; Motomiya, K.; Tohji, K.; Sato, Y. Work function, carrier type, and con-ductivity of nitrogen-doped single-walled carbon nanotube catalysts prepared by annealing via defluorination and efficient oxygen reduction reaction. Carbon 2019, 142, 518–527. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Z.; Li, B.; Wang, G.; Leu, P. Identification of efficient active sites in nitrogen-doped carbon nanotubes for oxygen reduction reaction. J. Phys. Chem. C 2020, 124, 8689–8696. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Tian, J.; Jin, C.; Yang, R. Sulfur-doped carbon spheres as efficient metal-free electrocatalysts for oxygen reduction reaction. Electrochim. Acta 2015, 178, 806–812. [Google Scholar] [CrossRef]
- Wong, C.H.A.; Sofer, Z.; Klímová, K.; Pumera, M. Microwave exfoliation of graphite oxides in H2S plasma for the synthesis of sulfur-doped graphenes as oxygen reduction catalysts. ACS Appl. Mater. Interfaces 2016, 8, 31849–31855. [Google Scholar] [CrossRef] [PubMed]
- Van, T.T.; Kang, S.G.; Babu, K.F.; Oh, E.-S.; Lee, S.G.; Choi, W.M. Synthesis of B-doped graphene quantum dots as a metal-free electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 10537–10543. [Google Scholar]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wang, Z.; Zhou, T.; Liu, Y.; Wang, X.; Zhao, Z.; Qiu, J. General synthesis of zeolitic imidazolate frame-work-derived planar-N-doped porous carbon nanosheets for efficient oxygen reduction. Energy Storage Mater. 2017, 7, 181–188. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.-X.; Chen, Y.-G.; Yang, L.-J.; Yang, X.-D.; Ye, J.-Y.; Xia, H.-P.; Zhou, Z.-Y.; Sun, S.-G. Identifying the active site of n-doped graphene for oxygen reduction by selective chemical modification. ACS Energy Lett. 2018, 3, 986–991. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Z.; Dai, L. Graphitic carbon nitrides supported by nitrogen-doped graphene as efficient metal-free electrocatalysts for oxygen reduction. J. Electroanal. Chem. 2015, 753, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Li, L.; Xiong, K.; Wang, Y.; Li, W.; Nie, Y.; Chen, S.; Qi, X.; Wei, Z. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5414–5420. [Google Scholar] [CrossRef]
- Wang, W.; Luo, J.; Chen, W.; Li, J.; Xing, W.; Chen, S. Synthesis of mesoporous Fe/N/C oxygen reduction catalysts through NaCl crystallite-confined pyrolysis of polyvinylpyrrolidone. J. Mater. Chem. A 2016, 4, 12768–12773. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Yang, P.; Xiao, L.; Du, L.; Lu, X.; Li, R.; Zhang, J.; An, M. A dual-template strategy to engineer hierarchically porous Fe-N-C electrocatalysts for the high-performance cathodes of Zn-air batteries dagger. J. Mater. Chem. A 2021, 9, 9761–9770. [Google Scholar] [CrossRef]
- Sa, Y.J.; Seo, D.-J.; Woo, J.; Lim, J.T.; Cheon, J.Y.; Yang, S.Y.; Lee, J.M.; Kang, D.; Shin, T.J.; Shin, H.S.; et al. A general approach to preferential formation of active Fe-N-x sites in Fe-N/C electrocatalysts for efficient oxygen reduction reaction. J. Am. Chemical Soc. 2016, 138, 15046–15056. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.-C.; Wu, Z.-Y.; Chu, S.-Q.; Zhu, H.-W.; Liang, H.-W.; Zhang, J.; Yu, S.-H. SiO2-protected shell mediated templating synthesis of Fe–N-doped carbon nanofibers and their enhanced oxygen reduction reaction performance. Energy Environ. Sci. 2018, 11, 2208–2215. [Google Scholar] [CrossRef]
- Meng, F.-L.; Wang, Z.-L.; Zhong, H.-X.; Wang, J.; Yan, J.-M.; Zhang, X.-B. Reactive multifunctional template-induced prepa-ration of Fe-N-doped mesoporous carbon microspheres towards highly efficient electrocatalysts for oxygen reduction. Adv. Mater. 2016, 28, 7948–7955. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wen, S.; Chen, J.; Zhao, Q.; Wang, L. Large-scale fabrication of biomass-derived N, S co-doped porous carbon with ultrahigh surface area for oxygen reduction. Mater. Chem. Phys. 2021, 267, 124601. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sun, Y.; Guo, L.K.; Sun, X.N.; Huang, N.B. Ball-Milling Effect on biomass-derived nanocarbon catalysts for the oxygen reduction reaction. Chemistry 2021, 6, 6019–6028. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wei, X.; Fan, H.; Li, L.; Geng, K.; Wang, J. Nitrogen-doped carbon shell structure derived from natural leaves as a potential catalyst for oxygen reduction reaction. Nano Energy 2015, 13, 518–526. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.F.; Knop-Gericke, A.; et al. tuning the acid/base properties of nanocarbons by functionalization via amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Zhang, L.; Chen, L.; Cui, X.; Hua, Z.; Wang, M.; Wang, J.; Chen, Y.; Shi, J. N-doped hierarchically macro/mesoporous carbon with excellent electrocatalytic activity and durability for oxygen reduction reaction. Carbon 2015, 86, 108–117. [Google Scholar] [CrossRef]
- Ferre-Vilaplana, A.; Herrero, E. Understanding the chemisorption-based activation mechanism of the oxygen reduction reac-tion on nitrogen-doped graphitic materials. Electrochim. Acta 2016, 204, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, W.; Wang, X.; Zheng, H.; Li, R.; Wu, R.; Chen, J.S. Molten-Salt-Assisted Synthesis of Nitrogen-Doped Carbon Nanosheets Derived from Biomass Waste of Gingko Shells as Efficient Catalyst for Oxygen Reduction Reaction. Processes 2021, 9, 2124. https://doi.org/10.3390/pr9122124
Hong W, Wang X, Zheng H, Li R, Wu R, Chen JS. Molten-Salt-Assisted Synthesis of Nitrogen-Doped Carbon Nanosheets Derived from Biomass Waste of Gingko Shells as Efficient Catalyst for Oxygen Reduction Reaction. Processes. 2021; 9(12):2124. https://doi.org/10.3390/pr9122124
Chicago/Turabian StyleHong, Wei, Xia Wang, Hongying Zheng, Rong Li, Rui Wu, and Jun Song Chen. 2021. "Molten-Salt-Assisted Synthesis of Nitrogen-Doped Carbon Nanosheets Derived from Biomass Waste of Gingko Shells as Efficient Catalyst for Oxygen Reduction Reaction" Processes 9, no. 12: 2124. https://doi.org/10.3390/pr9122124
APA StyleHong, W., Wang, X., Zheng, H., Li, R., Wu, R., & Chen, J. S. (2021). Molten-Salt-Assisted Synthesis of Nitrogen-Doped Carbon Nanosheets Derived from Biomass Waste of Gingko Shells as Efficient Catalyst for Oxygen Reduction Reaction. Processes, 9(12), 2124. https://doi.org/10.3390/pr9122124








