Adsorption of Cr(OH)n(3−n)+ (n = 1–3) on Illite (001) and (010) Surfaces: A DFT Study
Abstract
:1. Introduction
2. Computational Details
2.1. Model Construction
2.2. Calculation Method
2.3. Calculation of Adsorption Energy
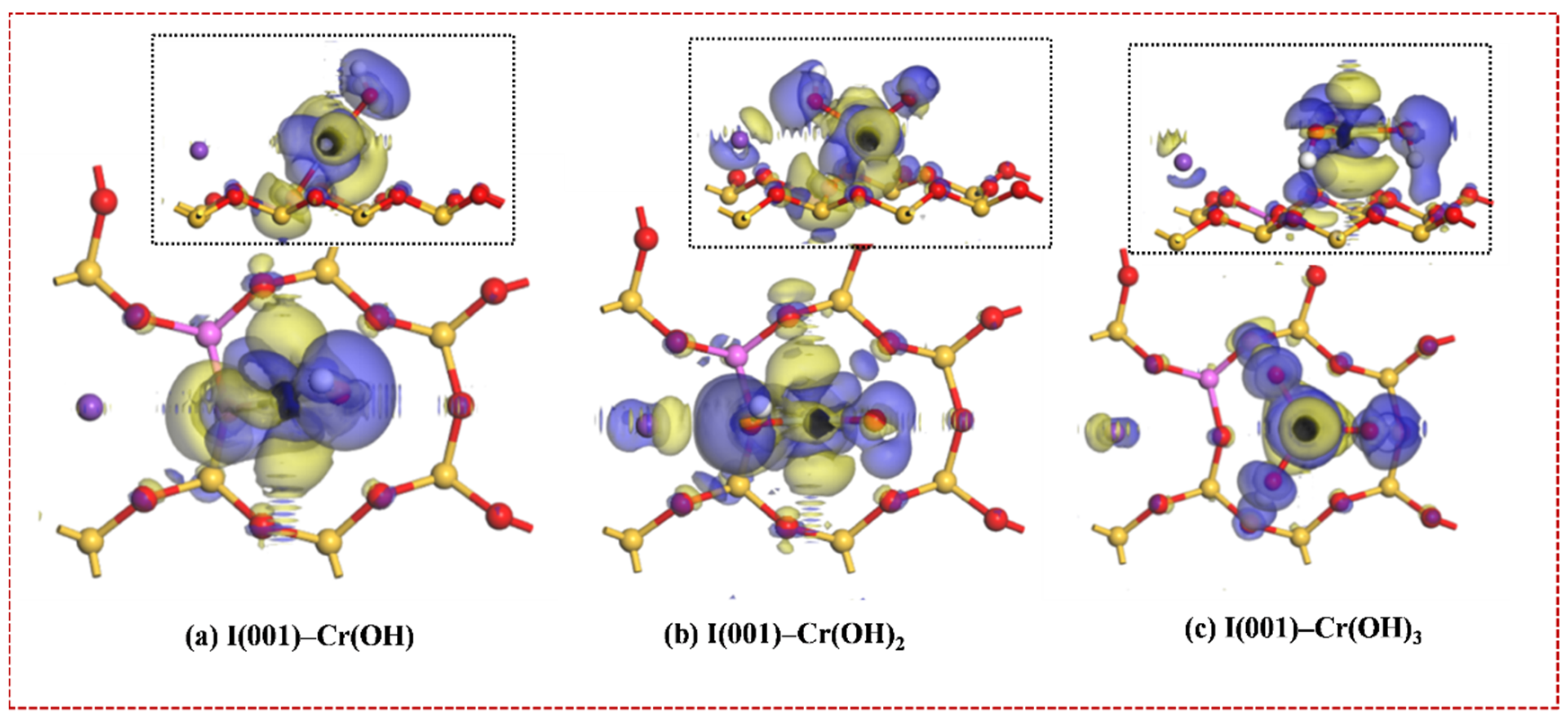
3. Results and Discussion
3.1. Adsorption Geometries on the Illite (001) Surface
3.2. Adsorption Geometries on the Illite (010) Surface
3.3. Charge Analysis
3.4. State Density Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Narir, B.U.; Ramasami, T. Bioaccumulation of chromium from tannery wastewater: An approach for chrome recocovery and reuse. Environ. Sci. Technol. 2004, 1, 300–306. [Google Scholar] [CrossRef]
- Sudha Bai, R.T.E.A. Biosorption of Cr (VI) from aqueous solution by rhizopus nigricans. Bioresour. Technol. 2001, 79, 73–81. [Google Scholar]
- Suwalsky, M.; Castro, R.; Villena, F.; Sotomayor, C. Cr(III) exerts stronger structural effects than Cr(VI) on the human erythrocyte membrane and molecular models. J. Inorg. Biochem. 2008, 102, 842–849. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, J.; Xu, R. Adsorption of Cr(III) from acidic solutions by crop straw derived biochars. J. Environ. Sci. 2013, 25, 1957–1965. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Chou, C. Adsorption of Cr(III) from wastewater by wine processingwaste sludge. J. Colloid Interf. Sci. 2004, 273, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Pehlivan, E. Adsorption of Cr(III) ions by turkish brown coals. Fuel Process. Technol. 2005, 86, 875–884. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Arnfalk, P.; Wasay, S.A.; Tokunaga, S. A comparative study of Cd, Cr(III), Cr(VI), Hg, and Pb uptake by minerals and soil materials. Water Air Soil Pollut. 1996, 87, 131–148. [Google Scholar] [CrossRef]
- Turan, N.G.; Ozgonenel, O. Study of montmorillonite clay for the removal of copper (II) by adsorption: Full factorial design approach and cascade forward neural network. Sci. World J. 2013, 2013, 342628. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Bhattacharyya, K. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef]
- Gui, Y.; Shi, J.; Yang, P.; Li, T.; Tang, C.; Xu, L. Platinum modified MoS2 monolayer for adsorption and gas sensing of SF6 decomposition products: A DFT study. High Volt. 2020, 5, 454–462. [Google Scholar] [CrossRef]
- Wang, J.; Xia, S.; Yu, L. Adsorption of Pb(II) on the kaolinite (001) surface in aqueous system: A DFT approach. Appl. Surf. Sci. 2015, 339, 28–35. [Google Scholar] [CrossRef]
- Peng, C.; Min, F.; Liu, L.; Chen, J. The adsorption of CaOH+ on (001) basal and (010) edge surface of na-montmorillonite: A DFT study. Surf. Interface Anal. 2016, 49, 267–277. [Google Scholar] [CrossRef]
- Fang, F.; Min, F.; Liu, L.; Chen, J.; Ren, B.; Liu, C. Adsorption of Al(OH)n(3−n)+(n = 2–4) on Kaolinite (001) Surfaces: A DFT study. Appl. Clay Sci. 2020, 187, 105455. [Google Scholar] [CrossRef]
- Drits, V.A.; Zviagina, B.B.; Mccarity, D.K. Factors responsible for crystal-chemical variations in the solid solutions from illite to alu-minoceladonite and from glauconite to celadonite. Am. Mineral. 2010, 95, 348–361. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Wang, R.; Meijer, E.J.; Zhou, H.; He, H. Atomic scale structures of interfaces between kaolinite edges and water. Geochim. Cosmochim. Acta 2012, 92, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.; Pickard, C.J.; Hasnip, P.; Clark, S.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximationmade made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- HMonkhorst, J.; Pack, J.D. Special points for Brillon-zone integrations. Phys. Rev. B. 1976, 16, 1748–1749. [Google Scholar]
- Du, J.; Min, F.F.; Zhang, M.X.; Peng, C.L.; Liu, C.F. Mechanism of H2O adsorption on ammonium-illite surface based on density functional theory. J. China Univ. Min. Technol. 2017, 46, 1349–1356. [Google Scholar]
- Churakov, S.V. Ab Initio Study of Sorption on Pyrophyllite: Structure and Acidity of the Edge Sites. J. Phys. Chem. B 2006, 110, 4135–4146. [Google Scholar] [CrossRef]
- Rosso, K.M.; Rustad, J.R.; Bylaska, E.J. The Cs/K exchange in muscovite interlayers:an ab initio treatment. Clay Clay Miner. 2001, 49, 500–513. [Google Scholar] [CrossRef]
- Lavikainen, L.P.; Tanskanen, J.T.; Schatz, T.; Kasa, S.; Pakkanen, T.A. Montmorillonite interlayer surface chemistry: Effect of magnesium ion substitution on cation adsorption. Theor. Chem. Acc. 2015, 134, 51–58. [Google Scholar] [CrossRef]
- Peng, C.; Min, F.; Liu, L.; Chen, J. A periodic DFT study of adsorption of water on sodium-montmorillonite (001) basal and (010) edge surface. Appl. Surf. Sci. 2016, 387, 308–316. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Sprik, M.; Lu, X.; Wang, R. Surface acidity of 2:1 type dioctahedral clay. Geochim. Cosmochim. Ac. 2014, 140, 410–417. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Wei, D. DFT study of interactions between calcium hydroxyl ions and pyrite, marcasite, pyrrhotite surfaces. Appl. Surf. Sci. 2015, 355, 577–587. [Google Scholar]
- Yu, F.-S.; Wang, Y.-H.; Wang, J.-M.; Xie, Z.-F.; Zhang, L. First-principle investigation on mechanism of Ca ion activating flotation of spodumene. Rare Met. 2014, 33, 358–362. [Google Scholar] [CrossRef]
- Moon, H.S.; Lee, J.H.; Kwon, S.; Kim, L.T.; Lee, S.G. Mechanisms of Na adsorption on graphene and graphene oxidedensity functional theory approach. Carbon Lett. 2015, 16, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Ramadugu, S.K.; Mason, S. DFT study of antimony(v) oxyanion adsorption on α-Al2O3(11̅02). J. Phys. Chem. C 2015, 119, 18149–18159. [Google Scholar] [CrossRef]




| Mode | OS1–Cr | OSn···Cr | OS···H | Cr–OCr(OH)n | Eads (kJ/mol) |
|---|---|---|---|---|---|
| I(001)–Cr(OH) | 2.152 [0.04] | 3.054–4.381, n = 2–6 | - | 1.904 (1.792) | −235.78 |
| I(001)–Cr(OH)2 | 2.185 [0.02] | 2.987–4.451, n = 2–6 | - | 1.834–1.9, 114.007 (1.759–1.757, 156.006) | −128.36 |
| I(001)–Cr(OH)3 | - | 3.597–4.049, n = 1–6 | 1.829–1.996 | 1.776–1.832, 117.551–121.617 (1.789–1.787, 119.304–120.719) | −78.89 |
| Mode | OS1–Cr | OS2–Cr | O1···HS | Cr–OCr(OH)n | Eads (kJ/mol) |
|---|---|---|---|---|---|
| I(010)–Cr(OH) | 2.050 [0.18] | 2.064 [0.13] | 1.615–1.975 | 1.904 (1.792) | −348.18 |
| I(010)–Cr(OH)2 | 2.053 [0.14] | 2.111 [0.07] | 1.596 | 1.839–1.916, 98.771 (1.757–1.759, 156.006) | −282.20 |
| I(010)–Cr(OH)3 | 2.088 [0.09] | 2.157 [0.04] | 1.638–1.810 | 1.850–1.942, 96.842–111.035 (1.789–1.787, 119.304–120.719) | −206.63 |
| Model | Name | Before | After | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| s | p | d | Total | Charge/e | s | p | d | Total | Charge/e | ||
| I(001)-Cr(OH) | OS1 | 1.86 | 5.34 | 0 | 7.2 | −1.2 | 1.85 | 5.29 | 0 | 7.14 | −1.14 |
| OS2 | 1.87 | 5.31 | 0 | 7.18 | −1.18 | 1.87 | 5.29 | 0 | 7.16 | −1.16 | |
| OS3 | 1.85 | 5.29 | 0 | 7.14 | −1.14 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | |
| OS4 | 1.84 | 5.34 | 0 | 7.18 | −1.18 | 1.84 | 5.33 | 0 | 7.17 | −1.17 | |
| OS5 | 1.85 | 5.28 | 0 | 7.14 | −1.14 | 1.85 | 5.27 | 0 | 7.13 | −1.13 | |
| OS6 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | 1.85 | 5.27 | 0 | 7.12 | −1.12 | |
| Cr | 2.84 | 6.16 | 4.51 | 13.5 | 0.51 | 2.63 | 6.19 | 4.73 | 13.55 | 0.45 | |
| H | 0.58 | 0 | 0 | 0.58 | 0.42 | 0.6 | 0 | 0 | 0.6 | 0.4 | |
| O | 1.88 | 5.05 | 0 | 6.93 | −0.93 | 1.88 | 5.06 | 0 | 6.94 | −0.94 | |
| I(001) | 0 | +0.09 | |||||||||
| Cr(OH)2+ | 0 | −0.09 | |||||||||
| I(001)-Cr(OH)2 | K | 1.97 | 5.88 | 0 | 7.85 | 1.15 | 2.05 | 5.88 | 0 | 7.93 | 1.07 |
| OS1 | 1.86 | 5.34 | 0 | 7.2 | −1.2 | 1.85 | 5.29 | 0 | 7.14 | −1.14 | |
| OS2 | 1.87 | 5.31 | 0 | 7.18 | −1.18 | 1.87 | 5.29 | 0 | 7.16 | −1.16 | |
| OS3 | 1.85 | 5.29 | 0 | 7.14 | −1.14 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | |
| OS4 | 1.84 | 5.34 | 0 | 7.18 | −1.18 | 1.84 | 5.32 | 0 | 7.16 | −1.16 | |
| OS5 | 1.85 | 5.28 | 0 | 7.14 | −1.14 | 1.85 | 5.27 | 0 | 7.13 | −1.13 | |
| OS6 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | 1.85 | 5.27 | 0 | 7.12 | −1.12 | |
| Cr | 2.49 | 6.12 | 4.51 | 13.12 | 0.88 | 2.42 | 6.14 | 4.48 | 13.04 | 0.96 | |
| H1 | 0.52 | 0 | 0 | 0.52 | 0.48 | 0.59 | 0 | 0 | 0.59 | 0.41 | |
| H2 | 0.52 | 0 | 0 | 0.52 | 0.48 | 0.59 | 0 | 0 | 0.59 | 0.41 | |
| O1 | 1.86 | 5.07 | 0 | 6.92 | −0.92 | 1.88 | 5.02 | 0 | 6.9 | −0.9 | |
| O2 | 1.85 | 5.07 | 0 | 6.92 | −0.92 | 1.89 | 4.97 | 0 | 6.85 | −0.85 | |
| I(001) | 0 | −0.03 | |||||||||
| Cr(OH)2+ | 0 | 0.03 | |||||||||
| I(001)-Cr(OH)3 | OS1 | 1.86 | 5.34 | 0 | 7.2 | −1.2 | 1.86 | 5.33 | 0 | 7.19 | −1.19 |
| OS2 | 1.87 | 5.31 | 0 | 7.18 | −1.18 | 1.87 | 5.3 | 0 | 7.16 | −1.16 | |
| OS3 | 1.85 | 5.29 | 0 | 7.14 | −1.14 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | |
| OS4 | 1.84 | 5.34 | 0 | 7.18 | −1.18 | 1.84 | 5.32 | 0 | 7.16 | −1.16 | |
| OS5 | 1.85 | 5.28 | 0 | 7.14 | −1.14 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | |
| OS6 | 1.85 | 5.28 | 0 | 7.13 | −1.13 | 1.85 | 5.27 | 0 | 7.12 | −1.12 | |
| Cr | 2.27 | 6.09 | 4.38 | 12.74 | 1.26 | 2.29 | 6.19 | 4.39 | 12.87 | 0.99 | |
| H1 | 0.55 | 0 | 0 | 0.55 | 0.45 | 0.64 | 0 | 0 | 0.64 | 0.36 | |
| H2 | 0.55 | 0 | 0 | 0.55 | 0.45 | 0.59 | 0 | 0 | 0.59 | 0.36 | |
| H3 | 0.55 | 0 | 0 | 0.55 | 0.45 | 0.58 | 0 | 0 | 0.58 | 0.35 | |
| O1 | 1.88 | 4.99 | 0 | 6.87 | −0.87 | 1.87 | 4.98 | 0 | 6.84 | −0.73 | |
| O2 | 1.89 | 4.99 | 0 | 6.87 | −0.87 | 1.86 | 4.94 | 0 | 6.81 | −0.67 | |
| O3 | 1.88 | 4.99 | 0 | 6.87 | −0.87 | 1.87 | 4.95 | 0 | 6.82 | −0.67 | |
| I(001) | 0 | 0.01 | |||||||||
| Cr(OH)3 | 0 | −0.01 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Fan, L.; Wang, Q.; Min, F. Adsorption of Cr(OH)n(3−n)+ (n = 1–3) on Illite (001) and (010) Surfaces: A DFT Study. Processes 2021, 9, 2048. https://doi.org/10.3390/pr9112048
Du J, Fan L, Wang Q, Min F. Adsorption of Cr(OH)n(3−n)+ (n = 1–3) on Illite (001) and (010) Surfaces: A DFT Study. Processes. 2021; 9(11):2048. https://doi.org/10.3390/pr9112048
Chicago/Turabian StyleDu, Jia, Leilei Fan, Qinghe Wang, and Fanfei Min. 2021. "Adsorption of Cr(OH)n(3−n)+ (n = 1–3) on Illite (001) and (010) Surfaces: A DFT Study" Processes 9, no. 11: 2048. https://doi.org/10.3390/pr9112048
APA StyleDu, J., Fan, L., Wang, Q., & Min, F. (2021). Adsorption of Cr(OH)n(3−n)+ (n = 1–3) on Illite (001) and (010) Surfaces: A DFT Study. Processes, 9(11), 2048. https://doi.org/10.3390/pr9112048





