Adsorption and Desorption Behavior of Ectoine Using Dowex® HCR-S Ion-Exchange Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Pretreatment of Resin
2.2. Batch Adsorption of Ectoine
2.2.1. Kinetic Study of Ectoine Adsorption
2.2.2. Preparation of Saturation Concentration of Ectoine in Ion-Exchange Resin
2.2.3. Desorption of Ectoine
2.3. Swelling Index of the Ion Exchanger
2.4. Determination of the Zeta Potential of Ectoine in the Solution
3. Models of Adsorption and Desorption
3.1. Adsorption Kinetic Models
- (i)
- Pseudo-first-order model
- (ii)
- Pseudo-second-order model
- (iii)
- Intraparticle diffusion model
- (iv)
- Elovich model
- (v)
- Arrhenius activation energy
3.2. Adsorption Isotherm Models
- (i)
- Langmuir isotherm
- (ii)
- Freundlich isotherm
- (iii)
- Temkin isotherm
- (iv)
- Dubinin–Radushkevich isotherm
- (v)
- Redlich–Peterson isotherm
- (vi)
- Sips isotherm
3.3. Adsorption Thermodynamics
3.4. Desorption Kinetic Models
4. Results and Discussion
4.1. Adsorption of Ectoine Using Ion-Exchange Resin
4.1.1. Effect of Types of Ion-Exchange Resin on the Adsorption of Ectoine
4.1.2. Effect of pH on Ectoine Adsorption
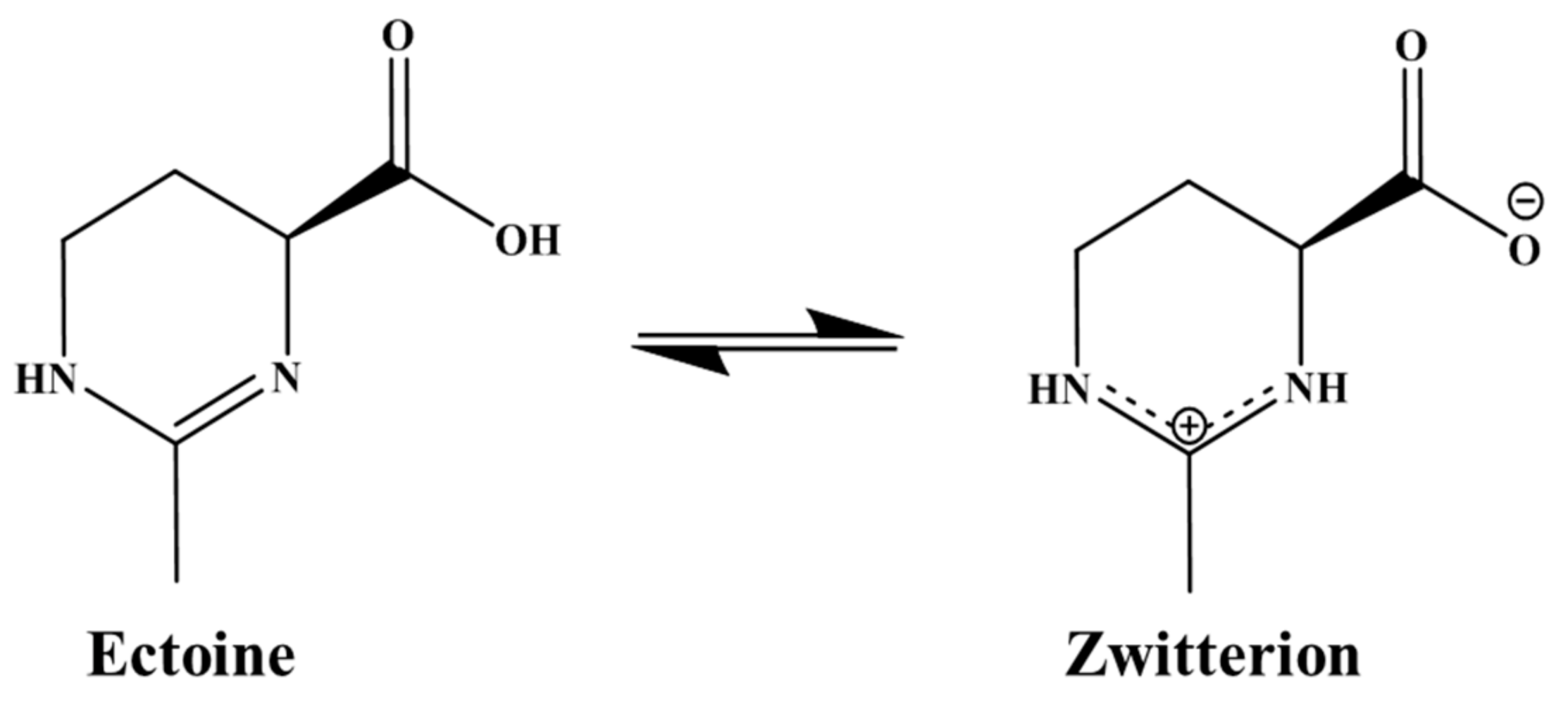
4.1.3. Zeta Potential in Ectoine Solution
4.1.4. Effect of Initial Ectoine Concentration
4.1.5. Isotherm Studies of Ectoine
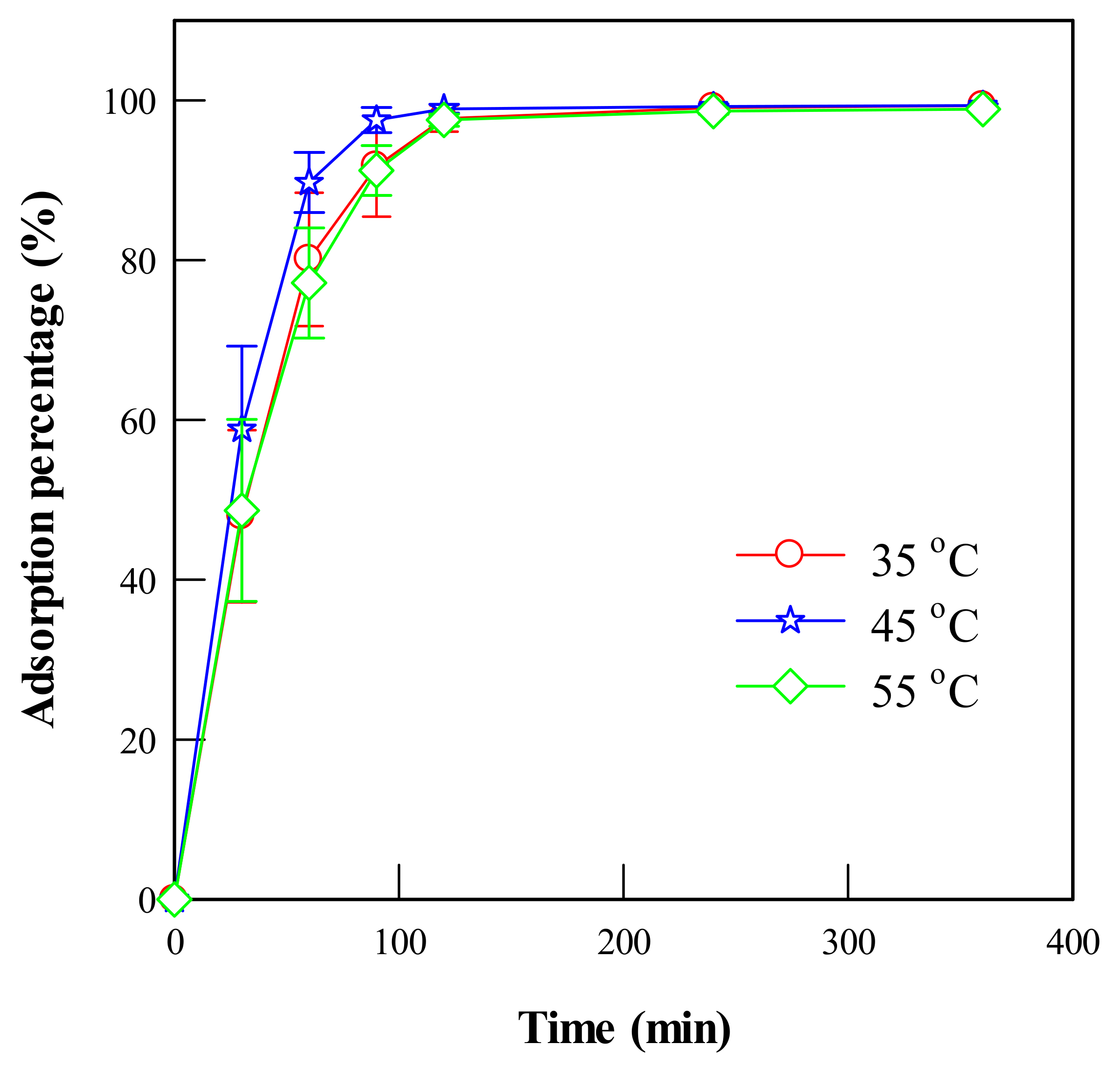
4.1.6. Effect of Adsorption Time
4.1.7. Adsorption Kinetics of Ectoine onto the Ion-Exchanger
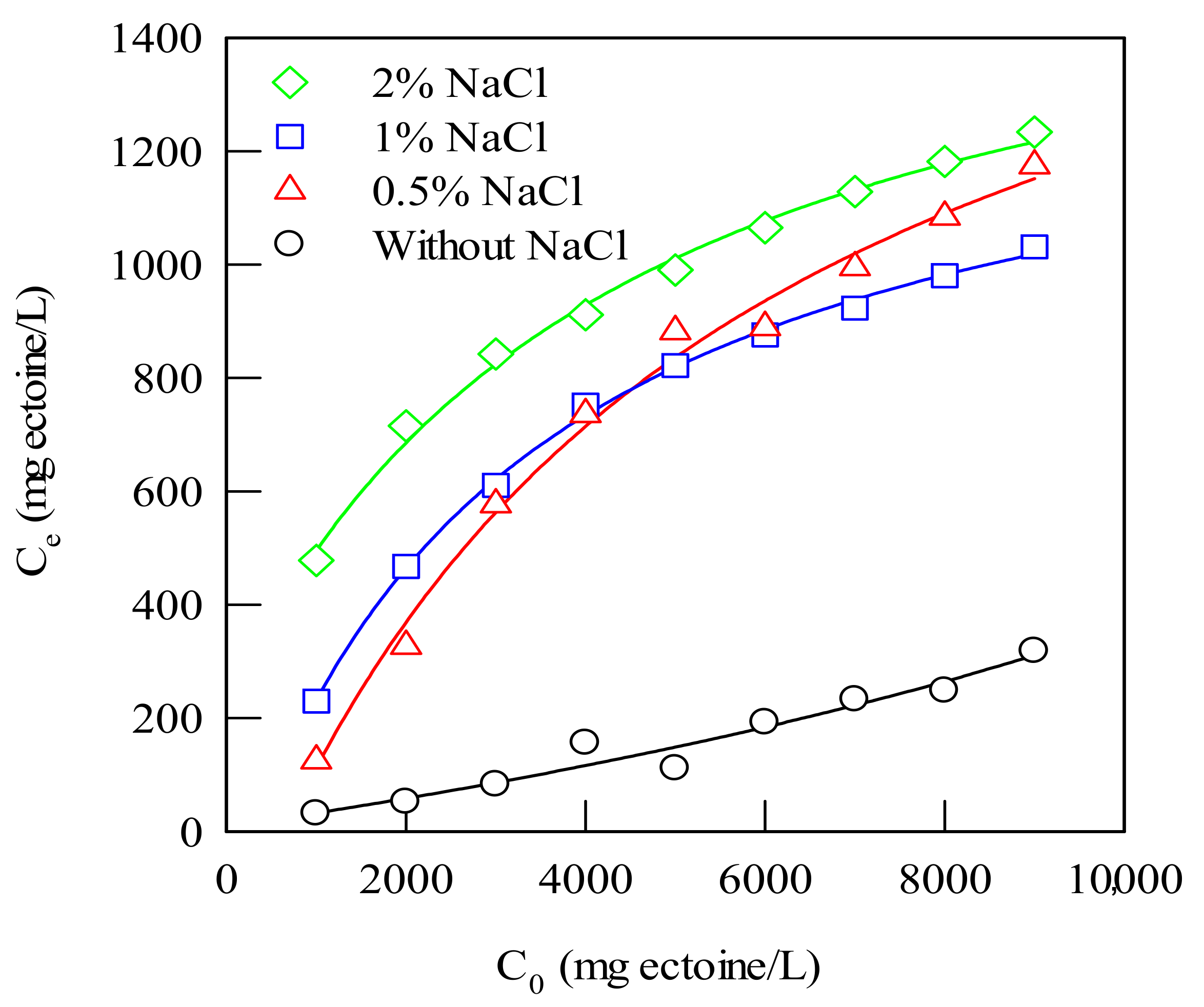
4.1.8. Effect of Salt Concentration
4.1.9. Adsorption Thermodynamics
4.2. Saturation of Ectoine in the Ion-Exchange Resin
4.3. Desorption of Ectoine from Saturated Ion-Exchange Resin
4.3.1. Effect of pH on Ectoine Desorption
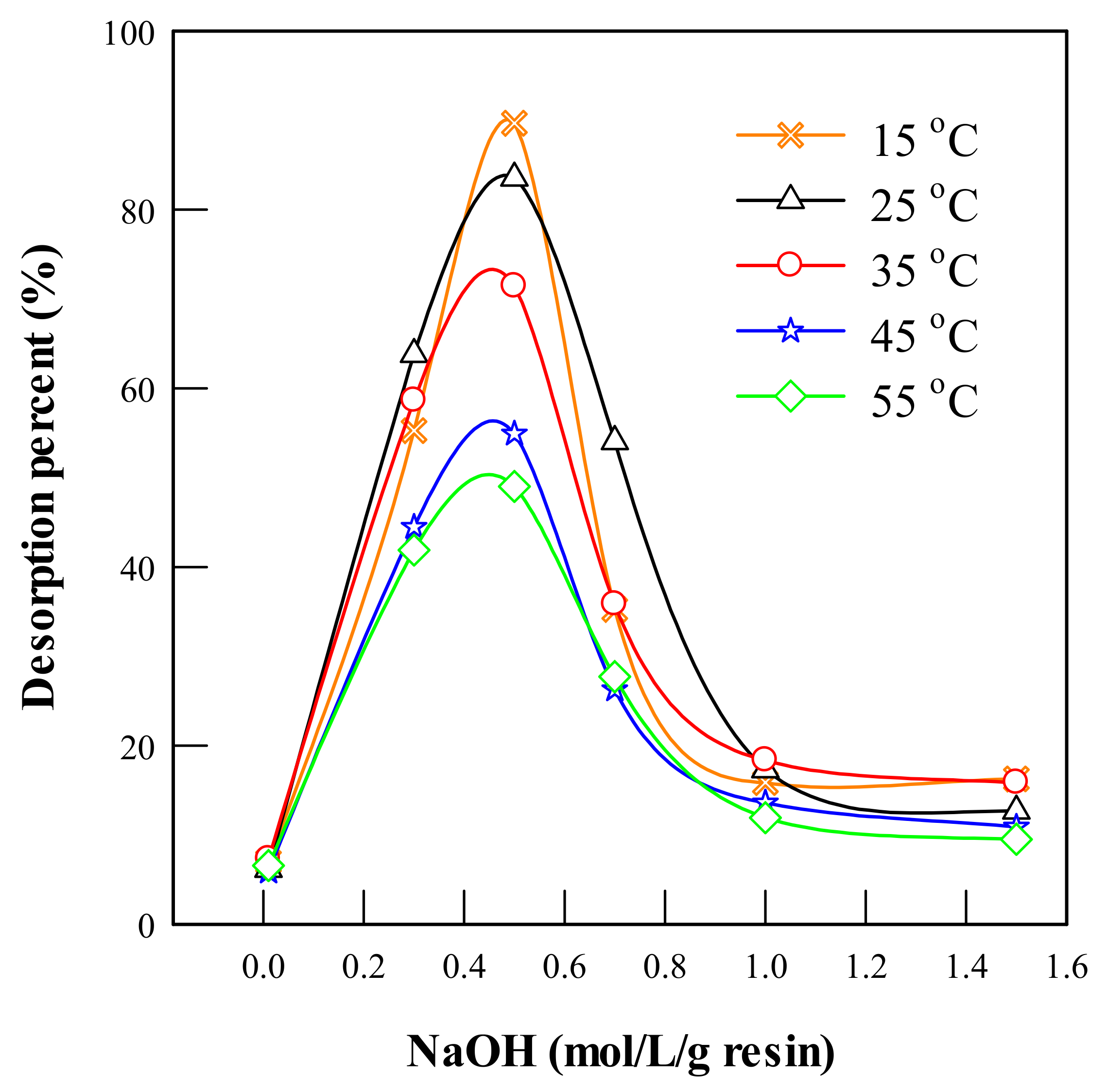
4.3.2. Effect of NaOH Concentration
4.3.3. Effect of Desorption Time and Temperature
4.3.4. Desorption Kinetics of Ectoine from the Ion-Exchange Resin
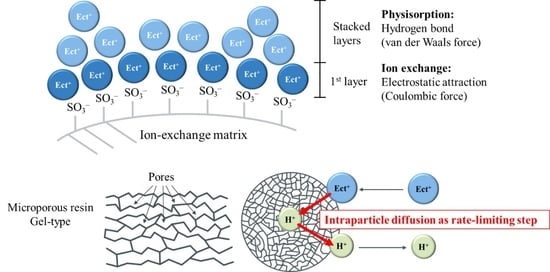
4.3.5. Mechanism of Ectoine Desorption from Ion-Exchange Resin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Becker, J.; Wittmann, C. Microbial production of extremolytes—High-value active ingredients for nutrition, health care, and well-being. Curr. Opin. Biotechnol. 2020, 65, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhu, L.; Lv, L.; Yao, S.; Li, B.; Qian, J. Optimization of the extraction and purification of the compatible solute ectoine from Halomonas elongate in the laboratory experiment of a commercial production project. World J. Microbiol. Biotechnol. 2017, 33, 116. [Google Scholar] [CrossRef] [PubMed]
- Kunte, H.J.; Lentzen, G.; Galinski, E.A. Industrial Production of the Cell Protectant Ectoine: Protection Mechanisms, Processes, and Products. Curr. Biotechnol. 2014, 3, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Pastor, J.M.; Salvador, M.; Argandoña, M.; Bernal, V.; Reina-Bueno, M.; Csonka, L.N.; Iborra, J.L.; Vargas, C.; Nieto, J.J.; Cánovas, M. Ectoines in cell stress protection: Uses and biotechnological production. Biotechnol. Adv. 2010, 28, 782–801. [Google Scholar] [CrossRef] [PubMed]
- Behazin, R.; Ebrahimi, A. The physicochemical properties and tyrosinase inhibitory activity of ectoine and its analogues: A theoretical study. Comput. Theor. Chem. 2018, 1130, 6–14. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Lv, P.; Sun, S.; Ma, C.; Xu, P.; Su, H.; Yang, C. High ectoine production by an engineered Halomonas hydrothermalis Y2 in a reduced salinity medium. Microb. Cell Factories 2019, 18, 184. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hsu, C.-C.; Lan, J.C.-W.; Chang, Y.-K.; Wang, L.-F.; Wei, Y.-H. Production and characterization of ectoine using a moderately halophilic strain Halomonas salina BCRC17875. J. Biosci. Bioeng. 2018, 125, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Yuan, F.-W.; Wang, L.-F.; Chien, C.-C.; Wei, Y.-H. Ectoine production with indigenous Marinococcus sp. MAR2 isolated from the marine environment. Prep. Biochem. Biotechnol. 2020, 50, 74–81. [Google Scholar] [CrossRef]
- Chen, J.; Liu, P.; Chu, X.; Chen, J.; Zhang, H.; Rowley, D.C.; Wang, H. Metabolic pathway construction and optimization of Escherichia coli for high-level ectoine production. Curr. Microbiol. 2020, 77, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Sauer, T.; Galinski, E.A. Bacterial milking: A novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 1998, 57, 306–313. [Google Scholar] [CrossRef]
- Gießelmann, G.; Dietrich, D.; Jungmann, L.; Kohlstedt, M.; Jeon, E.J.; Yim, S.S.; Sommer, F.; Zimmer, D.; Mühlhaus, T.; Schroda, M. Metabolic engineering of Corynebacterium glutamicum for high-level ectoine production: Design, combinatorial assembly, and implementation of a transcriptionally balanced heterologous ectoine pathway. Biotechnol. J. 2019, 14, 1800417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, Y.-j.; Bai, L.; Ren, Y.-n.; Zhang, L.-h.; Nagata, S. Production of ectoine through a combined process that uses both growing and resting cells of Halomonas salina DSM 5928 T. Extremophiles 2011, 15, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Fülberth, K.; Müssig, H.; Wesp, B. Method for the Chromatographic Isolation of Ectoine. European Patent EP1461322A1, 2004. [Google Scholar]
- LeVan, M.D.; Carta, G.; Yon, C.M. Adsorption and ion exchange. Energy 1997, 16, 17. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Removal of copper ions from aqueous solution by tree fern. Water Res. 2003, 37, 2323–2330. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- McKay, G.; Otterburn, M.; Sweeney, A. The removal of colour from effluent using various adsorbents—III. Silica: Rate processes. Water Res. 1980, 14, 15–20. [Google Scholar] [CrossRef]
- Aharoni, C.; Tompkins, F. Kinetics of adsorption and desorption and the Elovich equation. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1970; Volume 21, pp. 1–49. [Google Scholar]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Prasad, R.K.; Srivastava, S. Sorption of distillery spent wash onto fly ash: Kinetics and mass transfer studies. Chem. Eng. J. 2009, 146, 90–97. [Google Scholar] [CrossRef]
- Osmari, T.A.; Gallon, R.; Schwaab, M.; Barbosa-Coutinho, E.; Severo Jr, J.B.; Pinto, J.C. Statistical analysis of linear and non-linear regression for the estimation of adsorption isotherm parameters. Adsorpt. Sci. Technol. 2013, 31, 433–458. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 122383. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem 1906, 57, 385. [Google Scholar]
- Ho, Y.S. Absorption of Heavy Metals from Waste Streams by Peat; University of Birmingham: Birmingham, UK, 1995. [Google Scholar]
- Dubinin, M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Inglezakis, V.J.; Zorpas, A.A. Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalination Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Staudt, J.; Scheufele, F.B.; Ribeiro, C.; Sato, T.Y.; Canevesi, R.; Borba, C.E. Ciprofloxacin desorption from gel type ion exchange resin: Desorption modeling in batch system and fixed bed column. Sep. Purif. Technol. 2020, 230, 115857. [Google Scholar] [CrossRef]
- Ismail, M.; Hanafiah, K.M. Kinetic, isotherm, and possible mechanism of Pb (II) ion adsorption onto xanthated neem (Azadirachta indica) leaf powder. Sains Malays. 2020, 49, 1585–1596. [Google Scholar] [CrossRef]
- Nagy, B.; Mânzatu, C.; Măicăneanu, A.; Indolean, C.; Barbu-Tudoran, L.; Majdik, C. Linear and nonlinear regression analysis for heavy metals removal using Agaricus bisporus macrofungus. Arab. J. Chem. 2017, 10, S3569–S3579. [Google Scholar] [CrossRef] [Green Version]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Aksu, Z.; Kutsal, T. A bioseparation process for removing lead (II) ions from waste water by using C. vulgaris. J. Chem. Technol. Biotechnol. 1991, 52, 109–118. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of adsorption kinetic processes—errors, theory and application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2018. [Google Scholar]








 : adsorbed ectoine ion,
: adsorbed ectoine ion,  : free ectoine ion,
: free ectoine ion,  : sodium ion,
: sodium ion,  : hydroxide ion.
: hydroxide ion.
 : adsorbed ectoine ion,
: adsorbed ectoine ion,  : free ectoine ion,
: free ectoine ion,  : sodium ion,
: sodium ion,  : hydroxide ion.
: hydroxide ion.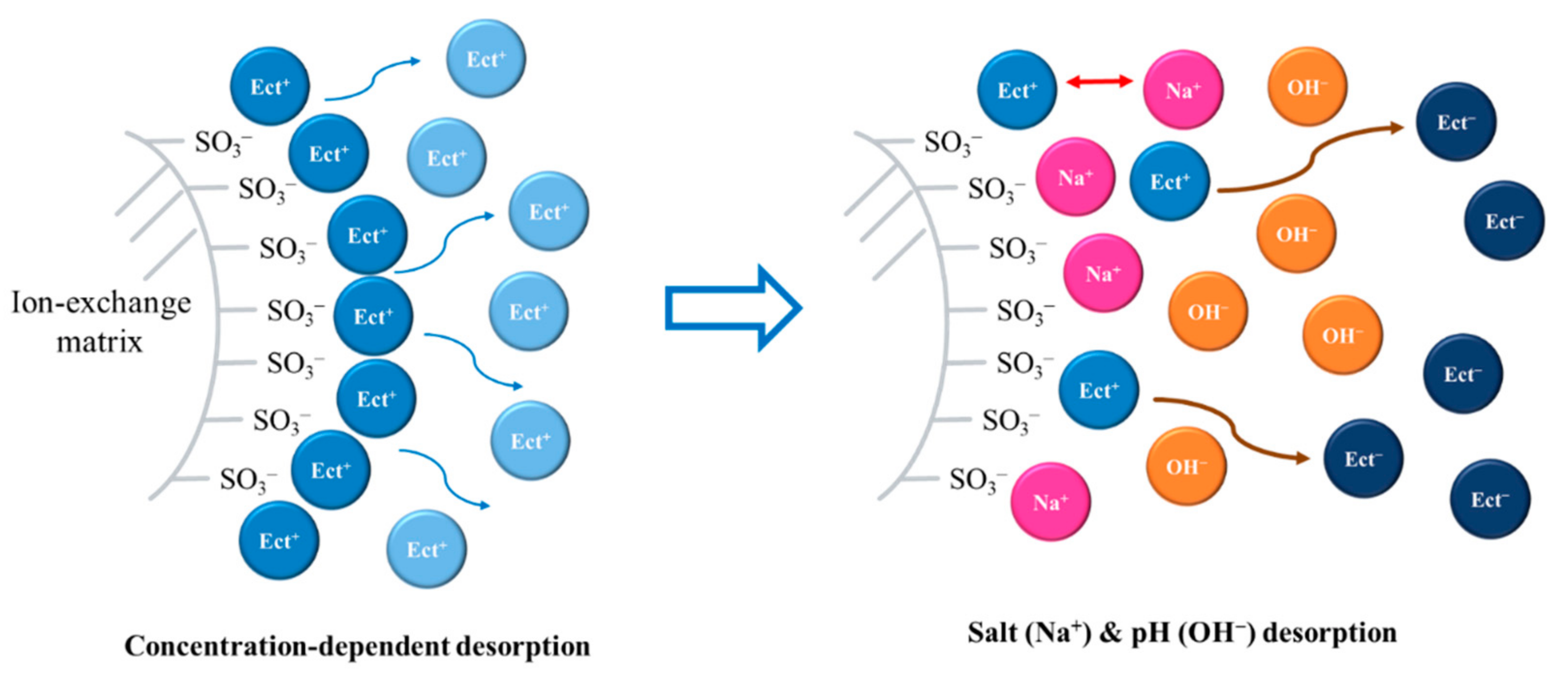
| Kinetic Model | Ref. | |
|---|---|---|
| Elovich | [21] | |
| Intraparticle diffusion | [18] | |
| Pseudo-first-order | [15] | |
| Pseudo-second-order | [29] | |
| Isothermal Model | Ref. | |
| Dubinin–Radushkevich | [30] | |
| Freundlich | [28] | |
| Langmuir | [31] | |
| Redlich–Peterson | [32] | |
| Sips | [33] | |
| Temkin | [34] |
| Initial pH without Resin | pH after Adsorption | Ce (mg/L) | qe (mg/g) | Initial pH with Resin | pH after Adsorption | Ce (mg/L) | qe (mg/g) |
|---|---|---|---|---|---|---|---|
| 2.00 | 1.98 | 15.73 | 9.84 | 2.00 | 1.95 | 19.90 | 9.80 |
| 3.00 | 2.82 | 15.94 | 9.84 | 3.00 | 2.90 | 18.04 | 9.82 |
| 4.00 | 3.18 | 14.44 | 9.86 | 4.00 | 3.80 | 80.23 | 9.20 |
| 5.00 | 3.29 | 16.87 | 9.83 | 5.00 | 6.70 | 740.76 | 2.59 |
| 6.00 | 3.30 | 20.79 | 9.79 | 6.00 | 6.89 | 869.34 | 1.31 |
| 7.00 | 3.33 | 15.23 | 9.85 |
| Temperature (°C) | Freundlich Model | Langmuir Model | Dubinin–Radushkevich Model | Temkin Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | KF (mg L−1) | R2 | qm (mg g−1) | KL (L mg−1) | R2 | RL | qm (mg g−1) | E a (kJ mol−1) | R2 | bT (J mol−1) | AT (L g−1) | R2 | |
| 25 | 1.35 | 1.43 | 0.98 | 201 | 0.0028 | 0.98 | 0.038–0.454 | 79.7 | 0.011 | 0.87 | 81.0 | 0.051 | 0.95 |
| 35 | 1.22 | 0.80 | 0.95 | 299 | 0.0013 | 0.95 | 78.3 | 0.009 | 0.82 | 79.1 | 0.035 | 0.91 | |
| 45 | 1.24 | 0.74 | 0.95 | 259 | 0.0013 | 0.95 | 81.5 | 0.007 | 0.87 | 81.1 | 0.029 | 0.91 | |
| 55 | 1.17 | 0.71 | 0.98 | 348 | 0.0012 | 0.98 | 84.3 | 0.008 | 0.90 | 77.6 | 0.034 | 0.95 | |
| 65 | 1.15 | 0.88 | 0.97 | 357 | 0.0016 | 0.97 | 87.7 | 0.010 | 0.94 | 67.3 | 0.038 | 0.98 | |
| Temperature(°C) | Redlich–Peterson Model | Sips Model | |||||||||||
| KR (L g−1) | αR (L mg−1) | g | R2 | KS (L g−1) | αS (L mg−1) | βS | R2 | ||||||
| 25 | 0.58 | 0.0045 | 0.92 | 0.98 | 0.55 | 0.0028 | 1.00 | 0.98 | |||||
| 35 | 0.52 | 0.0530 | 0.49 | 0.95 | 0.62 | 0.0009 | 0.88 | 0.98 | |||||
| 45 | 0.37 | 0.0076 | 0.74 | 0.95 | 0.37 | 0.0013 | 0.98 | 0.95 | |||||
| 55 | 0.41 | 0.0009 | 1.05 | 0.98 | 0.27 | 0.0012 | 1.11 | 0.98 | |||||
| 65 | 0.51 | 9E-09 | 3.17 | 0.98 | 0.05 | 0.0004 | 1.63 | 0.99 | |||||
| T (°C) | Pseudo-First-Order | Pseudo-Second-Order | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe a (mg g−1) | kpf (Min−1) | qe b (mg g−1) | R2 | Ea (kJ Mol−1) | kps (g mg−1 Min−1) | qe b (mg g−1) | R2 | Ea (kJ Mol−1) | |
| 35 | 10.03 | 0.04 | 18.00 | 0.98 | 3.72 | 0.0012 | 14.90 | 0.97 | 36.5 |
| 45 | 10.01 | 0.07 | 32.34 | 0.99 | 0.0049 | 11.67 | 0.99 | ||
| 55 | 9.95 | 0.04 | 16.50 | 0.92 | 0.0028 | 12.25 | 1.00 | ||
| T (°C) | Elovich | Intraparticle diffusion | |||||||
| α (mg g−1 min−1) | β (mg g−1) | R2 | Ea (kJ mol−1) | kid (initial) (mg g−1 min1/2) | R2 (initial) | E (kJ mol−1) | |||
| 35 | 0.49 | 0.27 | 0.97 | 1.12 0.77 0.79 | 0.97 0.93 0.94 | 35.1 | |||
| 45 | 1.86 | 0.44 | 0.90 | 23.1 | |||||
| 55 | 0.83 | 0.35 | 0.97 | ||||||
| Co (g/L) | ∆H (J/mol) | ∆S (J/mol) | ∆G (kJ/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | I | II | 25 °C | 35 °C | 45 °C | 55 °C | 65 °C | |
| 1 | −302 | 129 | −19.22 | 74.8 | −15.2 | −14.2 | −13.9 | −14.3 | −14.4 |
| 2 | −352 | 319 | −29.74 | 116.0 | −15.4 | −14.7 | −13.9 | −14.6 | −15.1 |
| 3 | −401 | 422 | −41.39 | 137.5 | −15.4 | −14.5 | −13.6 | −14.6 | −15.3 |
| 4 | −243 | 401 | −7.08 | 133.9 | −14.9 | −13.7 | −13.9 | −14.8 | −15.4 |
| 5 | −118 | 240 | 24.31 | 101.8 | −15.4 | −15.1 | −14.9 | −15.0 | −15.9 |
| 6 | −248 | 430 | −8.16 | 138.7 | −14.7 | −14.2 | −13.6 | −14.3 | −15.2 |
| 7 | −217 | 450 | −1.55 | 143.4 | −14.5 | −14.1 | −13.6 | −14.4 | −15.3 |
| 8 | −231 | 390 | −4.19 | 130.5 | −14.7 | −14.2 | −13.7 | −14.4 | −15.2 |
| 9 | −193 | 379 | 3.35 | 127.6 | −14.3 | −13.9 | −13.5 | −14.3 | −14.9 |
| Average | −256 | 351 | −9.30 | 122.7 | −14.6 | ||||
| pH | Ce (g/L) | mect,e (g) | mect,e (meq) | Desorption (%) |
|---|---|---|---|---|
| 2 | 5.42 | 0.06 | 0.42 | 9.96 |
| 3 | 4.05 | 0.04 | 0.31 | 7.44 |
| 4 | 3.72 | 0.04 | 0.29 | 6.84 |
| 5 | 3.58 | 0.04 | 0.28 | 6.58 |
| 6 | 3.99 | 0.04 | 0.31 | 7.33 |
| Temp. (°C) | kd (min−1) | R2 |
|---|---|---|
| 15 | 0.31 | 0.92 |
| 25 | 1.08 | 0.82 |
| 35 | 4.90 | 0.64 |
| 45 | 1.64 | 0.91 |
| 55 | 1.48 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Wei, Y.-H.; Wu, H.-S. Adsorption and Desorption Behavior of Ectoine Using Dowex® HCR-S Ion-Exchange Resin. Processes 2021, 9, 2068. https://doi.org/10.3390/pr9112068
Wu Y-C, Wei Y-H, Wu H-S. Adsorption and Desorption Behavior of Ectoine Using Dowex® HCR-S Ion-Exchange Resin. Processes. 2021; 9(11):2068. https://doi.org/10.3390/pr9112068
Chicago/Turabian StyleWu, Yu-Chi, Yu-Hong Wei, and Ho-Shing Wu. 2021. "Adsorption and Desorption Behavior of Ectoine Using Dowex® HCR-S Ion-Exchange Resin" Processes 9, no. 11: 2068. https://doi.org/10.3390/pr9112068
APA StyleWu, Y.-C., Wei, Y.-H., & Wu, H.-S. (2021). Adsorption and Desorption Behavior of Ectoine Using Dowex® HCR-S Ion-Exchange Resin. Processes, 9(11), 2068. https://doi.org/10.3390/pr9112068