Abstract
Although the gas phase combustion of metallic magnesium (Mg) has been extensively studied, the vaporization and diffusive combustion behaviors of Mg have not been well characterized. This paper proposes an investigation of the vaporization, diffusion, and combustion characteristics of individual Mg microparticles in inert and oxidizing gases by a self-built experimental setup based on laser-induced heating and microscopic high-speed cinematography. Characteristic parameters like vaporization and diffusion coefficients, diffusion ratios, flame propagation rates, etc., were obtained at high spatiotemporal resolutions (μm and tens of μs), and their differences in inert gases (argon, nitrogen) and in oxidizing gases (air, pure oxygen) were comparatively analyzed. More importantly, for the core–shell structure, during vaporization, a shock wave effect on the cracking of the porous magnesium oxide thin film shell-covered Mg core was first experimentally revealed in inert gases. In air, the combustion flame stood over the Mg microparticles, and the heterogeneous combustion reaction was controlled by the diffusion rate of oxygen in air. While in pure O2, the vapor-phase stand-off flame surrounded the Mg microparticles, and the reaction was dominated by the diffusion rate of Mg vapor. The diffusion coefficients of the Mg vapor in oxidizing gases are 1~2 orders of magnitude higher than those in inert gases. However, the diffusive ratios of condensed combustion residues in oxidizing gases are ~1/2 of those in inert gases. The morphology and the constituent contents analysis showed that argon would not dissolve into liquid Mg, while nitrogen would significantly dissolve into liquid Mg. In oxidizing gases of air or pure O2, Mg microparticles in normal pressure completely burned due to laser-induced heating.
1. Introduction
The performance of Mg-based propellants largely depends on the combustion characteristics of metallic Mg particles such as accumulation, ignition, and diffusion of combustion products, etc. [1,2,3]. The combustion of metallic Mg in different atmospheres has been extensively studied in the literature, which revealed that the combustion of metallic Mg is a vapor-phase controlled process in oxidizing atmospheres [4,5,6]. Cassel et al. [7] experimentally presented that the burn time of Mg microparticles in pure O2 was one tenth of that in air, suggesting that the combustion of Mg in air was controlled by the diffusion rate of oxygen component, whereas it controlled by the vaporization rate of Mg vapor in pure O2. In high-temperature H2O [8,9,10] or CO/CO2 [11,12,13,14,15,16], despite millimeter-sized or micron-sized Mg particles, they both underwent gas phase combustion, and the only difference that existed was in terms of burn time. Gaseous combustion was related to the pressure of oxidizing atmospheres [17,18].
For the combustion of vapor-phase Mg, an estimate based on the rate constants [19] showed that the reaction rate was insufficiently fast to prevent oxygen diffusion through the vapor-phase stand-off flame to the particle surface. The heterogeneous reaction of oxygen dissolution begins simultaneously with the vapor-phase combustion and later the heterogeneous reaction becomes the primary combustion mechanism.
Although the gas phase combustion of Mg has been widely studied, the vaporization and diffusive combustion behaviors of Mg have not been deeply understood. The vaporization, diffusion, and combustion of Mg microparticles are interactive in order to affect the comprehensive performance of Mg-based propellants. Thus, it is imperative to characterize these behaviors. In this work, the vaporization, diffusive, and combustion processes of individual micron-sized Mg particles in oxidizing atmospheres of air and pure O2 were directly observed by a self-designed experimental apparatus based on laser-induced heating and microscopic high-speed cinematography. In comparison to the vaporization and diffusion characteristics without oxidation, the experiments of heating individual Mg microparticles in inert atmospheres of argon (Ar) and nitrogen (N2) were also carried out. Characteristic parameters such as vaporization and diffusion coefficients, diffusion ratios, flame propagation rates, etc., were obtained at high spatiotemporal resolutions (μm and tens of μs), and their differences between in inert gases and in oxidizing gases were comparatively analyzed. The combustion residues of individual Mg microparticles heated in each atmosphere were collected to characterize the morphology and constituents using a scanning electron microscope (SEM) and through energy dispersive spectroscopy (EDS) mapping. These characteristic parameters were extracted through digit image processing technology to demonstrate the vaporization, diffusion, and combustion characteristics and to unveil the combustion mechanism.
2. Materials and Experimental Details
2.1. Materials
Mg microparticles were prepared using the melt atomization technique and were characterized by multiple methodologies such as SEM, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and EDS. These results have been presented in our previous work [20], demonstrating that Mg microparticles are uniform and spherical, with a purity of approximately 100%. There is a thin but not compact oxide layer on the surface. In the current work, the size distribution of the Mg microparticles is shown in Figure 1, and ranged from ~45 to 75 µm.
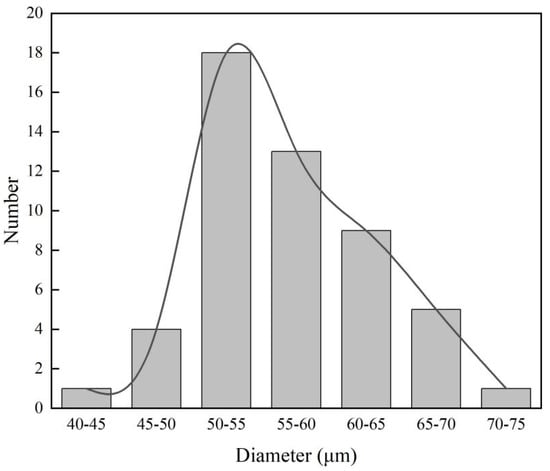
Figure 1.
Particle diameter distribution of individual Mg microparticles.
2.2. Experimental Setup
Figure 2 shows the schematic of the experimental apparatus. A beam launched from a laser was reflected through two totally reflecting mirrors and was transmitted through the dichroic to the objective. This laser (New Industries Optoelectronics Tech. Co. Ltd., Changchun, China) is a continuous wave Nd: YVO4 near-infrared with a wavelength of 1064 nm, TEM00 mode, beam divergence of 1.5 mrad (full angle), and variable output power of 0~600 mW. The dichroic was used to completely reflect the beam of 1064 nm to the objective and allowed the visible light from the illumination to transmit to the high-speed camera. The objective (20×, NA 0.40) was utilized to focus the beam to heat the Mg microparticles.
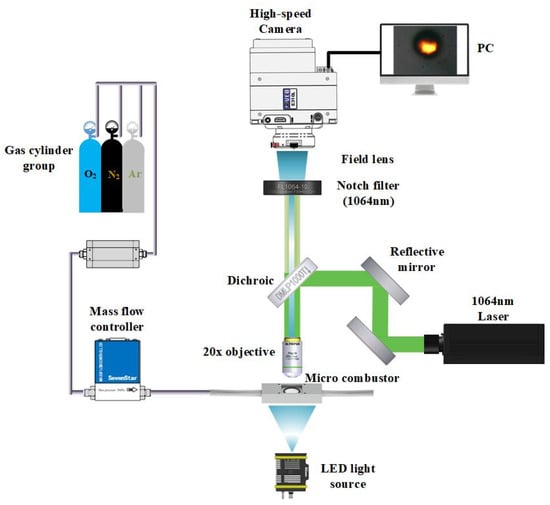
Figure 2.
Schematic of experimental apparatus.
Individual Mg microparticles dispersed in the combustion chamber were magnified by the same objective and were imaged by a high-speed camera (Phantom Micro E310, Vision Research Inc., Wayne, NJ, USA). A matching field lens and a notch filter were installed in front of a high-speed camera to eliminate the influence of the laser beam. A LED lamp and a condenser were used to supply uniformly transmitted bright field illumination so that the high-speed camera could take clear images. The laser and high-speed camera were triggered by a synchronizer.
The combustor consisted of a self-designed 3D printed combustion chamber body, an observation window, and seals. The upper and lower sides were transparent glass slides (observation windows), and the sealing ring was installed to ensure the airtightness of the circular combustion chamber. The gas inlet and outlet were set at the two ends of the combustion chamber. A mass flow controller was installed at the left side of the combustion chamber, connecting to the gas cylinder and the inlet of the combustion chamber to accurately control the intake volume of desired gas. The atmosphere could be exchanged by connecting different gas cylinders.
2.3. Experimental Procedure
In the experiments, the Mg powders were dried in an oven at 120 °C for about 1 h. Then, they were fetched to cool to room temperature. An optical fiber was used to adhere the Mg microparticles by the electrostatic adsorption between the optical fiber and Mg microparticles. Then, individual Mg microparticles were solely dispersed on the bottom wall of combustor, with a slight oscillation of the optical fiber. A sealing ring and a lid of transparent glass slide were installed on the combustion chamber. A desired atmosphere entered and purged the combustor for ~30 min to remove any residual gas. As the combustor was filled with the desired gas, the intake valve was closed. The temperature and pressure of desired atmosphere in the combustor was kept at room temperature (~25 °C) and at atmospheric pressure (0.1 MPa).
An individual Mg microparticle appeared in the field of vision of the high-speed camera after moving a 3-axis translation platform. Moreover, it was certain that there were no other microparticles around the microparticle that could be observed. This was to avoid interaction among the microparticles affecting the observation of combustion phenomenon and the judgement of the combustion process. At that time, the laser and high-speed camera were simultaneously trigged to heat the individual Mg microparticle and to record its burning process.
In air and pure O2, the resolution of the high-speed camera was set to 640 × 480, and the frame rate was 10,000 fps. However, in Ar and N2, since there were larger diffusive zones of vapor-phase Mg, the resolution of the high-speed camera needed to be 1280 × 800, and the frame rate was 3200 fps.
In each case, the combustion chamber body was replaced with a new one, and the combustion residues were collected to characterize the morphology and constituent contents.
2.4. Image and Data Processing
The vaporization of Mg microparticles and diffusion of gaseous Mg in inert and oxidizing atmospheres were recorded by high-speed camera. The representative snapshots of the vaporization and diffusion were then extracted. These images were cropped, filtered and binarized using self-programming software. Finally, the binarizated images were used to measure the area, perimeter, and diameter of individual Mg microparticles by calibration. Figure 3a represents the original image. After binarization, the corresponding binarizated image could be seen in Figure 3b.
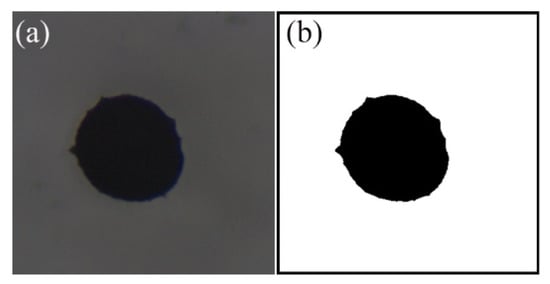
Figure 3.
The example of image and data processing: (a) original image; (b) binarized image.
For the combustion characteristics of the Mg microparticles, their propagation displacement and flame sizes can be measured by contrasting the images. The propagation velocity and diffusion ratio can be calculated based on the measured sizes and the time.
3. Results and Discussion
3.1. Vaporization and Diffusion
In Figure 4, under the same operation conditions, the vaporization of the Mg microparticles and diffusion of gaseous Mg in inert and oxidizing atmospheres demonstrated different characteristics. Regardless of whether they were in inert gases or in oxidizing gases, as the Mg microparticles were heated by the laser, the Mg melted and then vaporized. In Ar or N2, the Mg vapor homogeneously diffused and gradually condensed. While in air or pure O2, gaseous Mg diffused heterogeneously and then quickly ignited and underwent gas phase combustion.
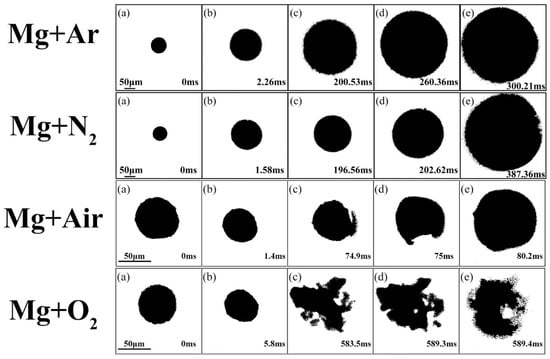
Figure 4.
Representative snapshots of gasification of Mg microparticles and diffusion process of Mg vapor in inert and oxidizing atmospheres.
In inert gases, the temperature rise of the Mg microparticles follows the energy conversation equation:
where ρp, Cp, and dp are the density, specific heat, and diameter of the Mg microparticle, respectively. and are the laser heating power and the heat loss containing the convection, the conduction, and the thermal radiation heat losses to the chamber wall, respectively.
In oxidizing gases, the heterogeneous oxidation reaction has to be considered in the energy conversation equation; thus, the temperature rise of Mg microparticles is as follows:
is the released heat of heterogeneous oxidation reaction.
By contrastively analyzing Equations (1) and (2), it is easily testified that due to the exothermic reaction, the temperature rise rate of Mg microparticles in oxidizing gases is higher than that in inert gases.
Figure 5 illustrates that the diffusion rate of Mg vapor varied with the heating time in different atmospheres. The diffusion rate of Mg vapor in Ar was significantly larger than that in N2. The diffusion rate of Mg vapor in air increased at the early stage and then kept nearly constant, whereas the diffusion rate of the Mg vapor in O2 decreased.
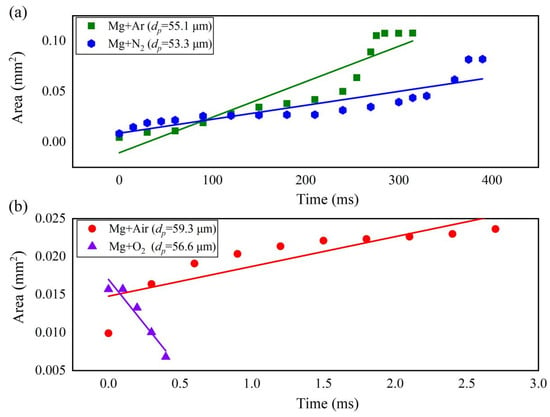
Figure 5.
The diffusion rates of Mg vapor in different atmospheres (a) in Ar and N2; (b) in Air and O2.
According to Fick’s second law, the concentration of Mg vapor for one-dimensional diffusion can be expressed as [21]:
where D is the diffusion coefficient. Assuming the diffusion coefficient is constant, Equation (3) can be simplified as:
In this work, the diffusion coefficient is defined as D = Adiff/t, in which Adiff and t is the diffusive area and time, respectively. By linearly fitting the parameters shown in Figure 5, the slope of the diffusion curve is the diffusion coefficient. the diffusion coefficients in Ar, N2, air, and O2 at 0.1 MPa are ~3.5 × 10−7 m2/s, 1.4 × 10−7 m2/s, 3.9 × 10−6 m2/s, and −2.4 × 10−5 m2/s, respectively. The diffusion coefficients of Mg vapor in oxidizing gases are 1~2 orders of magnitude higher than those in inert gases.
3.2. Shock Wave Effect on the Cracking of Porous Oxide Shell during Vaporization in Inert Gases
The structure of Mg microparticles is a core–shell type, similar to metallic aluminum. However, since the Pilling–Bedworth ratio is less than 1, the magnesium oxide film surface is not compact but is instead porous [22]. As the temperatures of Mg microparticles are enhanced to melting temperature and gasification temperature points, Mg microparticles melt and vaporize. The densities of solid and liquid Mg decrease almost linearly with the rise of temperature. However, the density of Mg suddenly drops during the solid–liquid phase transition. The density difference between solid Mg and liquid Mg at the melting temperature point (~921 K) reaches 4.89% [23]. This results in a significant change in the volume of the Mg core. As the liquid Mg temperature is continuously enhanced until the boiling point (~1380 K) [24], the liquid Mg vaporizes. At that moment, the oxide film (MgO) does not reach the melting temperature point (~3125 K) [6]. This means that the internal pressure sharply increases between the core and shell, which will lead to the cracking of the porous oxide film [25].
Figure 6 experimentally demonstrates a shock wave effect on the cracking of the porous magnesium oxide film during vaporization in the gases of Ar and N2. Owing to the release of loaded internal pressure, the Mg vapor homogeneously diffused towards ambient gas. It is worthy to note that the shock wave effect did not occur in the gases of air or O2. It is possible that the oxygen atoms and magnesium cations interactively diffuse through the porous oxide thin film during melting and vaporization. The pathway in the porous oxide thin film promoted the reaction and inhibited the formation of shock pressure. Additionally, as the thickness of the oxide film is its beyond critical value, the shock wave effect can be easily found.
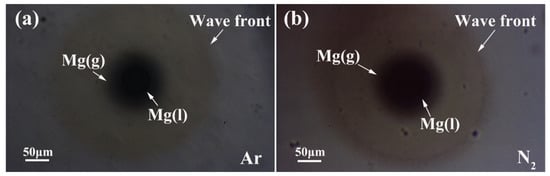
Figure 6.
Pictures of the shock wave effect on the cracking of the porous magnesium oxide film, (a) in Ar; (b) in N2.
The propagation displacement of shock wave is shown in Figure 7. Despite in Ar or in N2, the shock wave propagated exponentially, and the propagation displacement can be expressed as:
where Ds is the propagation displacement of the front of the shock wave, and t is the propagation time. D0, a, and k are the coefficients.
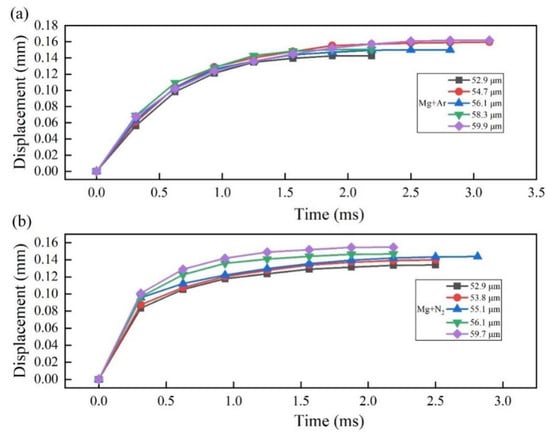
Figure 7.
The propagation displacements of the front of the shock wave (a) in Ar; (b) in N2.
In each atmosphere, the phenomena of multiple individual Mg microparticles with different diameters (dp) were repeatedly recorded, and the coefficients in Equation (5) and the correlation coefficients (R) for fitting are listed into Table 1. The coefficient k in Ar is smaller than that in N2, which suggests that the loaded internal pressure due to the phase transition in N2 was higher than that in Ar. In the early propagation stage, if the data in Figure 7 were fitted by a linear function, the propagation velocity of the shock wave in Ar ranged from 0.158~0.175 m/s, which is much lower than that in N2, which ranged from 0.266~0.322 m/s. The data in Table 1 show that at under the same operation conditions, the propagation velocity had a weak correlation with the initial diameter of the Mg microparticle.

Table 1.
The fitting coefficients and correlation coefficients in Equation (5).
3.3. Diffusive Combustion of Mg Vapor
In oxidizing gases, the combustion of the Mg microparticles is classically referred to as vapor-phase burning. In this work, although the Mg microparticles underwent diffusive gas phase combustion, the burning behavior in pure O2 was significantly different from that in air, as shown in Figure 8. It can be obviously observed that the combustion flame in air stood over the Mg microparticle, and the flame area was further smaller than the diffusion zone of Mg vapor. This suggests that the heterogeneous combustion reaction is controlled by the diffusion rate of oxygen in air. In pure O2, the vapor-phase stand-off flame surrounded the Mg microparticle, and the heterogeneous combustion reaction is controlled by the diffusion rate of Mg vapor. In the literature [19], an estimate showed that the reaction rate in air is insufficiently fast to prevent oxygen gas diffusion through the vapor-phase stand-off flame to the Mg particle surface.
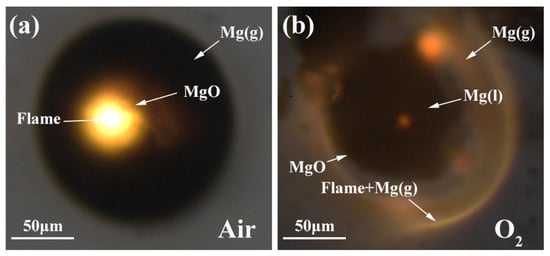
Figure 8.
Representative snapshots of the vapor-phase stand-off flame of burning Mg microparticles, (a) in air; (b) in O2.
In the O2 atmosphere, the combustion was more intense than that in air, and the gasification time was extremely short and almost occurred at the same time as the combustion, indicating that the oxygen content greatly affected the burning rate of Mg vapor [3,26,27]. During combustion in air, the flame is relatively homogeneous. By using the data processing technique shown in Section 2.4, the outer contour of the flame in Figure 8 could be obtained. The number of pixels in the contour were calculated, and the flame radius was obtained and is shown in Figure 9. From the pictures shown in Figure 9, it can be obviously observed that the produced magnesium oxide covered the partial flame. The distances between the flame front and center (called as flame radius) were measured and are illustrated in Figure 9. After ignition, the flame front quickly propagated, then remained stable, and finally gradually drew back. In the two cases, the propagation rates were ~11.2 and 16.5 mm/s, respectively, which are one order of magnitude lower than the diffusion rate of the shock wave in Section 3.2. In comparison to the flame in air, the flame in pure O2 is thicker and more heterogeneous.
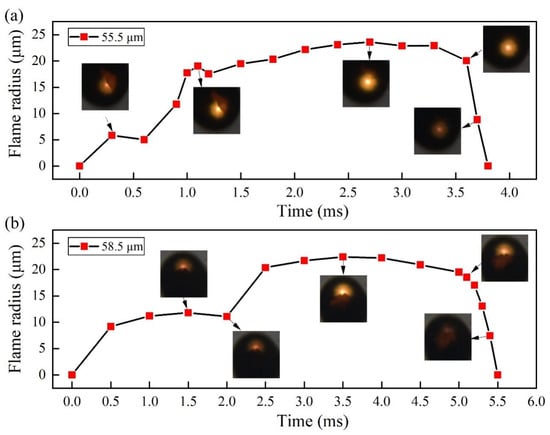
Figure 9.
Flame propagation rates of individual Mg microparticles burned in air. (a) particle of 55.5 μm; (b) particle of 58.5 μm.
3.4. Analysis of Combustion Residues
In four groups of atmospheres, the combustion residues were collected and characterized by SEM and EDS mapping techniques. Figure 10 shows the morphology of the combustion residues of Mg microparticles. In Ar or N2, the distribution of the condensed combustion residues was slightly circular in shape, suggesting that Mg vapor homogeneously diffused towards the ambient gases. In air or O2, the residues nonuniformly distributed at the bottom wall of the combustion chamber. This means that the diffusive combustion was heterogeneous and that combustion particulate matters randomly migrated with the diffusive gases.
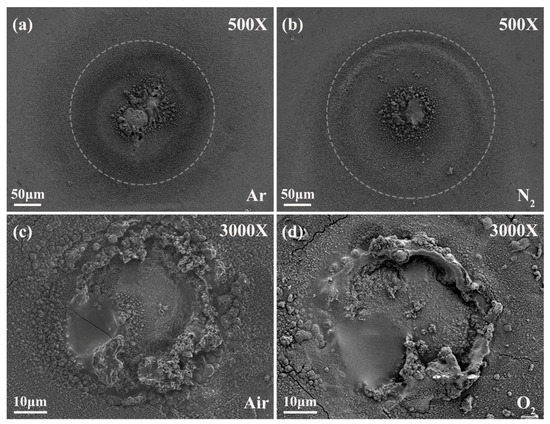
Figure 10.
SEM morphology of combustion residues of Mg microparticles in inert and oxidizing atmospheres. (a) 500×, Ar; (b) 500×, N2; (c) 3000×, Air; (d) 3000×, O2.
To further recognize the diffusive ranges of Mg vapor in inert and oxidizing atmospheres, the zones covered by condensed combustion residues were measured by an optical microscope. A coefficient called the diffusion ratio (β) is defined as:
where Ares and Aini are the area of condensed combustion residues and the initial area of an individual microparticle, respectively.
Figure 11 shows the diffusion ratios of Mg microparticles burned in four types of gases. In Ar or N2, the diffusion ratios were 4~6, while in air or O2, they were 2~3. A lower diffusion ratio in oxidizing gases suggests that the combustion reaction consumes the Mg vapor and O2, thus hindering long-distance diffusion.
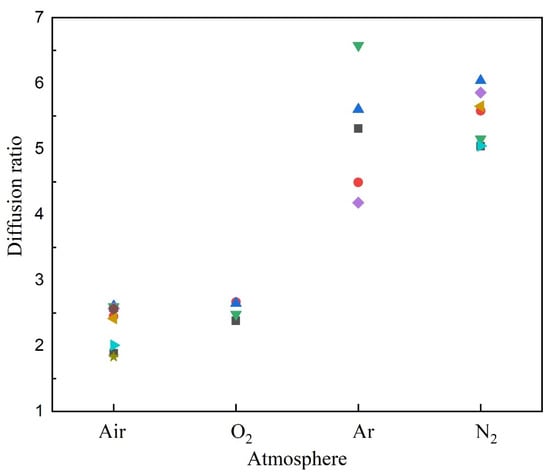
Figure 11.
Diffusion ratios of Mg microparticles burned in inert and oxidizing atmospheres.
The constituent contents in the combustion residues of individual Mg microparticles were obtained by EDS analysis. The pictures of the mapping results in inert and oxidizing atmospheres are shown in Figure 12, and the weight and atomic percentages are listed in Table 2. It was found that in Ar, the content of Ar in the residues was tiny, suggesting that Ar atoms scarcely dissolved into the liquid Mg. While when the Mg microparticles were heated in N2, the N atom content reached 25.54%, which could be attributed to the dissolution of the N atom into the liquid Mg. It is possible that the reaction between Mg vapor and N2 was trigged, i.e., 3Mg (g) + N2 (g) → Mg3N2 (s) [28]. In air, the weight percentage of Mg in residues was 69.97%, that of O element is 27.73%, and those of the elements N and C are tiny. The theoretical mass percentage ratio of Mg: O in MgO is 0.6; in this case, the mass ratio of Mg: O was ~0.6. This indicates that Mg element almost reacted with oxygen completely, and a reaction between Mg and CO2 possibly occurred in air, i.e., 2Mg (g) + CO2 (g) → 2MgO (s) + C (s) [11]. In pure O2, the mass percentage ratio of the Mg and O in the residues was also equal to that in air and the theoretical value. Therefore, in oxidizing gases of air or pure O2, Mg microparticles in normal pressure can completely burnout by laser-induced heating.
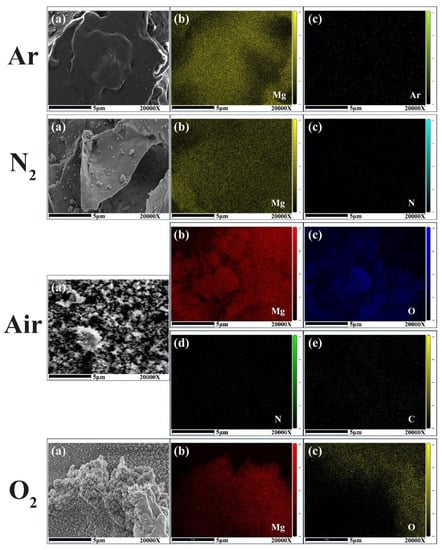
Figure 12.
Pictures of EDS mapping of combustion residue constituents of Mg microparticles in inert and oxidizing atmospheres.

Table 2.
The content of elements in combustion residues of individual Mg microparticles.
4. Conclusions
In this work, the vaporization, diffusion, and combustion of individual Mg microparticles in inert and oxidizing atmospheres were characterized and compared in detail. Some conclusions can be obtained from the experimental results:
- (1)
- The vaporization and diffusion in inert gases are homogeneous, and the diffusion rate of Mg vapor in Ar is significantly larger than that in N2. In oxidizing gases, owing to the heat release of heterogeneous oxidation reaction, the diffusion of Mg vapor is heterogeneous, and their diffusion coefficients are one or two orders of magnitude higher than those in inert gases. The higher the oxygen content is, the larger the diffusion rate becomes. In inert atmospheres, during laser-induced heating in the Mg microparticles with a porous oxide film, when the film thickness is beyond critical value, there is a shock wave effect that results in the cracking of the oxide film shell-covered Mg core due to the release of loaded internal pressure.
- (2)
- Regardless of air or O2 atmosphere, Mg microparticles both burn in a diffusive, vapor-phase mode of combustion The combustion flame in air stands over the Mg microparticle, and the flame area is even smaller than the diffusion zone of the Mg vapor. In pure O2, the vapor-phase stand-off flame surrounds the Mg microparticles. The heterogeneous combustion reaction is controlled by the diffusion rate of oxygen in air, and it is controlled by the diffusion rate of the Mg vapor in the O2.
- (3)
- From the images from the optical microscope and SEM, the diffusive ratios of condensed combustion residues at normal pressure in inert gases are 4~6, higher than those (2~3) in oxidizing gases. The constituent content analysis based on EDS mapping shows that argon does not dissolve into the liquid Mg, while nitrogen dissolves it significantly. In oxidizing gases of air or pure O2, Mg microparticles that underwent laser-induced heating at normal pressure completely burned.
Author Contributions
Conceptualization, S.L. and X.H.; Data curation, F.Y. and X.L.; Funding acquisition, S.L. and X.H.; Investigation, F.Y., X.L., and H.L.; Methodology, S.L., X.H., J.Z. and J.X.; Writing—original draft, F.Y. and S.L.; Writing—review & editing, S.L. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52027809 and 51976050.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (52027809 and 51976050).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pang, W.; Fan, X.; Zhao, F.; Xu, H.; Zhang, W.; Yu, H.; Li, Y.; Liu, F.; Xie, W.; Yan, N. Effects of different metal fuels on the characteristics for htpb-based fuel rich solid propellants. Propellants Explos. Pyrotech. 2013, 38, 852–859. [Google Scholar] [CrossRef]
- Lv, Z.; Xia, Z.X.; Liu, B.; Liu, Y.C. Experimental and numerical investigation of a solid-fuel rocket scramjet combustor. J. Propuls. Power 2016, 32, 1–6. [Google Scholar] [CrossRef]
- Li, L.B.; Chen, X.; Musa, O.; Zhou, C.S.; Zhu, M. The effect of pressure and oxygen concentration on the ignition and combustion of aluminum-magnesium fuel-rich propellant. Aerosp. Sci. Technol. 2018, 76, 394–401. [Google Scholar] [CrossRef]
- Maghsoudi, P.; Bidabadi, M. Evaluation of dynamic behavior of a falling porous magnesium particle over the ignition and combustion processes. J. Therm. Anal. Calorim. 2020, 141, 1605–1617. [Google Scholar] [CrossRef]
- Maghsoudi, P.; Lakzayi, H.; Bidabadi, M. Analytical investigation of magnesium aerosol combustion based on the asymptotic model of flame structure. Sustain. Energy Technol. Assess. 2021, 43, 100914. [Google Scholar] [CrossRef]
- Avdeev, K.A.; Frolov, F.S.; Borisov, A.A.; Frolov, S.M. A modified model of the ignition of a magnesium particle. Russ. J. Phys. Chem. B 2008, 2, 456–462. [Google Scholar] [CrossRef]
- Cassel, H.M.; Liebman, I. Combustion of magnesium particles I. Combust. Flame 1962, 6, 153–156. [Google Scholar] [CrossRef]
- Diwan, M.; Hanna, D.; Shafirovich, E.; Varma, A. Combustion wave propagation in magnesium/water mixtures: Experiments and model. Chem. Eng. Sci. 2010, 65, 80–87. [Google Scholar] [CrossRef]
- Huang, X.; Xia, Z.; Huang, L.; Hu, J. Experimental study on the ignition and combustion characteristics of a magnesium particle in water vapor. Sci. China Technol. Sci. 2012, 55, 2601–2608. [Google Scholar] [CrossRef]
- Ghassemi, H.; Fasih, H.F. Propulsive characteristics of metal fuel-rich pyrotechnics in hydro-reactive motors. Aerosp. Sci. Technol. 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Gol’dshleger, U.I.; Shafirovich, E.Y. Combustion regimes of magnesium in carbon oxides. 1. combustion in CO2. Combust. Explos. Shock. Waves 1999, 35, 637–644. [Google Scholar] [CrossRef]
- Gol’dshleger, U.I.; Shafirovich, E.Y. Combustion regimes of magnesium in carbon oxides. 2. combustion in CO. Combust. Explos. Shock. Waves 2000, 36, 220–226. [Google Scholar] [CrossRef]
- Abbud-Madrid, A.; Modak, A.; Branch, M.C.; Daily, J.W. Combustion of magnesium with carbon dioxide and carbon monoxide at low gravity. J. Propuls. Power 2015, 17, 852–859. [Google Scholar] [CrossRef]
- Li, Y.; Hu, C.; Zhu, X.; Hu, J.; Hu, X.; Li, C.; Cai, Y. Experimental study on combustion characteristics of powder magnesium and carbon dioxide in rocket engine. Acta Astronaut. 2018, 155, 334–349. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Guo, Y.; Ao, W.; Liu, S.; Hu, C. Experimental investigation on the ignition and combustion characteristics of moving micron-sized mg particles in CO2. Acta Astronaut. 2020, 169, 66–74. [Google Scholar] [CrossRef]
- King, M.K. A simplified two-reaction zone model of magnesium combustion in carbon dioxide. Proc. Combust. Inst. 2020, 29, 2931–2938. [Google Scholar] [CrossRef]
- Huang, H.T.; Zou, M.S.; Guo, X.Y.; Yang, R.J.; Zhang, P. Analysis of the solid combustion products of a Mg-based fuel-rich propellant used for water ramjet engines. Propellants Explos. Pyrotech. 2012, 37, 407–412. [Google Scholar] [CrossRef]
- Hu, J.; Han, C.; Xia, Z.; Huang, L.; Huang, X. Experimental investigation on combustion of high-metal magnesium-based hydroreactive fuels. J. Propuls. Power 2015, 29, 692–698. [Google Scholar] [CrossRef]
- Dreizin, E.L.; Berman, C.H.; Vicenzi, E.P. Condensed-phase modifications in magnesium particle combustion in air. Combust. Flame 2000, 122, 30–42. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, M.; Wang, Y.; Li, S.; Huang, X.; Xu, J.; Pan, S. Combustion of Laser-Induced Individual Magnesium Microparticles under Natural Convection. Processes 2021, 9, 1276. [Google Scholar] [CrossRef]
- Unlusu, B.; Sunol, A.K. Multicomponent interphase diffusion of carbon dioxide-methanol-water under near-critical conditions. Chem. Eng. Sci. 2004, 59, 1923–1929. [Google Scholar] [CrossRef]
- Pilling, N.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Met. 1923, 29, 529–591. [Google Scholar]
- Abdullaev, R.N.; Khairulin, R.A.; Kozlovskii, Y.M.; Agazhanov, A.S.; Stankus, S.V. Density of magnesium and magnesium-lithium alloys in solid and liquid states. Trans. Nonferrous Met. Soc. China 2019, 29, 507–514. [Google Scholar] [CrossRef]
- Liu, X.; Schoenitz, M.; Dreizin, E.L. Combustion of mg and composite mg· s powders in different oxidizers. Combust. Flame 2018, 195, 292–302. [Google Scholar] [CrossRef]
- Jona, F.; Marcus, P.M. Magnesium under pressure: Structure and phase transition. J. Phys. Condens. Matter 2003, 15, 7727. [Google Scholar] [CrossRef]
- Krupkin, V.G.; Shmelev, V.M.; Nikolaev, V.M.; Finyakov, S.V. Oxygen index of magnesium powder. Russ. J. Phys. Chem. B Focus Phys. 2019, 13, 596–602. [Google Scholar] [CrossRef]
- Li, G.; Yuan, C.M.; Fu, Y.; Zhong, Y.P.; Chen, B.Z. Inerting of magnesium dust cloud with Ar, N2 and CO2. J. Hazard. Mater. 2009, 170, 180–183. [Google Scholar] [CrossRef]
- Khajelakzay, M.; Bakhshi, S.R.; Borhani, G.H. Synthesis of magnesium silicon nitride nanopowder by employing two-step method. Adv. Appl. Ceram. 2015, 114, 321–325. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).