Abstract
Fe3O4 nanoparticles with cluster structure are superparamagnetic particles with applicability in various high-tech fields. In this study, the influence of surface modification with polyacrylic acid (PAA), a polymeric precursor, on the characteristics of Fe3O4 nanoparticles was investigated. The particles were synthesized by the polyol method and surface modified with various amounts of PAA. The surficial, structural, optical, and magnetic properties of the PAA-modified Fe3O4 nanoparticles were analyzed, confirming that negatively charged carboxyl groups were formed on the particle surface, and the particle dispersibility was enhanced by surface modification. This arises from an increase in the electrostatic repulsive force due to the surface functional groups. Functionalization promoted dissociation of the cluster particles, which became more pronounced as the PAA content increased. The optical parameters changed with the PAA content. Analysis of the magnetic properties showed that the saturation magnetization decreased as the PAA content increased. Overall, PAA modification induces structural changes of the Fe3O4 nanoparticles that enhance the dispersibility and influence the characteristics of the particles.
1. Introduction
Superparamagnetic nanoparticles are a group of materials that have received significant attention because of their unique physical properties, distinctive magnetic properties, and high magnetic susceptibility [1,2]. Fe3O4 nanoparticles, representing these superparamagnetic nanoparticles, are of interest in various high-tech fields, and specifically in medical science applications including biosensors [3], detection of cancer cells [4,5], magnetic resonance imaging (MRI) [6], drug delivery systems [7], hyperthermia treatment [8], next-generation separation material processes such as membrane filtration [9] and membrane adsorption [10], as well as eco-friendly inorganic pigments [11,12]. The utility of Fe3O4 is enhanced by conferring new functionality to the material itself. Therefore, to achieve new functions for innovative purposes, specific functional groups are appended the surface of the material via surface treatment; this allows the construction of organic/inorganic complexes and modification of the surface properties [13]. Thus, surface treatment is the basis for imparting special functions to particles or expanding the scope of application [14]. The functional groups formed by surface treatment serve as activation sites for the complexation of heterogeneous materials on the particle surface, or provide a function that enables bonding with a specific organic structure. Representative functional groups formed on the particle surface by surface treatment include hydroxyl, amine, and carboxyl groups. Among them, negatively charged carboxyl groups have high affinity for cations [15]. For this reason, studies have reported the introduction of carboxyl groups on the surface of Fe3O4, and applied carboxyl-functionalized Fe3O4 in various fields. Barick et al. reported the development of carboxyl-decorated Fe3O4 nanoparticles for MRI and hyperthermia applications [16]. In that study, carboxyl groups were introduced onto the surface of glycin-functionalized Fe3O4 to provide monodispersity, colloidal stability, and biocompatibility in aqueous and physiological media. After surface treatment, the Fe3O4 nanoparticles exhibited suitable colloidal stability in aqueous and physiological media due to the increased electrostatic repulsive force induced by the carboxyl groups on the surface, and it was confirmed that these particles exhibited resistance to bovine serum albumin (BSA) protein due to the negative surface charge. Functionalized Fe3O4 was also confirmed to be appropriate for application in various biomolecule/drug conjugates due to the high density of the surface functional groups. Kim et al. investigated the carboxyl functionalization of Fe3O4 nanoparticles for oligonucleotide immobilization [17]. Fe3O4 nanoparticles were functionalized with two types of reagents: 3-thiopheneacetic acid (3TA) and meso-2,3-dimercaptosuccinic acid (DMSA). After surface treatment, the hydrophilic properties of Fe3O4 were improved owing to the large amount of carboxyl groups formed on the surface, and high dispersibility in aqueous solution was demonstrated. Moreover, oligonucleotides were more efficiently immobilized on these particles than on Fe3O4 without surface treatment, and it was confirmed that DMSA was the most stable carboxylic chelate for effectively bonding and immobilizing the oligonucleotides. Tian et al. modified Fe3O4 with PAA and applied as nano additives to investigate the effect on the fluid loss and rheology of water-based drilling fluids (WBDF) [18]. After the surface modification, WBDF had enhanced filtration properties by decreased fluid loss about 31.8% and dense filter cake by incorporating 0.1 wt % of Fe3O4/PAA nanoparticles.
In the case of Fe3O4 nanoparticles, the internal structural characteristics of the particles may change due to changes in the surface charge of the internal particles via surface treatment. Therefore, the correlation between the structural changes and physical properties of cluster nanoparticles due to the surface treatment has been widely investigated. Marín et al. reported the influence of the surface treatment of Fe3O4 nanoparticles on the magnetic properties [19]. The surface of the Fe3O4 nanoparticles was treated using two types of salts, and the change in the magnetic properties after surface treatment was considered. After surface treatment, negatively charged carboxyl groups were formed on the surface of the particles, and the magnetic properties of the Fe3O4 nanoparticles changed according to the surface modifier. Radoń et al. investigated the influence of organic modifiers on the structure and optical properties of Fe3O4 nanoparticles synthesized by a co-precipitation method [20]. After surface treatment, the size of the Fe3O4 nanoparticles decreased due to the influence of the organic modifiers; the modified nanoparticles exhibited low crystallinity and volume size, and a high optical bandgap energy. The results confirmed that the particle size, shape, and optical properties were changed by surface treatment using organic modifiers. Thus, these prior studies consistently illustrate changes in the structure and properties of Fe3O4 nanoparticles via surface treatment. However, most of these prior studies have not focused on the specific change of structure and properties according to condition of surface functionalization such as amounts of surface modifier. For this reason, this study focused on the structural changes of Fe3O4 nanoparticles cluster structure synthesized by polyol method according to the amounts of carboxyl precursor, and the effect of the changes on the characteristics of Fe3O4.
In this study, the effect of surface treatment with carboxyl groups on the characteristics of Fe3O4 nanoparticles with cluster structure is reported. Fe3O4 nanoparticles are surface modified with various contents of polyacrylic acid (PAA), a representative polymer precursor. The correlation between the surface treatment conditions and morphological change of the particles and the formation of functional groups on the particle surface is discussed. Additionally, the effect of surface modification on the dispersibility of the particles in aqueous solution and on the surface properties with variation of the synthesis conditions is discussed. The color variations associated with changes in the cluster morphology and carboxylation are discussed based on optical analysis, and the change in the physical magnetic properties are evaluated. Therefore, the optimal conditions for carboxylation of Fe3O4 nanoparticles to achieve the desired optical and magnetic properties, as well as aqueous dispersibility, are presented by comprehensive analysis of the characteristics arising from controlled surface modification.
2. Materials and Methods
2.1. Materials
Ferric chloride hexahydrate (FeCl3·6H2O, >97.0%, Samchun Pure Chemical Co., Ltd., Gyeonggi-do, Korea), sodium acetate (NaOAc, >99.5%, Sigma Aldrich, St. Louis, MO, USA), ethylene glycol (EG, >99.5%, Samchun Pure Chemical Co., Ltd., Gyeonggi-do, Korea), and polyacrylic acid (PAA, average Mw ~450,000, Sigma Aldrich, St. Louis, MO, USA) were purchased from the named suppliers and were used without further purification.
2.2. Synthesis of Fe3O4 Magnetic Nanoparticles
Fe3O4 nanoparticles (approximately 300 nm in size) were synthesized by the polyol method proposed in a previous study [21]. FeCl3·6H2O (45 g) was dissolved in distilled water by stirring at room temperature for 1 h. The solution was poured into a 3 L three-neck round-bottom flask containing NaOAc (0.5 M) and EG (1000 mL). The mixture was completely mixed at 100 rpm for approximately 1 h using a mechanical stirrer and was continuously agitated at its boiling point for 18 h. The agitation was stopped when the mixture changed from yellow-brown to black, and the mixture was cooled naturally to room temperature. The synthesized Fe3O4 particles were separated from the liquid using an external magnet. The separated particles were rinsed 5 times by ultrasonication for 7 min with ethanol and distilled water.
2.3. Surface Modification of Fe3O4 Nanoparticles Using PAA Solutions
PAA solutions with various concentrations were prepared to modify the surface of the Fe3O4 particles. The PAA solutions (100 mL; 1, 3, 5, and 10 wt %) were prepared by dissolving the required amount of PAA in distilled water by stirring at 300–600 rpm, depending on the viscosity, using a magnetic stirrer. The as-prepared Fe3O4 nanoparticles (1 g) were mixed with the PAA solutions. Each suspension was transferred into a three-neck round-bottom flask and agitated at 300 rpm for 30 min. The mixture was allowed to react at 75 °C for 4 h while maintaining agitation. Thereafter, the mixture was allowed to cool naturally to room temperature. The reacted particles were separated by collection using an external magnet, and the residue was washed several times using distilled water.
2.4. Characterization
The surface characteristics of the samples modified with various PAA contents were analyzed using Fourier-transform infrared (FTIR) spectroscopy (IRAffinity-1, Shimadzu, Kyoto, Japan). The zeta potential, particle size distribution (PSD), and polydispersity index (PDI) of the samples were measured by the dynamic light scattering method (Zetasizer Nano ZSP, Malvern Instrument Ltd., Malvern, WR, UK) to analyze the changes in the surface charge and dispersion properties before and after modification. The change in the microstructure of the Fe3O4 particles after modification was observed using a scanning electron microscope (SEM) (S-4700, HITACHI, Tokyo, Japan) and transmission electron microscope (TEM) (Tecnai G2 F30 S-Twin, FEI, Eindhoven, Netherlands). The surface chemistry of samples was analyzed by the X-ray photoelectron spectroscope (XPS) (Sigma Probe, Thermo VG Scientific, Waltham, MA, USA) with an X-ray source using monochromatic Al Kα (1486.6 eV) under vacuum (<5×10-10 mbar). The Fe3O4 particles were fixed to the sample holder using carbon tape. All binding energies were calibrated with inherent carbon peak (C 1s, 284.8 eV). Peaks of the Fe 2p, C 1s, and O 1s spectra were fitted using a 30% Lorentzian and 70% Gaussian peak shape after the subtraction with use of the Shirley background using CasaXPS software. The CIE L*, a*, and b* color parameter measurements were performed using UV-visible spectroscopy (UV2600, Shimadzu, Kyoto, Japan). A vibrating sample magnetometer (VSM) (Lake Shore 7400, Lake Shore Cryotronics, Inc., Westerville, OH, USA) was used to measure the magnetic parameters of the as-prepared and modified particles at −10 to +10 kOe.
3. Results and Discussion
The FTIR spectra of as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA are shown in Figure 1. In the spectrum of neat PAA, the peaks were observed at 1448 and 2959 cm−1 attributed to CH2 stretching and C–H stretching modes, respectively. In addition, the peaks ascribed to C–O and C=O stretching modes in carboxyl groups were observed at 1160 and 1704 cm−1, respectively [22]. Near the range of 2760–3744 cm−1, the broad peak associated with the O–H stretching mode was observed. The spectra of Fe3O4 samples show the peak of the Fe–O stretching vibration at 594 cm−1, confirming that the particles were based on iron oxide [23]. After surface modification with PAA, new peaks associated with CH2 stretching and C–H stretching modes were observed at 1448 and 2959 cm−1, respectively. These peaks are ascribed to the organic polymer structure of PAA, the precursor. Other new peaks associated with the C–O symmetric stretching mode were observed at 1048 and 1406 cm−1. The peaks assigned to the C–OH stretching mode and C=O stretching mode were observed at 1048, 1642, and 1704 cm−1, respectively. In addition, the broad peak observed near 3435 cm−1 was associated with the O–H stretching mode. These peaks are attributed to carboxylic acid or carboxyl groups at the terminal of the PAA structure [24,25]. It is deduced that the surface was successfully modified, where the organic structures of PAA caused a change in the particle surface due to the reaction between the Fe3O4 nanoparticles and the precursor PAA, based on observation of the new peaks in the spectrum of the treated particles.
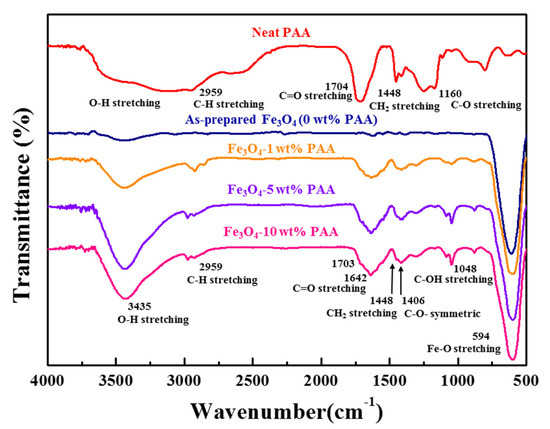
Figure 1.
FTIR spectra of as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA.
Figure 2 shows the results of the zeta potential and PSD measurements for as-prepared Fe3O4 and Fe3O4 modified with PAA. The zeta potential of as-prepared Fe3O4 was measured to be −1.8 mV. The zeta potential of PAA-modified Fe3O4 was −37.4 mV (1 wt %), −48.6 mV (5 wt %), and −48.3 mV (10 wt %). The zeta potential decreased as the PAA content increased, and the zeta potentials of Fe3O4 modified with 5 wt % and 10 wt % PAA were similar. The FTIR spectra (Figure 1), which contain information about the bonds on the particle surface, showed a change in the intensity of the adsorption of specific bonds as the precursor content changed. The peaks of the carboxyl groups of Fe3O4 modified with 5 wt % PAA were more intense than those of the particles modified with 1 wt % PAA. However, there were no significant differences in the intensity at the same wavenumber for the particles modified with 5 and 10 wt % PAA. The rate of change of the negative zeta potential also reflects this behavior.
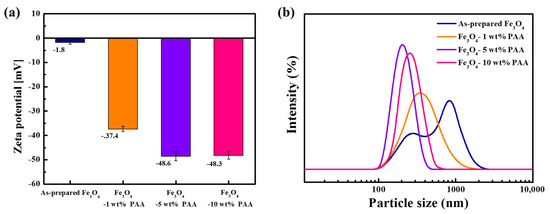
Figure 2.
(a) Zeta potentials; (b) PSDs of as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA.
The parabolic particle size distribution curve in Figure 2b indicates a bimodal particle size for as-prepared Fe3O4. The parabola had a broad distribution, and two peaks were observed at 271 and 838 nm. This indicates polydispersity of the particles, attributed to aggregation between the particles due to their inherent low surface charge and high surface energy derived from their small particle size and clustered structure. After surface modification, the parabola exhibited a monomodal shape, with a narrower distribution compared to that of as-prepared Fe3O4. In addition, the measured average particle size decreased. The average sizes after surface modification were 340 nm (1 wt %), 202 nm (5 wt %), and 256 nm (10 wt %). The peak shifted to a smaller particle size and the distribution became narrow as the PAA content increased.
The PDIs of as-prepared Fe3O4 and Fe3O4 modified with PAA are listed in Table 1. The PDI value for as-prepared Fe3O4 (0 wt % PAA) was 0.785 and that of Fe3O4 modified using PAA was 0.493 (1 wt %), 0.168 (5 wt %), and 0.212 (10 wt %). A smaller PDI value indicates that the particles were monodisperse. After surface modification, the PDI value decreased compared to that of as-prepared Fe3O4, and decreased as the content of PAA increased. It could be inferred that the dispersion of the Fe3O4 particles was enhanced by the inter-particle repulsive force from the carboxyl groups formed by surface modification. The PDI of Fe3O4 modified with 10 wt % PAA was higher than that of Fe3O4 modified with 5 wt % PAA. This trend is consistent with the results of the zeta potential and PSD analyses. As shown in Figure 2, the zeta potential of 10 wt % PAA-treated Fe3O4 was a similar value to that of 5 wt % PAA-treated Fe3O4, but the former had a larger average particle size and broader distribution. This is inferred to be due to the effects that appear on the particle surface, such as over-saturation with carboxyl groups or structural factors related to the particles. Consequently, the PDI values followed a trend similar to those of the zeta potential measurement as the PAA content changed.

Table 1.
PDIs of as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA.
The FE-SEM and TEM images of as-prepared Fe3O4 and Fe3O4 modified using PAA with various contents are presented in Figure 3. As shown in Figure 3a, the as-prepared Fe3O4 particles exhibited a spherical shape of approximately 300 nm in size. The particles dissociated after surface modification with 1 wt % PAA (Figure 3b), attributed to separation of the primary particles comprising the cluster by electrostatic repulsion derived from the influence of the external carboxyl precursor. In the case of the particles modified using over 5 wt % PAA, the dissociation of the particles was more extensive and the density of the outer part of the particles decreased. This was confirmed from the TEM images in Figure 3d,f. When the amount of precursor exceeded the critical value, separation of the primary particles from the surface of the cluster was accelerated. In comparison, partial agglomeration of the Fe3O4 particles was observed in the SEM and TEM images in Figure 3e,f, consistent with the PSD measurements. The parabolic size distribution curve of 10 wt % PAA-treated Fe3O4 (Figure 2b) revealed a broader distribution and larger average particle size compared to that of 5 wt % PAA-treated Fe3O4. In addition, an unspecified layer was formed on the particle surface, attributed to agglomeration of the particles owing to the bridging flocculation induced by excess PAA deposited on the surface [26,27].
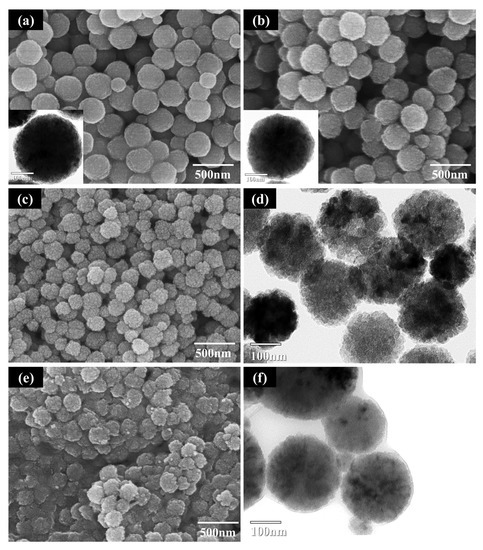
Figure 3.
FE-SEM and TEM images of Fe3O4 before and after surface modification: (a) as-prepared Fe3O4; (b) Fe3O4 modified using 1 wt % PAA; (c) 5 wt % PAA (FE-SEM image); (d) 5 wt % PAA (TEM image); (e) 10 wt % PAA (FE-SEM image); (f) 10 wt % PAA (TEM image).
The high resolution XPS spectra were measured for Fe 2p, C 1s, and O 1s of as-prepared Fe3O4 and Fe3O4 modified using various PAA contents (Figure 4). The spectra of Fe 2p exhibited two peaks at 710.1 and 723.5 eV corresponded to Fe 2p 3/2 and Fe 2p 1/2, respectively [28]. The spectra were deconvoluted into four component peaks located at 709.8 and 723.3 eV (Fe3+–O), 712.8 and 729.5 eV (Fe2+–O). According to previous studies, it has been reported that the satellite peak at 719.0 eV related to Fe3+ in γ-Fe2O3 is not observed in the Fe 2p spectra of Fe3O4 [29,30,31,32]. All the Fe 2p spectra obtained in this study do not have the satellite peak at 719.0 eV even after surface modification using PAA (Figure 4a). For this reason, it is deduced that the addition of PAA does not influence to oxidation of Fe3O4 to γ-Fe2O3.
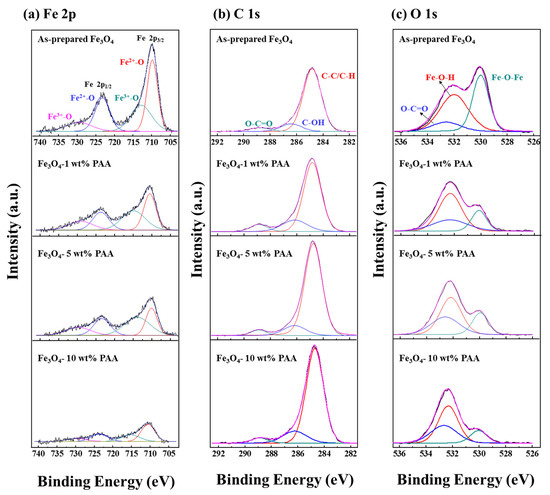
Figure 4.
XPS spectra of as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA: (a) Fe 2p; (b) C 1s; (c) O 1s.
After the surface modification using PAA, changes on the spectra were observed in the XPS results of Fe 2p, C 1s, and O 1s. Intensity of the peaks for the Fe 2p 3/2 and Fe 2p 1/2 decreased as the PAA content increased. In the spectra of C 1s, intensity of the peaks at 284.8 and 288.4 eV increased as the increase in PAA content. The peaks are related to C–C/C–H and O–C=O, respectively [33,34]. In the Figure 4c, the spectra of O 1s are consisted of three component peaks located at 531.1, 532.0, and 532.5 eV corresponded to Fe–O–Fe, Fe–O–H, and O–C=O, respectively [31]. As the PAA content increased, the spectra of O 1s exhibited the similar tendency with the spectra of Fe 2p and C 1s. Intensity of Fe–O–Fe peak increased and that of O–C=O peak decreased as the PAA content changed from 1 wt % to 10 wt %. Consequently, the peaks related to carboxyl group increased and the peaks about the Fe3O4 relatively decreased as the PAA content increased. It is considered to the influence of carboxyl groups formed on the surface of the particles by surface modification using PAA as shown in the results of above analyses.
The L*, a*, and b* values for the particles calculated from the CIE Lab color coordinates are presented in Figure 5 and Table 2. The three parameters L*, a*, and b* correspond to the brightness, red/green, and yellow/blue color intensities, respectively. The L*, a*, and b* values were in the range of 40.06–68.16, −1.11–4.22, and −7.10–3.99, respectively. For all Fe3O4–PAA samples, the L* and b* values were lower and a* values were higher than for the Fe3O4 sample before modification. This means that the brightness of the samples decreased and the color of the samples changed to red and blue. The L* and b* values decreased as the PAA content increased in the range of 0–5 wt %. In the same range, the value of a* changed to a positive value and increased with an increase in the PAA content. This is attributed to the acceleration of dissociation of the cluster particles owing to the electrostatic repulsion of the carboxyl groups. However, the opposite tendency was observed for all values at 10 wt % PAA content. The increase in the L* value is due to the opaque layer formed on the surface of the particles, as shown in Figure 3d-1 [35]. The reversal of the a* and b* values is inferred to be due to particle agglomeration, which is attributed to bridging flocculation caused by excessive PAA adsorbed on the particle surface.
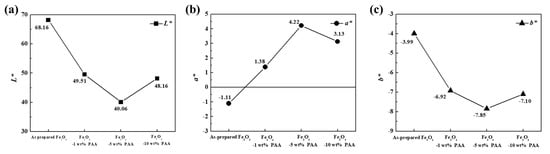
Figure 5.
Color parameters for as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA: (a) L*; (b) a*; (c) b*.

Table 2.
CIE Lab color coordinate data for the as-prepared Fe3O4 and Fe3O4–PAA.
Images of as-prepared Fe3O4 and modified particles are presented in Figure 6. The images show that the color of the samples changed after surface modification using PAA. The color of the modified particles was different from the dark brown color of as-prepared Fe3O4, where the color change was dependent on the PAA content. Fe3O4–1 wt % PAA exhibited a reddish-brown color compared to as-prepared Fe3O4. The color of Fe3O4–5 wt % PAA and Fe3O4–10 wt % PAA was close to purple. The observed color changes are consistent with the CIE Lab coordinates in Figure 5 and Table 2. This indicates that the optical properties of the particles can be adjusted by changing the structure of the particles through surface modification using PAA.

Figure 6.
Images of dried as-prepared Fe3O4 and Fe3O4–PAA: (a) as-prepared Fe3O4; (b) 1 wt % PAA; (c) 5 wt % PAA; (d) 10 wt % PAA.
The magnetic properties of as-prepared Fe3O4 and Fe3O4 modified using various PAA contents were analyzed using VSM. From the magnetization curves in Figure 7, it was determined that all samples had extremely low (nearly zero) remanence (Mr) and coercivity (H). In addition, in the liquid state, the samples maintained the dispersed state in the absence of an external magnetic field and could be separated from the liquid under an external applied magnetic field (images in Figure 7). It is deduced that Fe3O4 has superparamagnetic properties, and the Fe3O4–PAA samples also exhibit this property even after surface modification. The saturation magnetization (Ms) of the as-prepared Fe3O4 and Fe3O4 modified using 1, 5, and 10 wt % PAA was quantified to be 120.85, 108.11, 76.54, and 72.22 emu/g, respectively. The Ms value of Fe3O4−PAA decreased by 40.2% compared with that before surface modification. This can be attributed to the decrease in the density owing to dissociation of the particles. It was also inferred that the Ms value of Fe3O4−10 wt % PAA decreased because of the increase in the particle weight due to the formation of the surface layer. It was confirmed that the magnetic properties were affected by changes in the structural factors of the Fe3O4 nanoparticles due to surface modification.
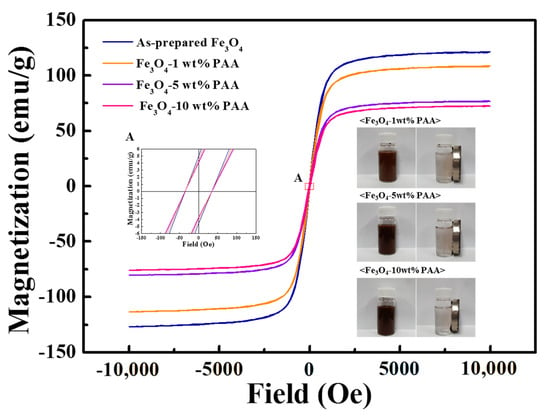
Figure 7.
Magnetization curves of as-prepared Fe3O4 and Fe3O4−PAA.
4. Conclusions
Carboxyl groups were formed on the surface of Fe3O4 nanoparticles by surface modification using PAA, a polymer-type precursor. After surface modification, the zeta potential of the particles decreased as the PAA content increased owing to the carboxyl groups formed on the surface. The dispersibility of the particles was improved by the enhanced repulsive force from the surface functional groups. FE-SEM and TEM analyses confirmed structural changes in the Fe3O4 cluster particles. A higher PAA content promotes dissociation of the particles, but induces agglomeration of the particles at 10 wt % PAA due to bridging flocculation through excess PAA on the surface. In the result of XPS, the peaks related to carboxyl group increased and the peaks concerning the Fe3O4 decreased by the influence of increases in the PAA content. It was inferred that the surface modification using PAA does not affect oxidation of Fe3O4 to γ-Fe2O3. The optical and magnetic properties were also affected by the dissociation and agglomeration of the particles. As the PAA content increased, the brightness of the particles decreased, and the colors became more red and blue. However, all the values were reversed when the particles were modified with 10 wt % PAA. The Ms value of the modified Fe3O4 decreased to 40.2% after PAA modification, attributed to dissociation of the particles and the formation of the surface layer. The Fe3O4 particles functionalized with 5 wt % PAA exhibited the highest absolute zeta potential value and the most stable dispersibility, as shown in the results of zeta potential, PSD, and PDI. In addition, partial agglomeration was observed at 10 wt % PAA in the results of SEM and TEM. The Fe3O4−10 wt % PAA exhibited a reversal tendency of the optical property values. Combining these results, 5 wt % of PAA content is considered to be the optimal condition of surface functionalization. In conclusion, the enhanced dispersibility in aqueous medium and controlled changes in the optical and magnetic properties of the Fe3O4 nanoparticles by surface modification using various PAA contents can potentially be exploited in various applications in medical diagnostics, next-generation separation material process, and eco-friendly inorganic fields.
Author Contributions
Conceptualization, methodology, validation, formal analysis, investigation, data curation, visualization, and writing—original draft preparation, J.R.S. and G.S.A.; writing—review and editing, resources, and supervision, G.S.A. and S.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute for Advancement of Technology (KIAT) grant funded by the Korean government (MOTIE) (P0017012, Human Resource Development Program for Industrial Innovation).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, A.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. 2007, 1222–1244. [Google Scholar] [CrossRef]
- Öztürk, M.; Okutan, M.; Coşkun, R.; Çolak, B.; Yalçın, O. Evaluation of the effect of dose change of Fe3O4 nanoparticles on electrochemical biosensor compatibility using hydrogels as an experimental living organism model. J. Mol. Liq. 2021, 322. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, L.; Hosmane, N.; Gong, Y.; Wu, A. Cancer cell detection and imaging: MRI-SERS bimodal splat-shaped Fe3O4/Au nanocomposites. Chinese Chem. Lett. 2019, 30, 87–89. [Google Scholar] [CrossRef]
- Du, T.; Zhao, C.; ur Rehman, F.; Lai, L.; Li, X.; Sun, Y.; Luo, S.; Jiang, H.; Selke, M.; Wang, X. Rapid and multimodal in vivo bioimaging of cancer cells through in situ biosynthesis of Zn&Fe nanoclusters. Nano Res. 2017, 10, 2626–2632. [Google Scholar]
- Kozenkova, E.; Levada, K.; Efremova, M.V.; Omelyanchik, A.; Nalench, Y.A.; Garanina, A.S.; Pshenichnikov, S.; Zhukov, D.G.; Lunov, O.; Lunova, M.; et al. Multifunctional Fe3O4-Au nanoparticles for the MRI diagnosis and potential treatment of liver cancer. Nanomaterials 2020, 10, 1646. [Google Scholar] [CrossRef]
- Han, C.; Cai, N.; Chan, V.; Liu, M.; Feng, X.; Yu, F. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly(lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 104–114. [Google Scholar] [CrossRef]
- Pourmiri, S.; Tzitzios, V.; Hadjipanayis, G.C.; Meneses Brassea, B.P.; El-Gendy, A.A. Magnetic properties and hyperthermia behavior of iron oxide nanoparticle clusters. AIP Adv. 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Yu, Y.; Wang, G.; Zhang, H.; Chen, A. Fe3O4 modified mesoporous carbon nanospheres: Magnetically separable adsorbent for hexavalent chromium. J. Alloys Compd. 2017, 698, 20–26. [Google Scholar] [CrossRef]
- Bassyouni, D.; Mohamed, M.; El-Ashtoukhy, E.S.; El-Latif, M.A.; Zaatout, A.; Hamad, H. Fabrication and characterization of electrospun Fe3O4/o-MWCNTs/polyamide 6 hybrid nanofibrous membrane composite as an efficient and recoverable adsorbent for removal of Pb (II). Microchem. J. 2019, 149, 103998. [Google Scholar] [CrossRef]
- Fitriawan, M.; Rahman, T.P.; Yulianto, A.; Ikono, R.; Nugroho, D.W.; Rochman, N.T. Characterization of Black Pigment Based on Iron Oxide from Mill Scale by Simple Burning Method. Unnes Phys. J. 2016, 5, 27–31. [Google Scholar]
- Ren, G.; Wang, X.; Zhang, Z.; Zhong, B.; Yang, L.; Yang, X. Characterization and synthesis of nanometer magnetite black pigment from titanium slag by microwave-assisted reduction method. Dye. Pigment. 2017, 147, 24–30. [Google Scholar] [CrossRef]
- An, G.S.; Chae, D.H.; Hur, J.U.; Oh, A.H.; Choi, H.H.; Choi, S.C.; Oh, Y.S.; Jung, Y.G. Hollow-structured Fe3O4@SiO2 nanoparticles: Novel synthesis and enhanced adsorbents for purification of plasmid DNA. Ceram. Int. 2018, 44, 18791–18795. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef] [PubMed]
- Carpio, A.; Mercader-Trejo, F.; Arce, L.; Valcárcel, M. Use of carboxylic group functionalized magnetic nanoparticles for the preconcentration of metals in juice samples prior to the determination by capillary electrophoresis. Electrophoresis 2012, 33, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Barick, K.C.; Singh, S.; Bahadur, D.; Lawande, M.A.; Patkar, D.P.; Hassan, P.A. Carboxyl decorated Fe3O4 nanoparticles for MRI diagnosis and localized hyperthermia. J. Colloid Interface Sci. 2014, 418, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jang, D.H.; Choa, Y.H. Preparation and characterization of carboxyl functionalization of magnetite nanoparticles for oligonucleotide immobilization. Phys. Scr. T 2010, T139. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Y.; Wang, Z.; Lin, Y.; Luo, P.; Guo, Q. Fe3O4 /poly (acrylic acid) nanoparticles as modifiers for improving rheological and filtration properties of water-based drilling fluids. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 393–402. [Google Scholar] [CrossRef]
- Marín, T.; Montoya, P.; Arnache, O.; Calderón, J. Influence of Surface Treatment on Magnetic Properties of Fe3O4 Nanoparticles Synthesized by Electrochemical Method. J. Phys. Chem. B 2016, 120, 6634–6645. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Oh, A.H.; Park, H.Y.; Jung, Y.G.; Choi, S.C.; An, G.S. Synthesis of Fe3O4 nanoparticles of various size via the polyol method. Ceram. Int. 2020, 46, 10723–10728. [Google Scholar] [CrossRef]
- Yan, J.C.; Zeng, X.Q.; Ren, T.H.; van der Heide, E. Exploring an alternative aqueous lubrication concept for biomedical applications: Hydration lubrication based on O/W emulsions combined with graphene oxide. Biosurface Biotribol. 2015, 1, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.U.; Choi, J.S.; Choi, S.C.; An, G.S. Highly dispersible Fe3O4 nanoparticles via anionic surface modification. J. Korean Ceram. Soc. 2020, 57, 80–84. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.A.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic Acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015, 320631. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, T.; Kang, I.K.; Park, S.Y. Poly(acrylic acid)-Grafted Graphene Oxide as an Intracellular Protein Carrier. Langmuir. 2014, 30, 402–409. [Google Scholar] [CrossRef]
- Benn, F.A.; Fawell, P.D.; Halewood, J.; Austin, P.J.; Costine, A.D.; Jones, W.G.; Francis, N.S.; Druett, D.C.; Lester, D. Sedimentation and consolidation of different density aggregates formed by polymer-bridging flocculation. Chem. Eng. Sci. 2018, 184, 111–125. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, K.M.; Maeng, W.Y.; Hur, D.H. Effects of environmental factors on the dispersion behavior of iron oxide in aqueous solutions with poly acrylic acid. Arch. Metall. Mater. 2015, 60, 1561–1564. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray Photoelectron Spectroscopy Studies of Solvated Metal Atom Dispersed Catalysts. Monometallic Iron and Bimetallic Iron-Cobalt Particles on Alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Muhler, M.; Schlögl, R.; Ertl, G. The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene. 2. Surface chemistry of the active phase. J. Catal. 1992, 138, 413–444. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by (formula presented)-assisted molecular-beam epitaxy. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Cuenca, J.A.; Bugler, K.; Taylor, S.; Morgan, D.; Williams, P.; Bauer, J.; Porch, A. Study of the magnetite to maghemite transition using microwave permittivity and permeability measurements. J. Phys. Condens. Matter 2016, 28. [Google Scholar] [CrossRef] [Green Version]
- Salman, F.; Zengin, A.; Çelik Kazici, H. Synthesis and characterization of Fe3O4-supported metal–organic framework MIL-101(Fe) for a highly selective and sensitive hydrogen peroxide electrochemical sensor. Ionics (Kiel) 2020, 26, 5221–5232. [Google Scholar] [CrossRef]
- Godo, O.; Gaskell, K.; Pathak, G.K.; Kyrtsos, C.R.; Ehrman, S.H.; Shah, S.B. Characterization of fluorescent iron nanoparticles—Candidates for multimodal tracking of neuronal transport. AIMS Bioeng. 2016, 3, 362–378. [Google Scholar] [CrossRef]
- Gupta, K.; Jha, P.K.; Dadwal, A.; Debnath, A.K.; Jaiswal, I.; Rana, S.; Joy, P.A.; Ballav, N. Embedding S = 1/2 Kagome-like Lattice in Reduced Graphene Oxide. J. Phys. Chem. Lett. 2019, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Petran, A.; Radu, T.; Culic, B.; Turcu, R. Tailoring the properties of magnetite nanoparticles clusters by coating with double inorganic layers. Appl. Surf. Sci. 2016, 390, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).