Multiobjective Optimization Based on “Distance-to-Target” Approach of Membrane Units for Separation of CO2/CH4
Abstract
:1. Introduction
2. Modeling
2.1. Model Development
- −
- The deformation of the hollow fibers under pressure is negligible;
- −
- The membrane permeability is independent of the concentration and pressure;
- −
- The pressure changes in the retentate and permeate streams in the lumen and shell sides are negligible;
- −
- The concentration polarization on both sides of the membrane is negligible;
- −
- The gas flows are evenly distributed, and the end effects resulting from flow direction changes are negligible;
- −
- The gas on the lumen and shell sides of the hollow fibers is in a plug flow;
- −
- The membrane module is operated at a steady state.
2.2. Model Validation: Determining the Number of Cells from a Reference System
3. Case Study to Optimize: Separation of CO2/CH4 with Both Components as Targets
4. Results and Discussion
4.1. Module Simulation
4.2. Module Optimization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| Ci | Components of the vector to be optimized |
| D | Euclidean distance |
| DN | Normalized distance to target (-) |
| Fin | Total feed flowrate to the module (m3(STP)/h) |
| FAin | Flow of the most permeable compound A in the feed (m3(STP)/h) |
| FBin | Flow of the least permeable compound B in the feed (m3(STP)/h) |
| Fin(i) | Total feed flowrate to the cell i (m3(STP)/h) |
| Fout | Total retentate flowrate leaving the module (m3(STP)/h) |
| FAout | Flow of the most permeable compound A in the final retentate (m3(STP)/h) |
| FBout | Flow of the least permeable compound B in the final retentate (m3(STP)/h) |
| Fout(i) | Total retentate flowrate leaving the cell i (m3(STP)/h) |
| Gi | Components of the target vector |
| J(i) | Total gas flowrate through the membrane in cell i (m3(STP)/h) |
| JA(i) | Flow of the most permeable compound A through the membrane in cell i (m3(STP)/h) |
| JB(i) | Flow of the least permeable compound B through the membrane in cell i (m3(STP)/h) |
| n | Number of objectives to be optimized (-) |
| PF | Pressure in the retentate lumen side (atm) |
| PQ | Pressure in the permeate shell side (atm) |
| Perm | Membrane permeability (GPU) |
| PermA | Specific permeability of the most permeable compound A (m3(STP)/h·m2·atm) |
| PermB | Specific permeability of the least permeable compound B (m3(STP)/h·m2·atm) |
| Qin(i) | Total permeate flowrate entering the cell i (m3(STP)/h) |
| Qout | Total permeate flowrate leaving the module (m3(STP)/h) |
| QAout | Flow of the most permeable compound A in the final permeate (m3(STP)/h) |
| QBout | Flow of the least permeable compound B in the final permeate (m3(STP)/h) |
| Qout(i) | Total permeate flowrate leaving the cell i (m3(STP)/h) |
| xA(i) | Molar fraction of compound A in the lumen side of cell i (-) |
| xAin | Molar fraction of compound A in the feed stream to the module (-) |
| xAin(i) | Molar fraction of compound A in the feed stream to cell i (-) |
| xAout | Molar fraction of compound A in the final retentate (-) |
| xB(i) | Molar fraction of compound B in the lumen side of cell i (-) |
| xBin | Molar fraction of compound B in the feed stream to the module (-) |
| xBin(i) | Molar fraction of compound B in the feed stream to cell i (-) |
| xBout | Molar fraction of compound B in the final retentate (-) |
| yA(i) | Molar fraction of compound A in the shell side of cell i (-) |
| yAin(i) | Molar fraction of compound A in the permeate stream entering the cell i (-) |
| yAout | Molar fraction of compound A in the final permeate (-) |
| yB(i) | Molar fraction of compound B in the shell side of cell i (-) |
| yBin(i) | Molar fraction of compound B in the permeate stream entering the cell i (-) |
| yBout | Molar fraction of compound B in the final permeate (-) |
| α | Membrane selectivity (-) |
References
- Salestan, S.K.; Pirzadeh, K.; Rahimpour, A.; Abedini, R. Poly (ether-block amide) thin-film membranes containing functionalized MIL-101 MOFs for efficient separation of CO2/CH4. J. Environ. Chem. Eng. 2021, 9, 105820. [Google Scholar] [CrossRef]
- Liao, Z.; Hu, Y.; Wang, J.; Yang, Y.; You, F. Systematic design and optimization of a membrane-cryogenic hybrid system for CO2 capture. ACS Sustain. Chem. Eng. 2019, 7, 17186–17197. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Hafiz Dzarfan Othman, M.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Demirciyeva, F. Comparison of biogas upgrading performances of different mixed matrix membranes. Chem. Eng. J. 2013, 222, 209–217. [Google Scholar] [CrossRef]
- Scholz, M.; Melin, T.; Wessling, M. Transforming biogas into biomethane using membrane technology. Renew. Sustain. Energy Rev. 2013, 17, 199–212. [Google Scholar] [CrossRef]
- Cerveira, G.S.; Borges, C.P.; de Kronemberger, F.A. Gas permeation applied to biogas upgrading using cellulose acetate and polydimethylsiloxane membranes. J. Clean. Prod. 2018, 187, 830–838. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Xiao, W.; Gao, P.; Dai, Y.; Ruan, X.; Jiang, X.; Wu, X.; Fang, Y.; He, G. Effciency separation process of H2/CO2/CH4 mixtures by a hollow fiber dual membrane separator. Processes 2020, 8, 560. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M.; Dose, M.E.; Freeman, B.D.; Paul, D.R. Analysis of the transport properties of thermally rearranged (TR) polymers and polymers of intrinsic microporosity (PIM) relative to upper bound performance. J. Membr. Sci. 2017, 525, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friess, K.; Lanč, M.; Pilnáček, K.; Fíla, V.; Vopička, O.; Sedláková, Z.; Cowan, M.G.; McDanel, W.M.; Noble, R.D.; Gin, D.L.; et al. CO2/CH4 separation performance of ionic-liquid-based epoxy-amine ion gel membranes under mixed feed conditions relevant to biogas processing. J. Membr. Sci. 2017, 528, 64–71. [Google Scholar] [CrossRef]
- Brunetti, A.; Cersosimo, M.; Kim, J.S.; Dong, G.; Fontananova, E.; Lee, Y.M.; Drioli, E.; Barbieri, G. Thermally rearranged mixed matrix membranes for CO2 separation: An aging study. Int. J. Greenh. Gas Control 2017, 61, 16–26. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, L.; Liu, L.; Deng, X.; Gao, Y.; Ding, Z. Ultrathin porous amine-based solid adsorbent incorporated zeolitic imidazolate framework-8 membrane for gas separation. RSC Adv. 2021, 11, 28863–28875. [Google Scholar] [CrossRef]
- Thomas, A.; Ahamed, R.; Prakash, M. Selection of a suitable ZIF-8/ionic liquid (IL) based composite for selective CO2 capture: The role of anions at the interface. RSC Adv. 2020, 10, 39160–39170. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Heydari, I.; Zhang, Z. Hybrid systems: Combining membrane and absorption technologies leads to more efficient acid gases (CO2 and H2S) removal from natural gas. J. CO2 Util. 2017, 18, 362–369. [Google Scholar] [CrossRef]
- Sampaio, A.M.; Nabais, A.R.; Tomé, L.C.; Neves, L.A. Impact of MOF-5 on Pyrrolidinium-Based Poly(ionic liquid)/Ionic Liquid Membranes for Biogas Upgrading. Ind. Eng. Chem. Res. 2020, 59, 308–317. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Mashhadikhan, S.; Sanaeepur, H.; Moghadassi, A.; Matsuura, T.; Ramakrishna, S. Substantial breakthroughs on function-led design of advanced materials used in mixed matrix membranes (MMMs): A new horizon for efficient CO2 separation. Prog. Mater. Sci. 2019, 102, 222–295. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Irabien, A. Effect of humidity on CO2/N2 and CO2/CH4 separation using novel robust mixed matrix composite hollow fiber membranes: Experimental and model evaluation. Membranes 2020, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Ameen, A.W.; Budd, P.M.; Gorgojo, P. Superglassy polymers to treat natural gas by hybrid membrane/amine processes: Can fillers help? Membranes 2020, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barquín, A.; Rea, R.; Venturi, D.; Giacinti-Baschetti, M.; De Angelis, M.G.; Casado-Coterillo, C.; Irabien, Á. Effect of relative humidity on the gas transport properties of zeolite A/PTMSP mixed matrix membranes. RSC Adv. 2018, 8, 3536–3546. [Google Scholar] [CrossRef] [Green Version]
- Casado-Coterillo, C. Mixed matrix membranes. Membranes 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casado-Coterillo, C.; Garea, A.; Irabien, Á. Effect of water and organic pollutant in CO2/CH4 separation using hydrophilic and hydrophobic composite membranes. Membranes 2020, 10, 405. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef] [Green Version]
- Ohs, B.; Lohaus, J.; Wessling, M. Optimization of membrane based nitrogen removal from natural gas. J. Membr. Sci. 2016, 498, 291–301. [Google Scholar] [CrossRef]
- Nasir, R.; Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F. Effect of ionic liquid inclusion and amino-functionalized SAPO-34 on the performance of mixed matrix membranes for CO2/CH4 separation. J. Environ. Chem. Eng. 2018, 6, 2363–2368. [Google Scholar] [CrossRef]
- Kammakakam, I.; Bara, J.E.; Jackson, E.M.; Lertxundi, J.; Mecerreyes, D.; Tomé, L.C. Tailored CO2-Philic Anionic Poly(ionic liquid) Composite Membranes: Synthesis, Characterization, and Gas Transport Properties. ACS Sustain. Chem. Eng. 2020, 8, 5954–5965. [Google Scholar] [CrossRef]
- Turgut, O.; Murat, A.E. Generating pareto surface for multi objective integer programming problems with stochastic objective coefficients. Procedia Comput. Sci. 2011, 6, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Abejón, R.; Garea, A.; Irabien, A. Multiobjective optimization of membrane processes for chemicals ultrapurification. Comput. Aided Chem. Eng. 2012, 30, 542–546. [Google Scholar] [CrossRef]
- Abejón, R.; Vázquez-Rowe, I.; Bala, A.; Fullana-i-Palmer, P.; MaríaMargallo; Aldaco, R. Application of the “Distance to Target” Approach to the Multiobjective Optimization of Nutritional and Economic Costs due to Food Loss and Waste. Comput. Aided Chem. Eng. 2020, 48, 1681–1686. [Google Scholar] [CrossRef]
- Kundu, P.K.; Chakma, A.; Feng, X. Simulation of binary gas separation with asymmetric hollow fibre membranes and case studies of air separation. Can. J. Chem. Eng. 2011, 90, 1253–1268. [Google Scholar] [CrossRef]
- Coker, D.T.; Freeman, B.D.; Fleming, G.K. Modeling multicomponent gas separation using hollow-fiber membrane contactors. AIChE J. 1998, 44, 1289–1302. [Google Scholar] [CrossRef]
- Limleamthong, P.; Guillén-Gosálbez, G. Rigorous analysis of Pareto fronts in sustainability studies based on bilevel optimization: Application to the redesign of the UK electricity mix. J. Clean. Prod. 2017, 164, 1602–1613. [Google Scholar] [CrossRef]
- Schädler, P.; Berdugo, J.D.; Hanne, T.; Dornberger, R. A Distance-Based Pareto Evolutionary Algorithm Based on SPEA for Combinatorial Problems. In Proceedings of the 4th International Symposium on Computational and Business Intelligence, Windisch, Switzerland, 5–7 September 2016; pp. 112–117. [Google Scholar]
- Feng, X.; Ivory, J.; Rajan, V.S.V. Air separation by integrally asymmetric hollow-fiber membranes. AIChE J. 1999, 45, 2142–2152. [Google Scholar] [CrossRef]
- Altintas, C.; Keskin, S. Molecular Simulations of MOF Membranes and Performance Predictions of MOF/Polymer Mixed Matrix Membranes for CO2/CH4 Separations. ACS Sustain. Chem. Eng. 2019, 7, 2739–2750. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Etxeberria-Benavides, M.; Zuñiga, J.; Irabien, A. Comparison of Flat and Hollow-Fiber Mixed-Matrix Composite Membranes for CO2 Separation with Temperature. Chem. Eng. Technol. 2017, 40, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.; Alders, M.; Lohaus, T.; Wessling, M. Structural optimization of membrane-based biogas upgrading processes. J. Membr. Sci. 2015, 474, 1–10. [Google Scholar] [CrossRef]
- Harasimowicz, M.; Orluk, P.; Zakrzewska-Trznadel, G.; Chmielewski, A.G. Application of polyimide membranes for biogas purification and enrichment. J. Hazard. Mater. 2007, 144, 698–702. [Google Scholar] [CrossRef]
- Molino, A.; Nanna, F.; Migliori, M.; Iovane, P.; Ding, Y.; Bikson, B. Experimental and simulation results for biomethane production using peek hollow fiber membrane. Fuel 2013, 112, 489–493. [Google Scholar] [CrossRef]
- Melone, L.; Giorno, L.; Brunetti, A.; Barbieri, G. Analysis of membrane unit performance in presence of wet CO2-containing mixtures. Chem. Eng. Res. Des. 2020, 153, 721–727. [Google Scholar] [CrossRef]
- Vilardi, G.; Bassano, C.; Deiana, P.; Verdone, N. Exergy and energy analysis of biogas upgrading by pressure swing adsorption: Dynamic analysis of the process. Energy Convers. Manag. 2020, 226, 113482. [Google Scholar] [CrossRef]
- Zhang, C.; Sheng, M.; Hu, Y.; Yuan, Y.; Kang, Y.; Sun, X.; Wang, T.; Li, Q.; Zhao, X.; Wang, Z. Efficient facilitated transport polymer membrane for CO2/CH4 separation from oilfield associated gas. Membranes 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Aliaga-Vicente, A.; Caballero, J.A.; Fernández-Torres, J. Synthesis and Optimization of Membrane Cascade for Gas Separation via Mixed-Integer Nonlinear Programming. AIChE J. 2017, 63, 1989–2006. [Google Scholar] [CrossRef] [Green Version]
- Seong, M.S.; Kong, C.I.; Park, B.R.; Lee, Y.; Na, B.K.; Kim, J.H. Optimization of pilot-scale 3-stage membrane process using asymmetric polysulfone hollow fiber membranes for production of high-purity CH4 and CO2 from crude biogas. Chem. Eng. J. 2020, 384, 123342. [Google Scholar] [CrossRef]
- Shin, M.S.; Jung, K.H.; Kwag, J.H.; Jeon, Y.W. Biogas separation using a membrane gas separator: Focus on CO2 upgrading without CH4 loss. Process Saf. Environ. Prot. 2019, 129, 348–358. [Google Scholar] [CrossRef]
- Favre, E.; Bounaceur, R.; Roizard, D. Biogas, membranes and carbon dioxide capture. J. Membr. Sci. 2009, 328, 11–14. [Google Scholar] [CrossRef]
- Singhal, S.; Agarwal, S.; Arora, S.; Sharma, P.; Singhal, N. Upgrading techniques for transformation of biogas to bio-CNG: A review. Int. J. Energy Res. 2017, 41, 1657–1669. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Winston Ho, W.S. Recent progress in the engineering of polymeric membranes for CO2 capture from flue gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef] [PubMed]




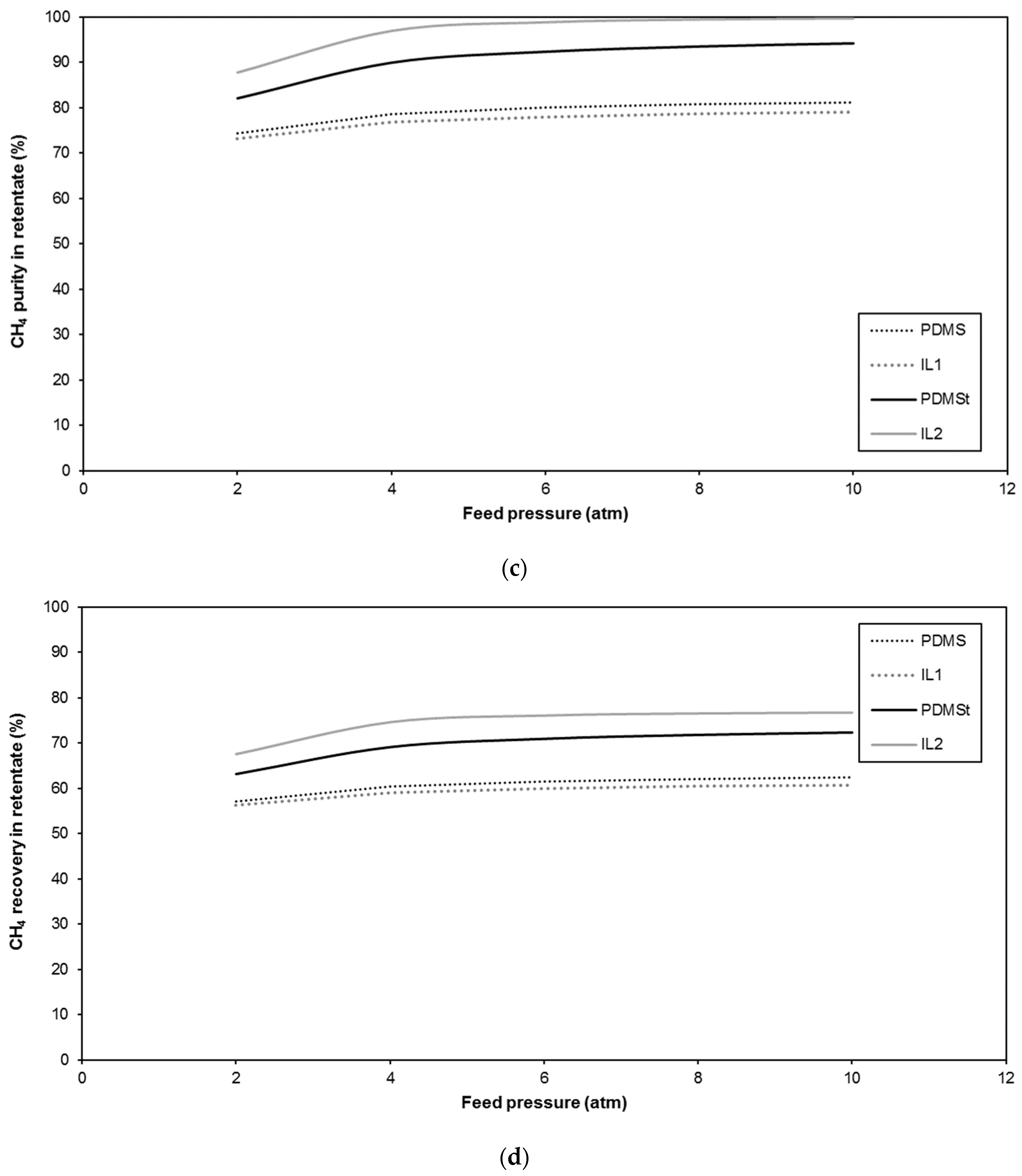

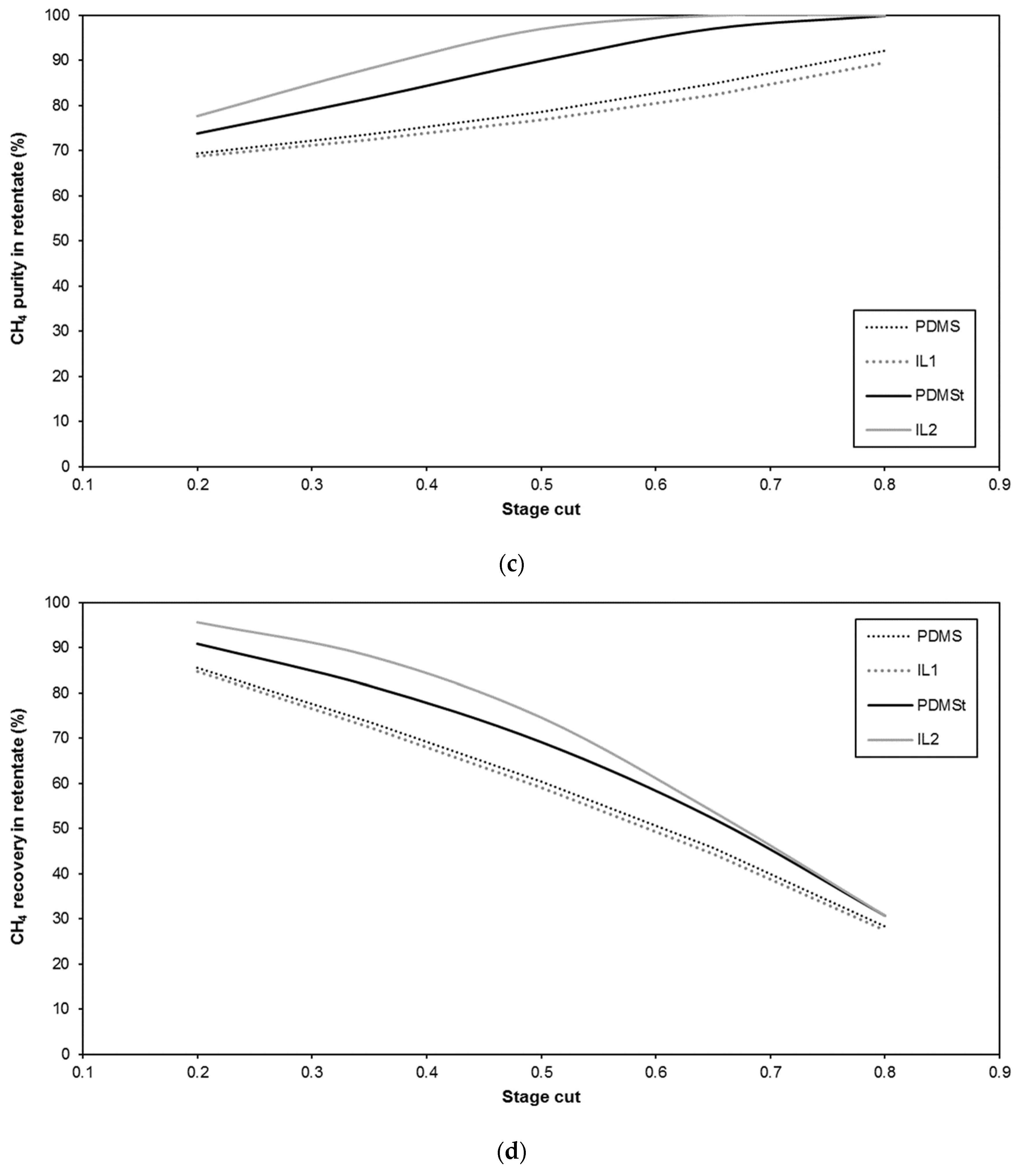
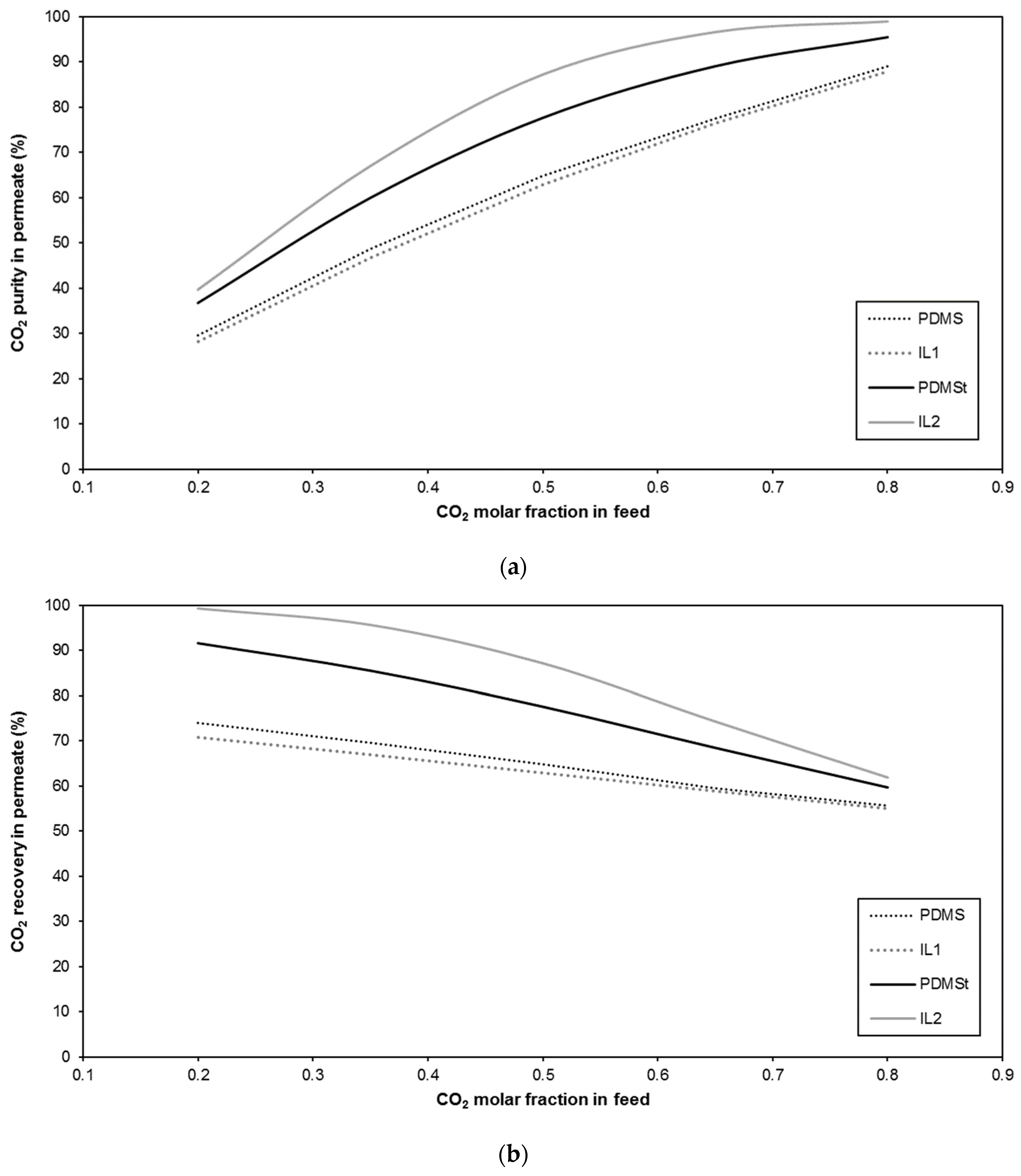
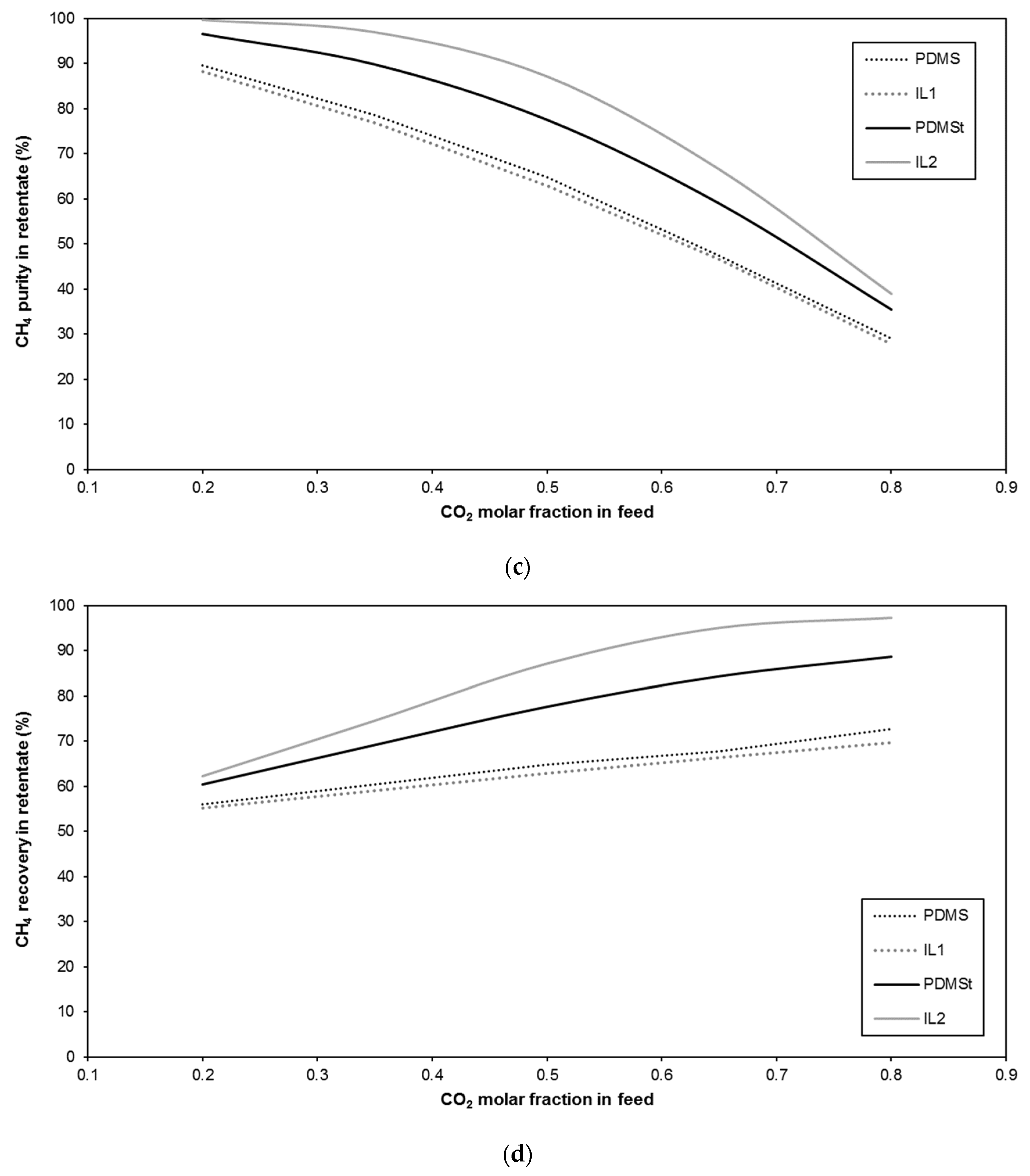
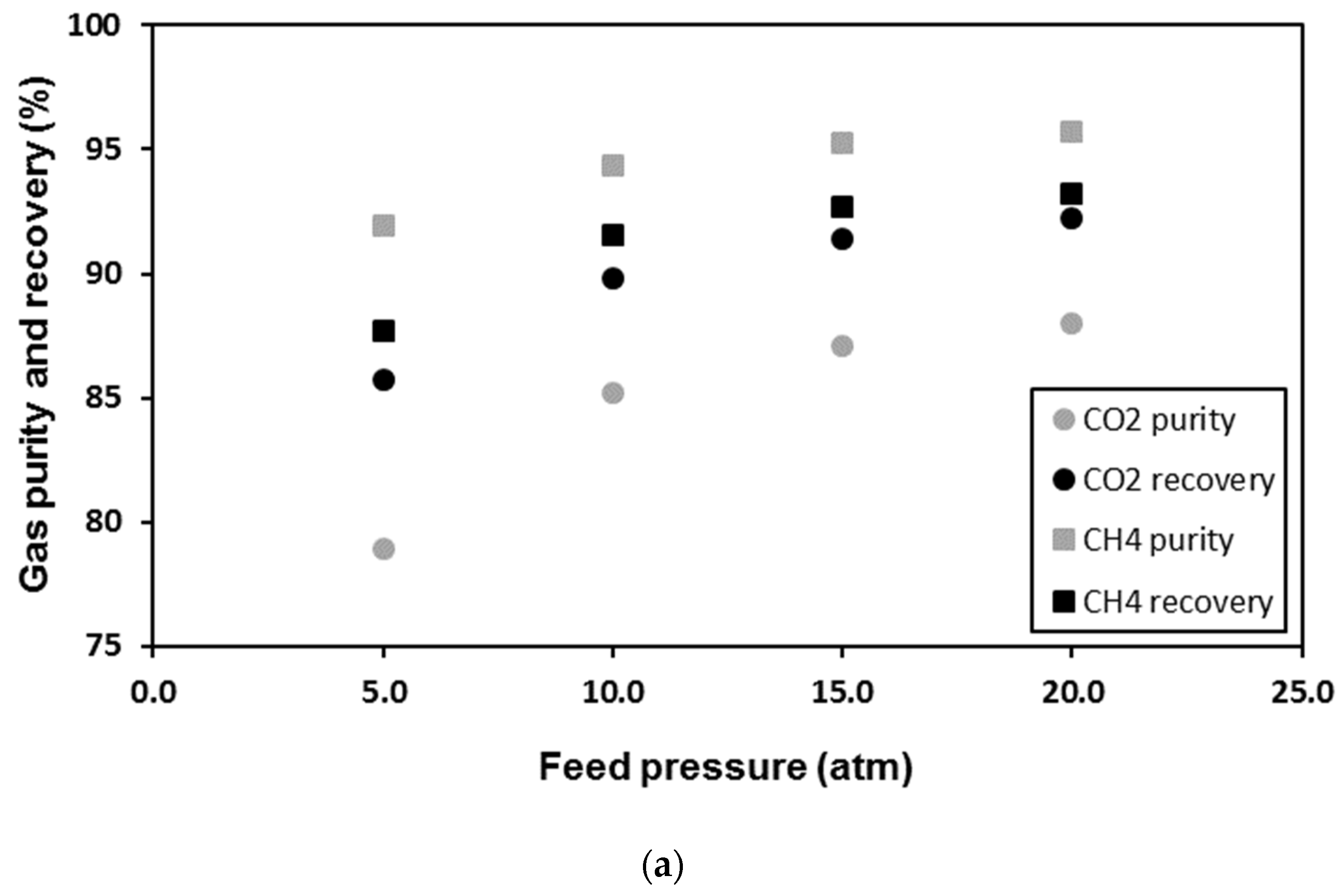
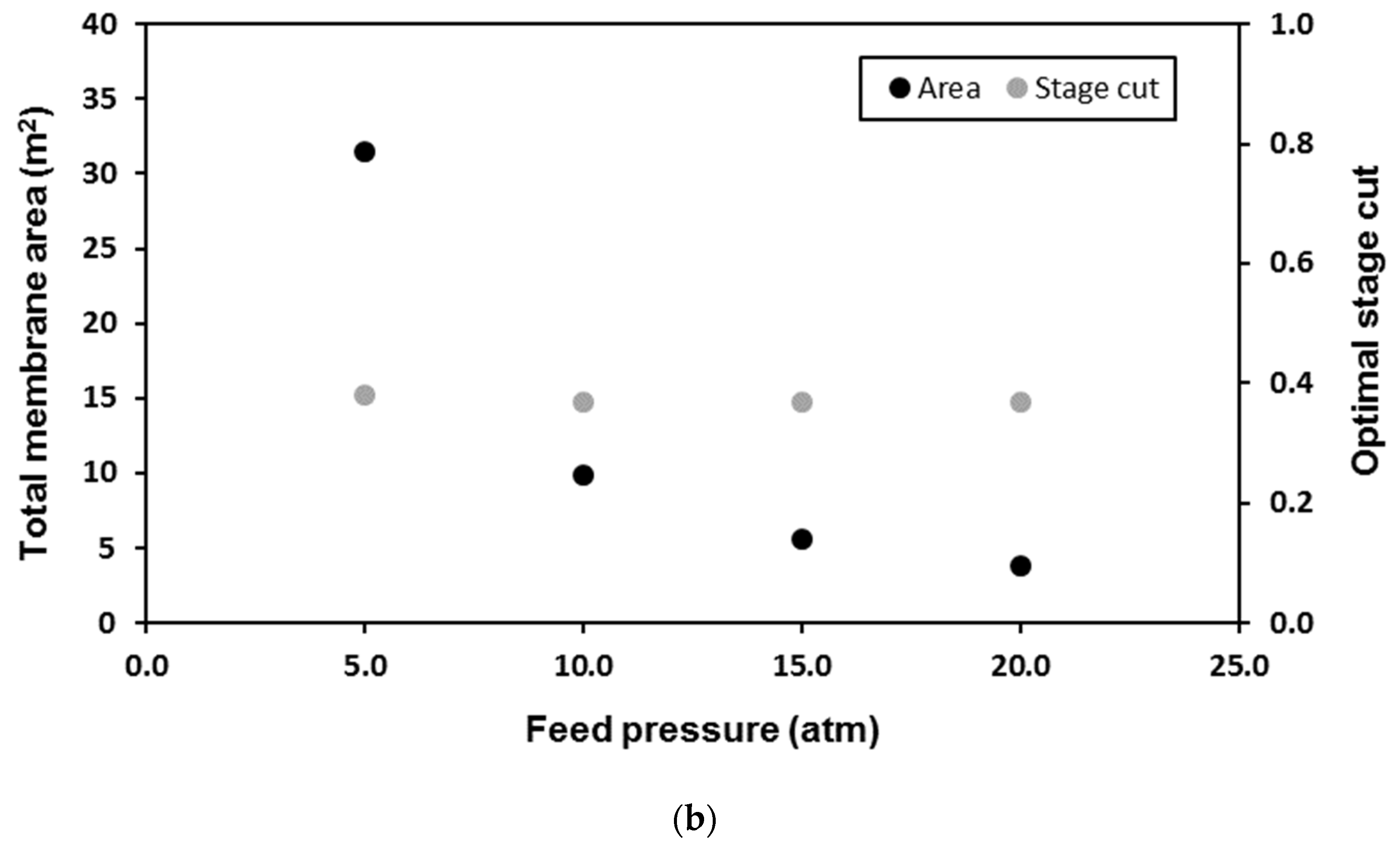
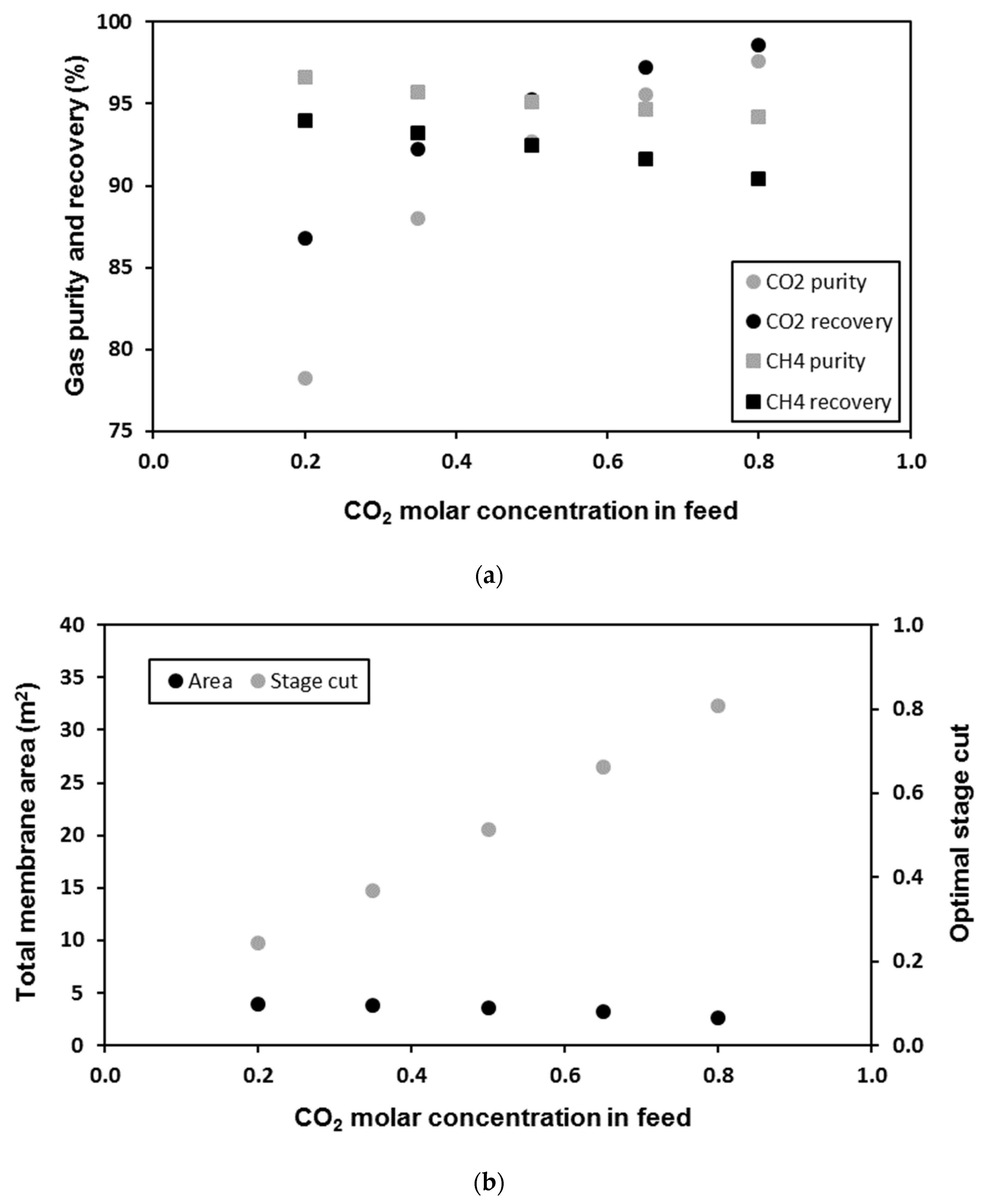
| Membrane | CO2 Permeance (GPU) | CO2/CH4 Selectivity (-) | Reference |
|---|---|---|---|
| PDMS (commercial) | 266 | 3.1 | [24] |
| PDMSt (modified) | 73.7 | 10.7 | [24] |
| IL1 (IL-CS self-standing) | 8.5 | 2.7 | [28,42] |
| IL2 (IL-CS/PES) | 102 | 54.8 | [28,42] |
| Pressure Ratio (PF/PQ) | 2.0/0.5 | 4.0/1.0 | 8.0/2.0 |
|---|---|---|---|
| CO2 purity (%) | 67.0 | ||
| CO2 recovery (%) | 95.8 | ||
| CH4 purity (%) | 97.0 | ||
| CH4 recovery (%) | 74.6 | ||
| Membrane area (m2) | 167.6 | 83.8 | 41.9 |
| Membrane | Objectives | Values | Stage Cut | PF (atm) | PQ (atm) | Area (m2) | DN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 Purity | CO2 Recovery | CH4 Purity | CH4 Recovery | CO2 Purity | CO2 Recovery | CH4 Purity | CH4 Recovery | ||||||
| PDMS | X | 56.2 | 8.0 | 66.1 | 96.6 | 0.050 | 1.0 | 0.2 | 1.2 | 0.537 | |||
| X | 36.8 | 99.9 | 99.2 | 7.6 | 0.950 | 1.0 | 0.2 | 28.7 | 0.560 | ||||
| X | X | 40.5 | 97.9 | 95.3 | 22.5 | 0.846 | 1.0 | 0.2 | 24.7 | 0.489 | |||
| X | 36.8 | 99.9 | 99.2 | 7.6 | 0.950 | 1.0 | 0.2 | 28.7 | 0.560 | ||||
| X | 56.2 | 8.0 | 66.1 | 96.6 | 0.050 | 1.0 | 0.2 | 1.2 | 0.537 | ||||
| X | X | 56.2 | 8.0 | 66.1 | 96.6 | 0.050 | 1.0 | 0.2 | 1.2 | 0.537 | |||
| X | X | X | X | 46.2 | 85.9 | 85.8 | 46.2 | 0.650 | 1.0 | 0.2 | 17.9 | 0.393 | |
| Minimal DN | 49.8 | 69.0 | 78.9 | 62.6 | 0.485 | 1.0 | 0.2 | 12.9 | 0.365 | ||||
| PDMSt | X | 76.5 | 10.9 | 67.2 | 98.2 | 0.050 | 1.0 | 0.2 | 7.6 | 0.489 | |||
| X | 42.2 | 100.0 * | 100.0 | 26.3 | 0.829 | 1.0 | 0.2 | 253.0 | 0.468 | ||||
| X | X | 56.1 | 95.3 | 96.0 | 59.9 | 0.594 | 1.0 | 0.2 | 144.3 | 0.299 | |||
| X | 42.2 | 100.0 | 100.0 * | 26.3 | 0.829 | 1.0 | 0.2 | 253.0 | 0.468 | ||||
| X | 76.5 | 10.9 | 67.2 | 98.2 | 0.050 | 1.0 | 0.2 | 7.6 | 0.489 | ||||
| X | X | 71.9 | 48.6 | 76.4 | 89.8 | 0.237 | 1.0 | 0.2 | 40.8 | 0.320 | |||
| X | X | X | X | 61.0 | 88.4 | 91.7 | 69.6 | 0.507 | 1.0 | 0.2 | 111.9 | 0.257 | |
| Minimal DN | 64.9 | 79.1 | 87.2 | 77.0 | 0.426 | 1.0 | 0.2 | 86.8 | 0.243 | ||||
| IL2 | X | 92.6 | 13.2 | 68.0 | 99.4 | 0.050 | 1.0 | 0.2 | 8.3 | 0.464 | |||
| X | 55.6 | 100.0 * | 100.0 | 56.9 | 0.630 | 1.0 | 0.2 | 526.6 | 0.310 | ||||
| X | X | 73.0 | 94.0 | 96.2 | 81.3 | 0.450 | 1.0 | 0.2 | 234.6 | 0.168 | |||
| X | 55.6 | 100.0 | 100.0 * | 56.9 | 0.630 | 1.0 | 0.2 | 526.6 | 0.310 | ||||
| X | 92.6 | 13.2 | 68.0 | 99.4 | 0.050 | 1.0 | 0.2 | 8.3 | 0.464 | ||||
| X | X | 80.5 | 82.7 | 90.5 | 89.2 | 0.360 | 1.0 | 0.2 | 139.0 | 0.149 | |||
| X | X | X | X | 76.0 | 90.5 | 94.3 | 84.6 | 0.417 | 1.0 | 0.2 | 194.9 | 0.153 | |
| Minimal DN | 78.9 | 85.7 | 91.9 | 87.7 | 0.380 | 1.0 | 0.2 | 157.2 | 0.147 | ||||
| Membrane | Objectives | Values | Stage Cut | PF (atm) | PQ (atm) | Area (m2) | DN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 Purity | CO2 Recovery | CH4 Purity | CH4 Recovery | CO2 Purity | CO2 Recovery | CH4 Purity | CH4 Recovery | ||||||
| PDMS | X | 60.6 | 8.7 | 66.3 | 97.0 | 0.050 | 20 | 1 | 0.050 | 0.525 | |||
| X | 36.8 | 99.9 | 99.6 | 7.7 | 0.950 | 20 | 1 | 1.206 | 0.559 | ||||
| X | X | 42.7 | 97.2 | 95.1 | 29.8 | 0.796 | 20 | 1 | 0.957 | 0.454 | |||
| X | 36.8 | 99.9 | 99.6 | 7.7 | 0.950 | 20 | 1 | 1.206 | 0.559 | ||||
| X | 60.6 | 8.7 | 66.3 | 97.0 | 0.050 | 20 | 1 | 0.050 | 0.525 | ||||
| X | X | 60.6 | 8.7 | 66.3 | 97.0 | 0.050 | 20 | 1 | 0.050 | 0.525 | |||
| X | X | X | X | 49.1 | 84.8 | 86.6 | 52.7 | 0.604 | 20 | 1 | 0.681 | 0.362 | |
| Minimal DN | 52.7 | 70.9 | 80.7 | 65.8 | 0.470 | 20 | 1 | 0.511 | 0.340 | ||||
| PDMSt | X | 82.8 | 11.8 | 67.5 | 98.7 | 0.050 | 20 | 1 | 0.260 | 0.478 | |||
| X | 45.7 | * 100.0 | 100.0 | 36.0 | 0.766 | 20 | 1 | 4.334 | 0.420 | ||||
| X | X | 64.7 | 94.4 | 96.0 | 72.3 | 0.511 | 20 | 1 | 4.392 | 0.227 | |||
| X | 45.7 | 100.0 | * 100.0 | 36.0 | 0.766 | 20 | 1 | 4.334 | 0.420 | ||||
| X | 82.8 | 11.8 | 67.5 | 98.7 | 0.050 | 20 | 1 | 0.260 | 0.478 | ||||
| X | X | 73.4 | 77.7 | 87.6 | 84.9 | 0.370 | 20 | 1 | 2.579 | 0.199 | |||
| X | X | X | X | 68.1 | 90.2 | 93.6 | 77.2 | 0.464 | 20 | 1 | 3.696 | 0.205 | |
| Minimal DN | 71.5 | 83.3 | 90.1 | 82.1 | 0.408 | 20 | 1 | 2.987 | 0.194 | ||||
| IL2 | X | 95.9 | 13.7 | 68.2 | 99.7 | 0.050 | 20 | 1 | 0.225 | 0.460 | |||
| X | 68.5 | * 100.0 | 100.0 | 75.2 | 0.511 | 20 | 1 | 12.959 | 0.200 | ||||
| X | X | 85.5 | 95.5 | 97.4 | 91.3 | 0.391 | 20 | 1 | 4.858 | 0.088 | |||
| X | 68.5 | 100.0 | * 100.0 | 75.2 | 0.511 | 20 | 1 | 12.959 | 0.200 | ||||
| X | 95.9 | 13.7 | 68.2 | 99.7 | 0.050 | 20 | 1 | 0.230 | 0.460 | ||||
| X | X | 87.4 | 93.3 | 96.2 | 92.7 | 0.374 | 20 | 1 | 4.114 | 0.082 | |||
| X | X | X | X | 86.2 | 94.8 | 97.0 | 91.9 | 0.385 | 20 | 1 | 4.560 | 0.085 | |
| Minimal DN | 88.0 | 92.3 | 95.7 | 93.2 | 0.367 | 20 | 1 | 3.865 | 0.082 | ||||
| Membrane | Permeability (GPU) | Selectivity CO2/CH4 | CO2 Purity | CO2 Recovery | CH4 Purity | CH4 Recovery | Stage Cut | PF (atm) | PQ (atm) | Area (m2) | DN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PDMS | 266 | 3.1 | 49.1 | 84.8 | 86.6 | 52.7 | 0.604 | 20 | 1 | 0.681 | 0.362 |
| PDMSt | 73.7 | 10.7 | 68.1 | 90.2 | 93.6 | 77.2 | 0.464 | 20 | 1 | 3.696 | 0.205 |
| IL2 | 102 | 54.8 | 86.2 | 94.8 | 97.0 | 91.9 | 0.385 | 20 | 1 | 4.560 | 0.085 |
| Ref [49] | 37 | 24.8 | 78.9 | 93.0 | 95.8 | 86.6 | 0.412 | 20 | 1 | 9.654 | 0.131 |
| Ref [50] | 103 | 39 | 83.4 | 94.1 | 96.6 | 89.9 | 0.395 | 20 | 1 | 4.023 | 0.103 |
| Ref [51] | 158 | 27 | 79.8 | 93.2 | 96.0 | 87.3 | 0.409 | 20 | 1 | 2.324 | 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abejón, R.; Casado-Coterillo, C.; Garea, A. Multiobjective Optimization Based on “Distance-to-Target” Approach of Membrane Units for Separation of CO2/CH4. Processes 2021, 9, 1871. https://doi.org/10.3390/pr9111871
Abejón R, Casado-Coterillo C, Garea A. Multiobjective Optimization Based on “Distance-to-Target” Approach of Membrane Units for Separation of CO2/CH4. Processes. 2021; 9(11):1871. https://doi.org/10.3390/pr9111871
Chicago/Turabian StyleAbejón, Ricardo, Clara Casado-Coterillo, and Aurora Garea. 2021. "Multiobjective Optimization Based on “Distance-to-Target” Approach of Membrane Units for Separation of CO2/CH4" Processes 9, no. 11: 1871. https://doi.org/10.3390/pr9111871
APA StyleAbejón, R., Casado-Coterillo, C., & Garea, A. (2021). Multiobjective Optimization Based on “Distance-to-Target” Approach of Membrane Units for Separation of CO2/CH4. Processes, 9(11), 1871. https://doi.org/10.3390/pr9111871








