Reducing the Internal Stress of Fe-Ni Magnetic Film Using the Electrochemical Method
Abstract
:1. Introduction
2. Experiment
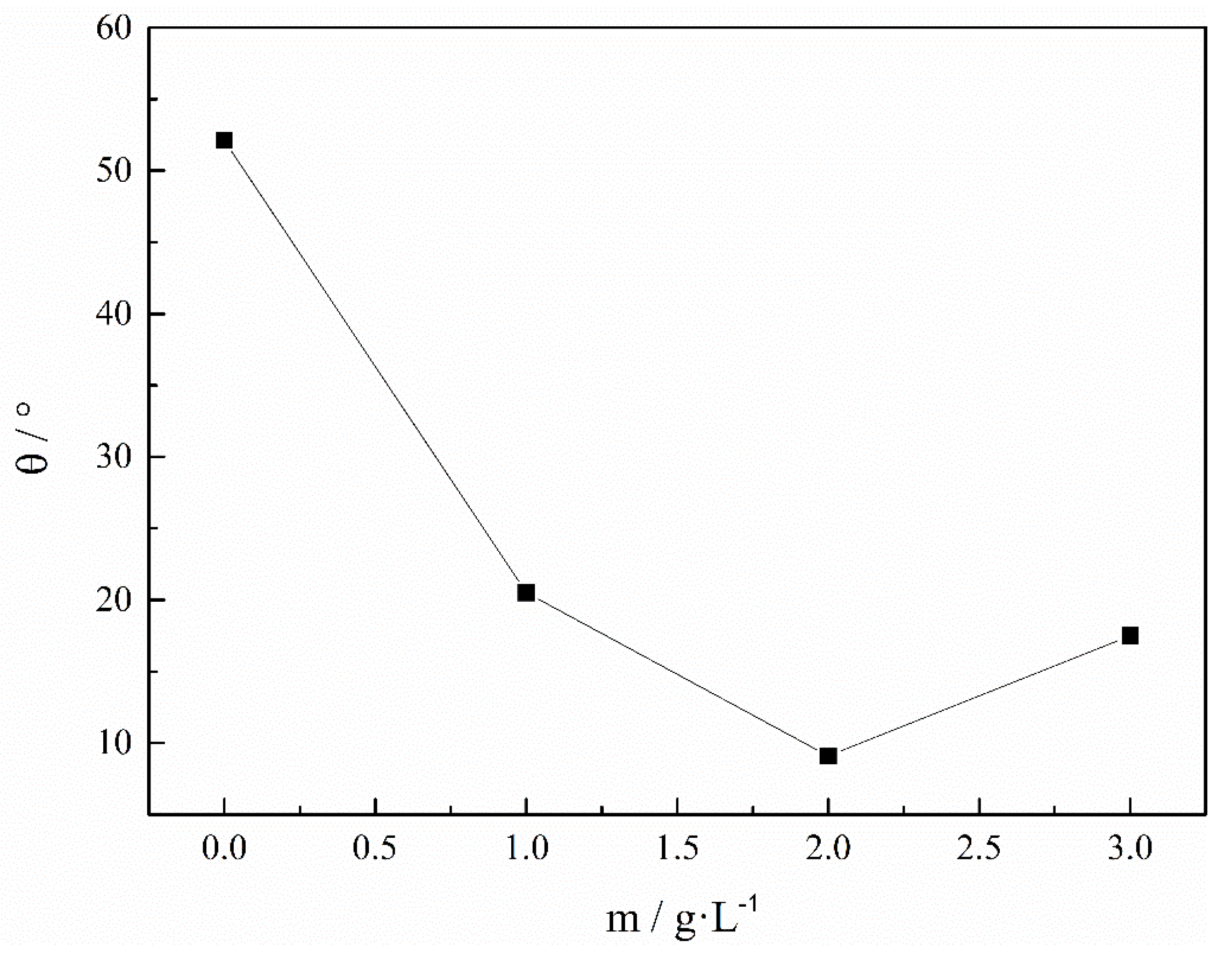
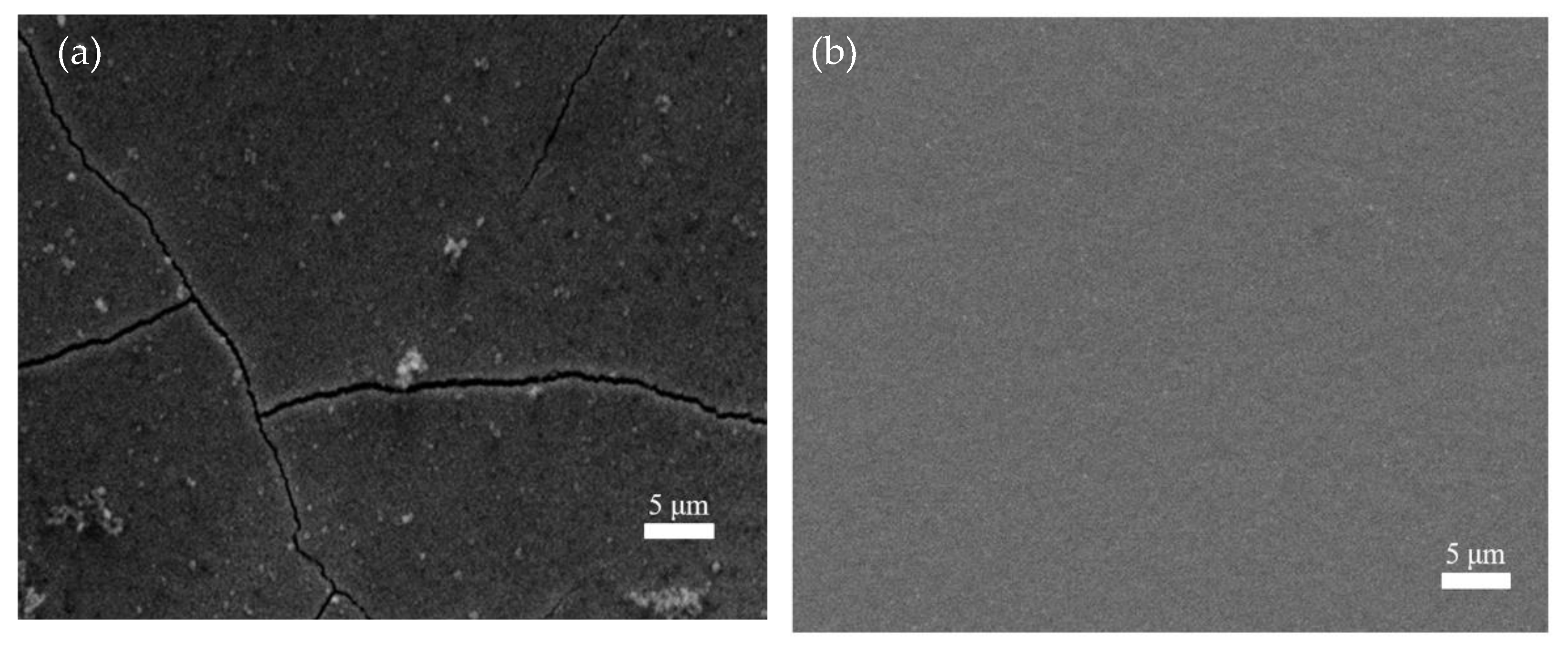
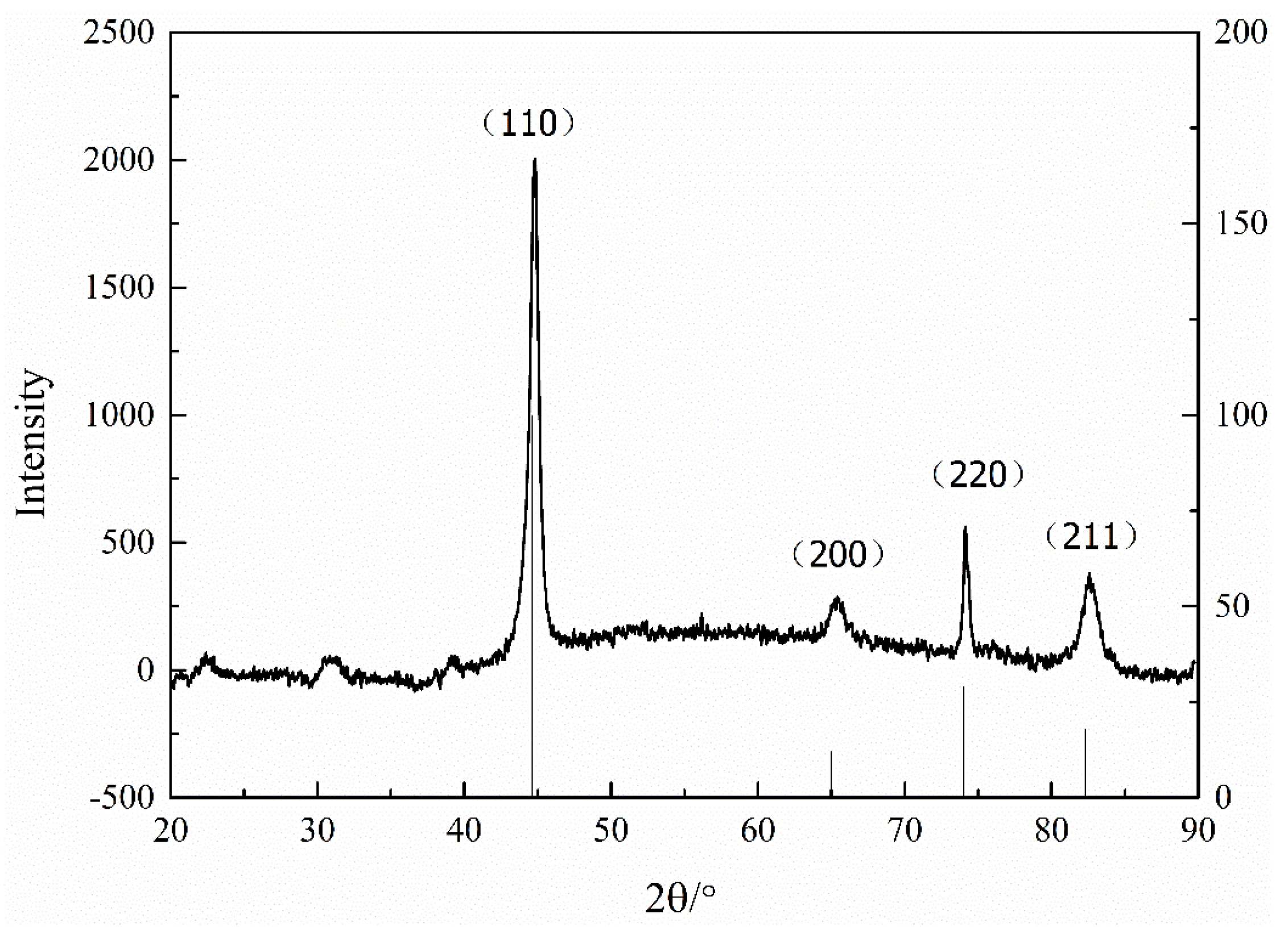
3. Result and Discuss
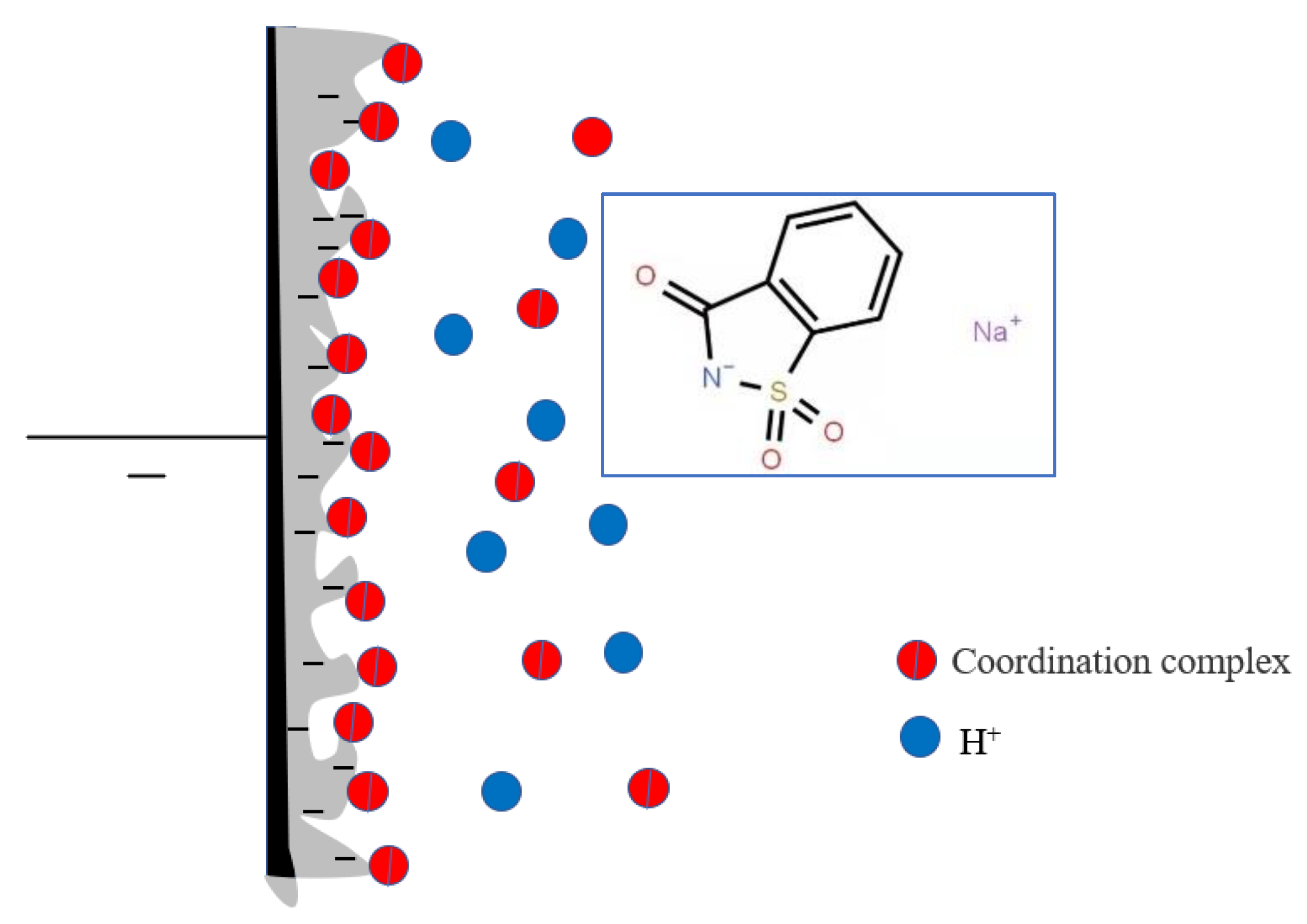
4. The Mechanism of Saccharin Sodium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Krings, A.; Boglietti, A.; Cavagnino, A.; Sprague, S. Soft magnetic ferrite core blank material conveying device. IEEE Trans. Ind. Electron. 2017, 64, 2405–2414. [Google Scholar] [CrossRef]
- Bhattacharya, S. Transforming the transformer. IEEE Spectr. 2017, 54, 38–43. [Google Scholar] [CrossRef]
- Krishnamurthy, S. Simplified loss analysis for high speed SiC MOSFET inverter. In Proceedings of the Electronics Conference and Exposition (APEC, IEEE), East Hartford, CT, USA, 12 June 2012; pp. 1414–1417. [Google Scholar]
- Kroposki, B. Benefits of Power Electronic Interfaces for Distributed Energy Systems. IEEE Trans. Energy Convers. 2010, 25, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Bose, B.K. Global Warming: Energy, Environmental Pollution, and the Impact of Power Electronics. IEEE Ind. Electron. Mag. 2010, 4, 6–17. [Google Scholar] [CrossRef]
- Waide, P.; Brunner, C.U. Energy-Efficiency Policy Opportunities for Electric Motor-Driven Systems; International Energy Agency (IEA): IEA Energy Papers: Paris, France, 2011. [Google Scholar]
- Popović-Gerber, J. Power Electronics Enabling Efficient Energy Usage: Energy Savings Potential and Technological Challenges. IEEE Trans. Power Electron. 2012, 27, 2338–2353. [Google Scholar] [CrossRef]
- Ahn, S.H.; Choi, I.; Park, H.-Y.; Hwang, S.J.; Yoo, S.J.; Cho, E.; Kim, H.-J.; Henkensmeier, D.; Nam, S.W.; Kim, S.-K. Effect of morphology of electrodeposited Ni catalysts on the behavior of bubbles generated during the oxygen evolution reaction in alkaline water electrolysis. Chem. Commun. 2013, 49, 9323–9325. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured threedimensional nickeleiron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [Green Version]
- Vicenzo, A. Structure and mechanical properties of electrodeposited nanocrystalline Ni-Fe alloys. J. Electrochem. Soc. 2013, 160, D570–D577. [Google Scholar] [CrossRef]
- Sohrabi, A.; Dolati, A.; Ghorbani, M.; Monfared, A.; Stroeve, P. Nanomechanical properties of functionally graded composite coatings: Electrodeposited nickel dispersions containing silicon micro-and nanoparticles. Mater. Chem. Phys. 2010, 121, 497–505. [Google Scholar] [CrossRef]
- Balachandran, R.; Yow, H.; Ong, B.; Tan, K.B.; Anuar, K.; Wong, H. Surface morphology and electrical properties of pulse electrodeposition of NiFe films on copper substrates in ultrasonic field. Int. J. Electrochem. Sci. 2011, 6, 3564–3579. [Google Scholar]
- Abdel-Karim, R.; Reda, Y.; Muhammed, M.; El-Raghy, S.; Shoeib, M.; Ahmed, H.J. Electrodeposition and characterization of nanocrystalline Ni-Fe alloys. Nanomaterials 2011, 10, 8. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Reuter, K.; Baker-O’Neal, B.; Huang, Q. Electroplating of Fe-Rich NiFe alloys in Sub-50 nm lines. J. Electrochem. Soc. 2014, 161, D301–D308. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, G.; Ge, H.; Meng, X. Study on preparation of NiFe films by galvanostatic electrodeposition. Surf. Eng. 2014, 30, 97–101. [Google Scholar] [CrossRef]
- Gong, J.; Riemer, S.; Kautzky, M.; Tabakovic, I. Composition gradient, structure, stress, roughness and magnetic properties of 5–500 nm thin NiFe films obtained by electrodeposition. J. Magn. Magn. Mater. 2016, 398, 64–69. [Google Scholar] [CrossRef]
- Dragos, O.; Chiriac, H.; Lupu, N.; Grigoras, M.; Tabakovic, I. Anomalous codeposition of fcc NiFe nanowires with 5–55% Fe and their morphology, crystal structure and magnetic properties. J. Electrochem. Soc. 2016, 163, D83–D94. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Luo, Y.; Asiri, A.M. Efficient electrochemical water splitting catalyzed by electrodeposited NiFe nanosheets film. Int. J. Hydrogen Energy 2016, 41, 8785–8792. [Google Scholar] [CrossRef]
- Kim, K.H.; Zheng, J.Y.; Shin, W.; Kang, Y.S. Preparation of dendritic NiFe films by electrodeposition for oxygen evolution. RSC Adv. 2012, 2, 4759–4767. [Google Scholar] [CrossRef]
- Kuru, H.; Kockar, H.; Alper, M.; Karaagac, O. Growth of binary NieFe films: Characterisations at low and high potential levels. J. Magn. Magn. Mater. 2015, 377, 59–64. [Google Scholar] [CrossRef]
- Wei-Su, C.; Yang, W.; Jun-Ming, G.; Feng-Jiao, H. Thermal stability of Ni-Fe alloy foils continuously electrodeposited in a fluorborate bath. Open J. Metal 2012, 2, 18–23. [Google Scholar]
- Torabinejad, V.; Rouhaghdam, A.S.; Aliofkhazraei, M.; Allahyarzadeh, M. Electrodeposition of NieFe and NieFe-(nano Al2O3) multilayer coatings. J. Alloys Compd. 2016, 657, 526–536. [Google Scholar] [CrossRef]
- Van Petegem, S.; Zimmermann, J.; Van Swygenhoven, H. Microstructure and deformation mechanisms in nanocrystalline NieFe. Part II. In situ testing during X-ray diffraction. Acta Mater. 2013, 61, 5846–5856. [Google Scholar] [CrossRef]
- Tabakovic, I.; Inturi, V.; Thurn, J.; Kief, M. Properties of Ni1_xFex (0.1 <x <0.9) and Invar (x¼0.64) alloys obtained by electrodeposition. Electrochim. Acta 2010, 55, 6749–6754. [Google Scholar]
- Deepthi, K.A.; Balachandran, R.; Ong, B.H.; Tan, K.B.; Wong, H.Y.; Yow, H.K.; Srimala, S. Physical and electrical characteristics of NiFe thin films using ultrasonic assisted pulse electrodeposition. Appl. Surf. Sci. 2016, 360, 519–524. [Google Scholar] [CrossRef]
- Ohta, M.; Yoshizawa, Y.J. Recent Progress in Fe-based nanocrystalline soft magnetic alloys and their applications. Phys. D Appl. Phys. 2011, 44, 064004. [Google Scholar] [CrossRef]
- Kong, F.; Men, H.; Liu, T.; Shen, B. Research progress and application prospects of Fe-based soft magnetic amorphous/nanocrystalline alloys. J. Appl. Phys. 2012, 111, 07A311. [Google Scholar] [CrossRef]
- Fan, X.; Men, H.; Ma, A.; Shen, B. Soft magnetic properties in Fe84-xB10C6Cux nanocrystalline alloys. J. Magn. Magn. Mater. 2013, 326, 22. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, A.; Zhao, C.; Men, H.; Wang, X.; Chang, C. The solubility of cerium in La2Ti2O7 by DFT + U calculations. J. Alloys Compd. 2015, 622, 1000. [Google Scholar] [CrossRef]
- Sharma, P.; Zhang, X.; Zhang, Y.; Makino, A. Competition driven nanocrystallization in high Bs and low coreloss Fe–Si–B–P–Cu soft magnetic alloys. Scr. Mater. 2015, 95, 3. [Google Scholar] [CrossRef]
- Jafari, S.; Beitollahi, A.; Yekta, B.E.; Ohkubo, T.; Sky, V.B.; Marsilius, M.; Herzer, G.; Hono, K. Atom probe analysis and magnetic properties of nanocrystalline Fe84.3Si4B8P3Cu0.7. J. Alloys Compd. 2015, 674, 136. [Google Scholar] [CrossRef]
- Suzuki, K.; Parsons, R.; Zang, B.; Onodera, K.; Kishimoto, H. Copper-free nanocrystalline soft magnetic materials with high saturation magnetization comparable to that of Si steel. Appl. Phys. Lett. 2017, 110, 012407. [Google Scholar] [CrossRef]
- Hasegawa, R.; Chien, C.L. Mössbauer and its r.f. sideband effects in iron-rich glassy alloys. Solid State Commun. 1976, 18, 913. [Google Scholar] [CrossRef]
- Pathak, S.; Guinard, M.; Vernooij, M.G.C.; Cousin, B.; Wang, Z.; Michler, J.; Philippe, L. Influence of lower current densities on the residual stress and structure of thick nickel electrodeposits. Surf. Coat. Technol. 2011, 205, 3651–3657. [Google Scholar] [CrossRef]
- Ortolani, M.; Zanella, C.; Ricardo, C.L.A.; Scardi, P. Elastic grain interaction in electrodeposited nanocomposite nickel matrix coatings. Surf. Coat. Technol. 2012, 206, 2499–2505. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Altamirano, L.G.; Pritzker, M.; Luna, R.S.; Cabrera, R.S. Experimental and modeling study of nickel electrodeposition including H+ and water reduction and homogeneous reactions. J. Electrochem. Soc. 2011, 158, D33–D41. [Google Scholar] [CrossRef]
- Xie, L.S.; Li, J.S.; Zhang, T.B.; Kou, H.C. Role of milling time and Ni content on dehydrogenation behavior of MgH2/Ni composite. Trans. Nonferr. Met. Soc. China 2017, 27, 569–577. [Google Scholar] [CrossRef]
- Dundálek, J.; Šnajdr, I.; Libánský, O.; Vrána, J. Zinc electrodeposition from flowing alkaline zincate solutions: Role of hydrogen evolution reaction. J. Power Sources 2017, 372, 221–226. [Google Scholar] [CrossRef]
- Hong, B.; Yu, X.Y.; Jiang, L.X.; Xue, H.T.; Liu, F.Y.; Liu, J.; Liu, Y.X. Hydrogen evolution inhibition with diethylenetriamine modification of activated carbon for a lead-acid battery. RSC Adv. 2014, 4, 33574–33577. [Google Scholar] [CrossRef]
- Liu, X.; Evans, P.; Zangari, G. Electrodeposited Co-Fe and Co-Fe-Ni alloy films for magnetic recording write heads. IEEE Trans. Magn. 2000, 36, 3479–3481. [Google Scholar]
- Djokic, S.S. Electrodeposition: Theory and Practice; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Boudinar, S.; Benbrahim, N.; Benfedda, B.; Kadri, A.; Chainet, E.; Hamadou, L. Electrodeposition of heterogeneous Mn−Bi thin films from a sulfate−nitrate bath: Nucleation mechanism and morphology. J. Electrochem. Soc. 2014, 161, D227–D234. [Google Scholar] [CrossRef]
- Mockute, D.; Butkiene, R.; Nivinskiene, O. Effect of chloride ions on the behavior of saccharin, N-methylsaccharin, and 2-butyne-1,4-diol during electrodeposition of nickel from acid electrolytes. Russ. J. Electrochem. 2001, 37, 376–381. [Google Scholar] [CrossRef]
- Lukomska, A.; Sobkowski, J. Potential of zero charge of monocrystalline copper electrodes in perchlorate solutions. J. Electroanal. Chem. 2004, 567, 95–102. [Google Scholar] [CrossRef]
- Bhandari, A.; Hearne, S.J.; Sheldon, B.W.; Soni, S.K. Microstructural origins of saccharin-induced stress reduction in electrodeposited Ni. J. Electrochem. Soc. 2009, 156, D279–D282. [Google Scholar] [CrossRef]
- Lallemand, F.; Ricq, L.; Wery, A.; Bercot, P. The influence of organic additives on the electrodeposition of irongroup metals and binary alloy from sulfate electrolyte. Appl. Surf. Sci. 2004, 228, 326–333. [Google Scholar] [CrossRef]
- Hassani, S.; Raeissi, K.; Golozar, M.A. Effects of saccharin on the electrodeposition of Ni-Co nanocrystalline coatings. J. Appl. Electrochem. 2008, 38, 689–694. [Google Scholar] [CrossRef]

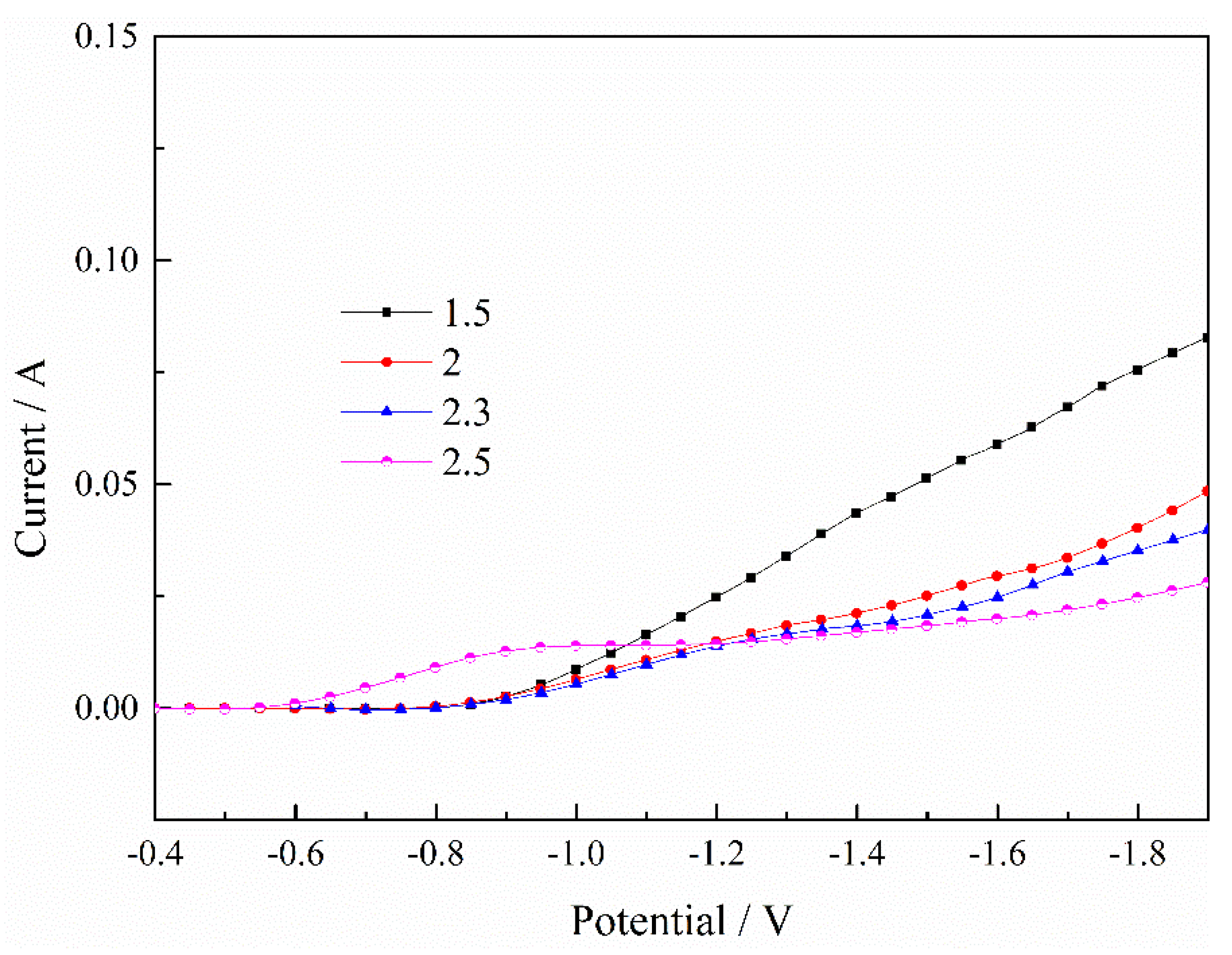
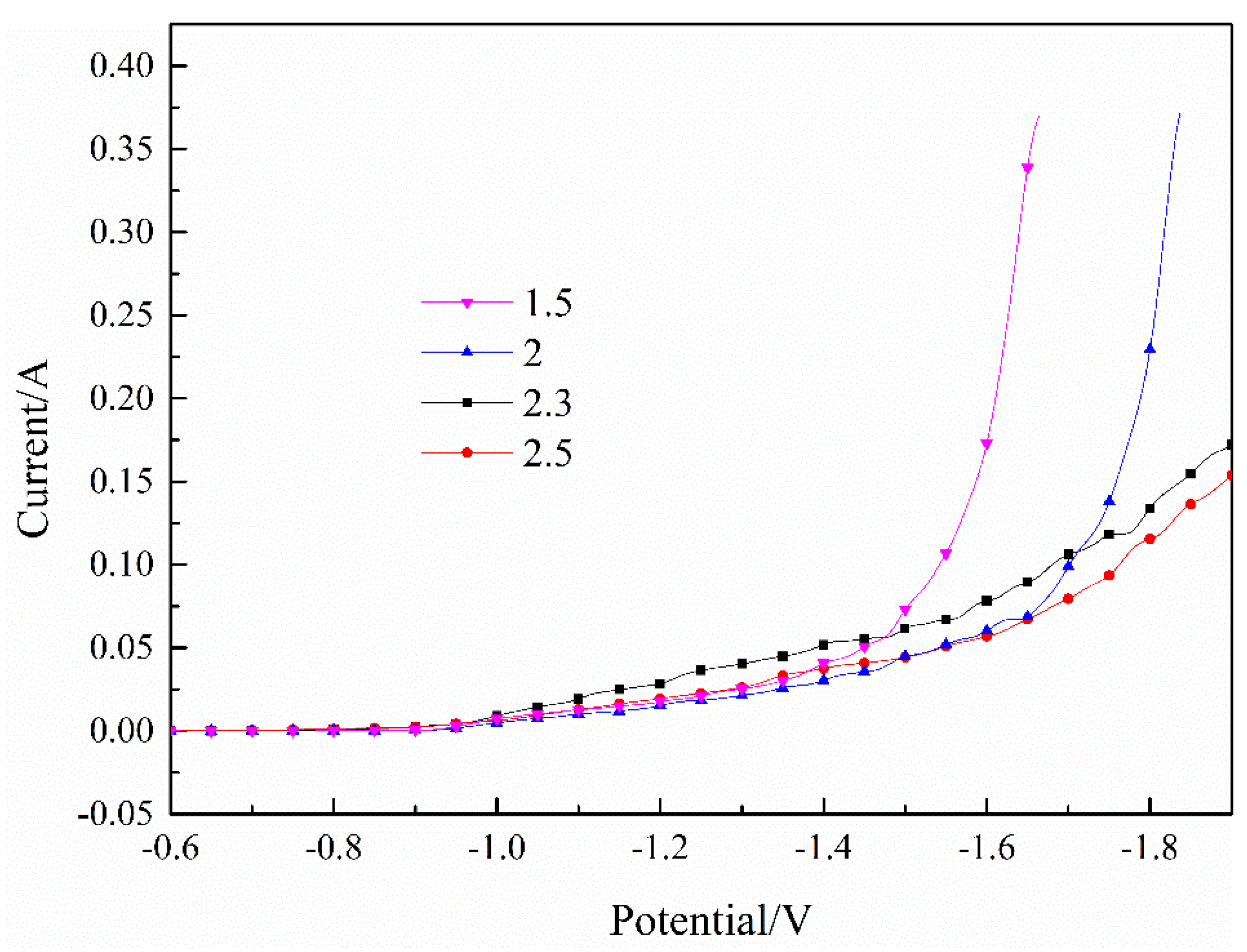
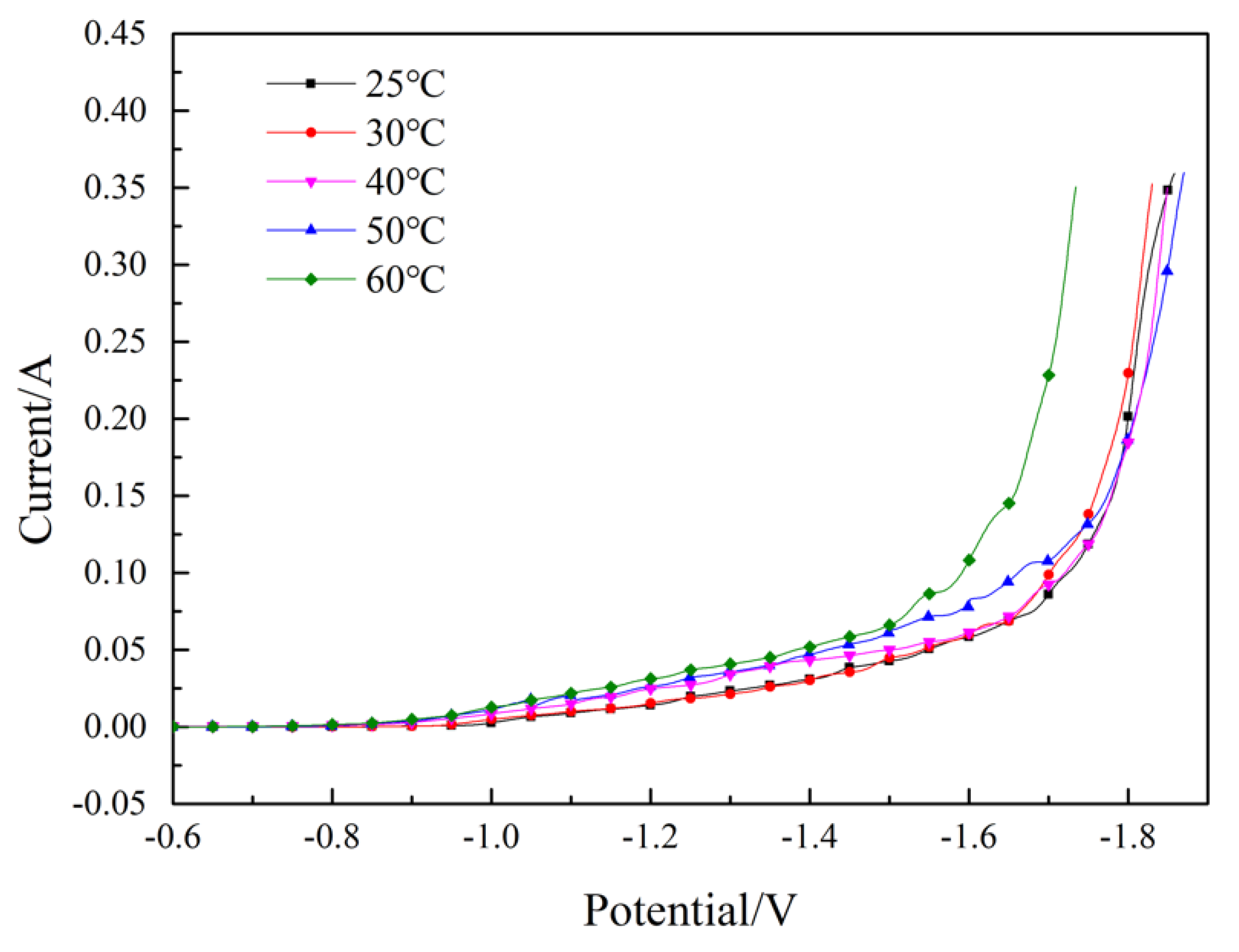
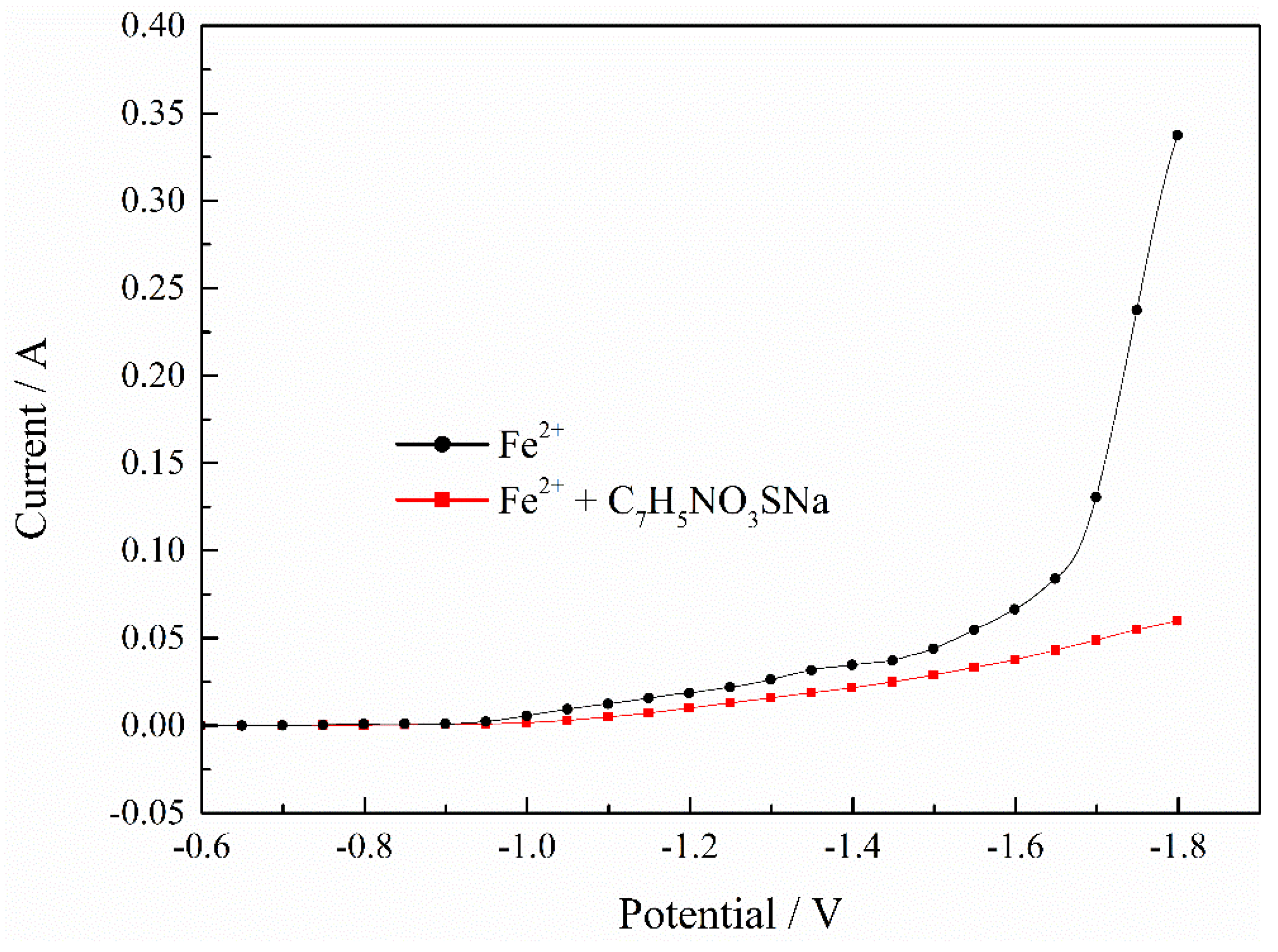


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Ji, B.; Wang, W. Reducing the Internal Stress of Fe-Ni Magnetic Film Using the Electrochemical Method. Processes 2021, 9, 1883. https://doi.org/10.3390/pr9111883
Wu Y, Ji B, Wang W. Reducing the Internal Stress of Fe-Ni Magnetic Film Using the Electrochemical Method. Processes. 2021; 9(11):1883. https://doi.org/10.3390/pr9111883
Chicago/Turabian StyleWu, Yan, Bin Ji, and Wei Wang. 2021. "Reducing the Internal Stress of Fe-Ni Magnetic Film Using the Electrochemical Method" Processes 9, no. 11: 1883. https://doi.org/10.3390/pr9111883
APA StyleWu, Y., Ji, B., & Wang, W. (2021). Reducing the Internal Stress of Fe-Ni Magnetic Film Using the Electrochemical Method. Processes, 9(11), 1883. https://doi.org/10.3390/pr9111883




