Abstract
Enzymatic hydration of oleic acid into 10-hydroxystearic acid (10-HSA) represents a theme of substantial scientific and practical interest. In this study, a fatty acid hydratase (OHase) from Lactococcus garvieae was cloned and expressed in Escherichia coli. The recombinantly expressed enzyme was identified as oleate hydratase (EC 4.2.1.53) confirming its highest hydration activity for oleic acid. The optimally yielded enzyme fraction was purified and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). A solitary band on SDS-PAGE confirmed the molecular weight of 65 kDa. Gas chromatography-mass spectrometry (GC-MS) analysis scrutinized the silylated hydroxy fatty acid products acquired from the hydration of oleic acid by the oleate hydratase from L. garvieae. Optimal reaction conditions for the enzymatic production of 10-HSA from oleic acid using the purified oleate hydratase were pH 7.5, 30 °C, 105.49 U/mL enzyme solution and 30 g/L oleic acid. In the presence of activity stimulators, that is, magnesium (II) (Mg2+), the oleate hydratase activity was found to be greatly improved at 30 °C. In conclusion, the results revealed the potential efficacy of recombinant enzyme for the biotechnological conversion of oleic acid to 10-HSA acid with high efficiency. The results would be useful for the improved industrial-scale biosynthesis of 10-HSA via an economical and environmentally friendly bioprocess approach.
1. Introduction
As a biocatalyst, hydratases have attracted more attention in recent years. These enzymes catalyze the hydrogenation of unsaturated fatty acids (UFAs) by stereo-selectively adding water to the carbon-carbon double bond [1]. Therefore, hydroxyl groups can be incorporated without the requirement for costly cofactors such as recycling or electron releasing groups [2]. These unique characteristics of hydratases render them interesting candidates for numerous applications in industrial bioprocesses. A large number of microorganisms have been recognized to synthesize oleic acid hydratase with different regioselectivity. For example, Escherichia coli hydratase shows the greatest activity against oleic acid. A recent study revealed that oleic acid hydratase necessitates flavin adenosine dinucleotide (FAD) as a cofactor for the production of 10-hydroxystearic acid [2]. Microscopic characterization revealed that the hydratase is an integral membrane protein, and therefore, enzymatic reaction carries out at the periphery of the cell. This reaction site might be beneficial in the detoxification process because the toxic linoleic acid molecule transformed to non-toxic 10-hydroxy-9-cis-octadecenoic acid by interacting with the hydratase before reaching the cell membrane. In addition, the amino-acid based structural analysis identified a binding site with linoleic acid during the reaction with the hydratase: Site 1, and Site 2, which are located in the external hydrophobic pocket of the protein and in the central contacting with FAD molecules. It was also interesting to note that linoleic acid molecules are arranged around the methionine residue at two sites (Met81 and Met154), which serve as a rigid pole and therefore plays a pivotal role in the binding of UFAs [3]. Hydroxy fatty acids have been discovered in plants, animals, and various microorganisms. However, the plants are considered the main source including plant seeds to get waxes and other lipids [4].
Hydroxy fatty acids (HFAs) are valuable raw materials that can be utilized to produce a range of industrially relevant compounds such as resins and polymers, plastics, nylons, cosmetics, biodiesel, lubricants, additives in paints and varnish formulations [5,6]. In addition, HFAs are also lactones precursors that indicate their wide applicability in the flavor and fragrance industries. Since its first discovery, more research on 10-hydroxystearic acid (10-HAS) has been undertaken with growing industrial interest. The 10-HSA can be obtained by whole cell transformation or enzymatic reaction. In the earlier case (whole cell transformation), Nocardia paraffinae [7], N. cholesterolicum [8], Selenomonas ruminantium [9], and Stenotrophomonas nitritireducens, Lactobacillus acidophilus [10] have been exploited to produce HFAs by the hydration of oleate acids. The hydration of UFAs is speculated to characterize a detoxification mechanism in bacteria, so the production of HFAs is very low. Kim et al. [11] used the whole cells of S. nitritireducens to produce higher titers of 10-hydroxystearic acid under optimal reaction conditions, that is, pH 7.5, 35 °C, 0.05% (w/v) Tween 80, 20 g cells/L, and 30 g/L oleic acid in an anaerobic atmosphere.
The enzymatic hydration of oleic acid (naturally occurring and one of the most abundant UFAs) into 10-HSA, has become a topic of substantial research and has received scientific attention in recent years. As the hydroxystearic acid is a precursor of γ-lactone (an important ingredient for perfumes and other cosmetics), biotransformation of oleic acid to 10-HAS is of great interest from an industrial perspective, particularly the flavor and fragrance industries [2]. In recent years, numerous studies have been carried out to convert various substrates into lactones, though using different materials (as a substrate) and strategies, such as olive oil, perilla oil, microalgae oil, and waste oil [12,13,14]. Herein, a putative oleate hydratase gene from L. garvieae was expressed in E. coli. The process operating conditions such as pH, temperature, the influence of metal ions, the concentration of substrate, and reaction duration were also optimized for the maximal bioconversion of oleic acid to 10-HSA by the oleic acid hydratase.
2. Materials and Methods
2.1. Strains, Vectors, Growth Conditions and Reagents
Escherichia coli DH5α and BL21 (DE3) strains procured from Novagen (Madison, WI, USA), were utilized as cloning and expression host cells, respectively. A pET28a vector from Novagen was used both for cloning as well as expression purposes. Gene encoding oleate hydratase (UniProt ID: 34204340) was derived from Lactococcus garvieae (China Center for Type Culture Collection, China). E. coli DH5α and BL21 (DE3) were cultivated in Luria-Bertani (LB) medium (tryptone 10 g/L, yeast extract 5 g/L, sodium chloride 10 g/L (2% agar powder, if solid) with a final concentration of 50-μg kanamycin at 37 °C. BU-Taq Plus 2X Master PCR Mix (Biouniquer Technology, Beijing, China), restriction endonuclease (Thermo Fisher Scientific, Waltham, MA, USA), and T4 DNA ligase (NEB, Ipswich, MA, USA), were used for molecular cloning. A His-Bind Purification kit was provided by the Sangon Biotech Company (Shanghai, China). All other reagents and solvents were of the highest-purity grade and used as such.
2.2. Gene Cloning
The genomic DNA of L. garvieae was extracted by using a bacterial genomic DNA extraction kit (Easy Pure Genomic DNA Kit, Transgen Biotech, Beijing, China). Gene encoding the oleate hydratase was polymerase chain reaction (PCR) amplified using genomic DNA as a template. The gene sequence of the upstream primer GF for gene cloning: 5′TTTCCATGGTGCGTTATACAAACGGAAATT3′ and downstream primer GR: 5′TTTCTCGAGAAGTAGCCCTGCATCTTTAA3′. Underlined are restriction sites by Nco1 and Xho1. Figure 1 illustrates the complete process for constructing oleate hydratase enzymes. The PCR-amplified DNA fragment and the pET-28a (+) plasmid vector were enzymatically digested, and the target gene was inserted into the pET-28a (+) vector followed by transformation into E. coli DH5α. Recombinant E. coli DH5α was subjected to double enzyme digestion verification and colony PCR verification, and plasmid sequencing was performed. After successful sequencing, the recombinant plasmids thus constructed were confirmed by sequence analysis and transformed into E. coli BL21 (DE3).
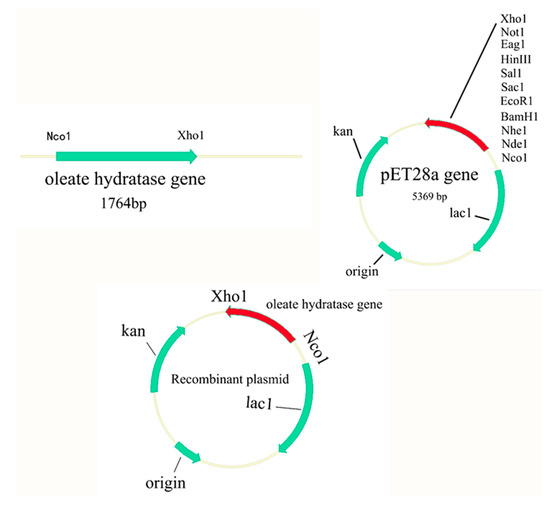
Figure 1.
A schematic representation for construction of the oleate hydratase.
2.3. Enzyme Purification
E. coli BL21(DE3) positive transformants were inoculated and grown into 500 mL of LB culture medium, and then induced by the incorporation of 0.5 mM β-D-thiogalactoside (IPTG) after OD600 reached 0.6–0.8. After 12 h, cultured cells were harvested and washed using sterile water and resuspended in a 20 mL binding buffer (Novagen, His Bind Purification kit). The cells were broken by sonication, centrifuged, and the supernatant was collected and used for purification. The fusion proteins with His (6×) tags were applied on His Bind Purification kit (Novagen) for purification. Briefly, the protein extract was mixed with the binding buffer, added to the column, and collected the flow through to the centrifuge tube. This operation was repeated three times for better protein combination with the resin. The column was then washed with a double column volume of binding/wash buffer and the flow through was collected. The histidine-tagged protein on the column was eluted twice with 50 mM imidazole. The eluate containing the fusion protein was then concentrated by ultrafiltration and stored at −20 °C. The concentration of fusion proteins was quantified with a BCA protein assay kit.
2.4. SDS-PAGE
Approximately 100 mL of cultured cells were concentrated to 10 mL with a citrate-sodium citrate buffer, sonicated for one sec, rested for 4 s, and worked for 45 min. After sonication, the cells were centrifuged at 12,000 rpm for 15 min. The supernatant was used as a sample for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Samples for SDS-PAGE were neutralized using 1.0 M Tris-HCl (pH 6.8) before mixing with an equal volume of Laemmli Sample Buffer that contained 1% SDS and 10 mM dithiothreitol. After heating at 95 °C for 5 min, all samples were separated on a 12% SDS-PAGE gel prepared in house. After electrophoresis, gels were fixed in a fixing solution (50% methanol, 10% acetic acid) for 10 min, washed with water for at least 40 min, and protein bands were visualized using a Coomassie brilliant blue G-250 staining.
2.5. Optimization of Reaction Conditions for 10-HSA Production
A range of buffers with a pH range of 5.5 to 8.0 was used in this assay to determine the optimal pH of the oleate hydratase. Optimal temperatures of the purified enzymes were investigated by incubating the reaction mixture at a temperature range of 20 to 45 °C and pH 7.5. The thermal stabilities of the oleate hydratase were measured by heating enzymes at different temperatures (20 °C–45 °C) for up to 120 h. Moreover, the pH stabilities of the oleate hydratase were also determined following incubating enzymes into the sodium citrate buffer (pH 5.5–pH 8.0) with a concentration of 100 mM at 30 °C for 90 min. Chemicals with a final concentration of 1 mM were added into the reaction combination to investigate their effects on the oleate hydratase activities. In order to determine the effect of substrate concentration on oleic acid hydratase, the amount of oleic acid incorporated was ranged from 10 g/L to 70 g/L at 30 °C for 10 min. All the optimization-related reactions were carried out at 30 °C in a 100 mM sodium citrate buffer (pH 7.5) comprising of 30 g/L oleic acid, 4% ethanol, and 10 µL enzyme solution (105.49 U/mL) for 10 min. The biotransformation of oleic acid to 10-HSA in the presence of oleate hydratase under optimized reaction conditions is shown in Scheme 1.
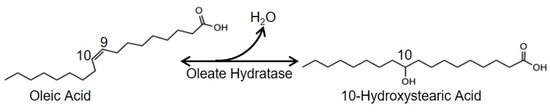
Scheme 1.
The biotransformation of oleic acid to 10-hydroxystearic acid in the presence of oleate hydratase.
2.6. Analytical Methods
The standard of 10-hydroxystearic acid was prepared in ethyl acetate solution at a concentration of 64 μg/mL. A 0.5 mL of the standard solution (or sample) was taken in a 2 mL sample vial, and 100 μL of pyridine and 50 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide were added, mixed, and allowed to react at 70 °C for 30 min to obtain silylated fatty acids [9]. After cooling to room temperate, the sample (1 μL) was injected in gas chromatography (SHIMADZU Excellence in Science GCMS-QP2010S, Tokyo Japan), and quantified by a DB-5 capillary column (30 m × 0.25 mm × 0.25 µm) coupled to a flame ionization detector (FID). He (purity: 99.999%) was used as the carrier gas with a constant flow rate of 1 mL/min. Injection temperature was 240 °C. The temperature program was as follows: the initial oven temperature at 120 °C was held for 0.5 min and increased at 20 °C/min to reach 250 °C for 8 min. The hydroxy fatty acid products were identified by GC–mass spectrometry (MS) with an electron impact ionization source. The ion source was operated at 70 eV and held at 230 °C.
2.7. Statistical Analysis
All the experiments were carried out in triplicate and the results were expressed as means ± standard deviation.
3. Results and Discussion
3.1. Cloning and Molecular Characterization of Fatty Acid Hydratase from Lactococcus garvieae
A gene encoding oleic acid hydratase from L. garvieae was cloned into the pET-28a (+) vector and then over-expressed in E. coli BL21(DE3). The oleic acid hydratase gene was transformed into E. coli BL21 by double enzyme digestion of Nco1 and Xho1, and verified by sequencing. The target gene and plasmid were approximately 1700 bp and 5000 bp, respectively (Figure 2A). The sequencing results corroborated that the target gene and the vector were transformed successfully into E. coli, resulting in the construction of recombinant E. coli. A commercially available Ni-NTA resin column purified the obtained crude enzyme solution. The protein was mainly over-expressed as a soluble form in the recombinant cells with the occasional observation of inclusion bodies. When eluted 8 times with imidazole at a concentration of 10 mM, the hybrid protein was almost eluted, and then the target protein was eluted with 50 mM imidazole. The purified protein was immediately concentrated by ultrafiltration because imidazole may precipitate the protein following a longer exposure. Notably, the concentration and purity of the protein were greatly increased by ultrafiltration. The purified enzyme elucidated a solitary band on SDS-PAGE with an approximate molecular weight of 65 kDa (Figure 2B), which was found to be in agreement with the calculated value of 67.2 kDa based on 587 amino acids residues and six histidine residues.
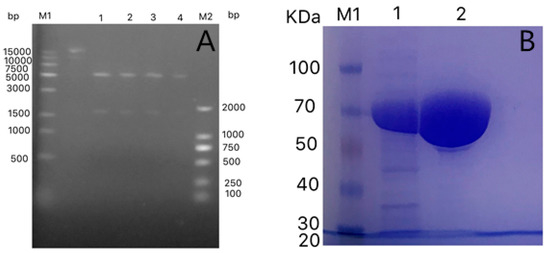
Figure 2.
(A) DNA gel electrophoresis, M1—15K DNA marker, M2—2K DNA marker, Lane 1 to 4—double digested bands; (B) Molecular mass determination of purified oleate hydratase enzyme from Lactococcus garvieae by SDS-PAGE stained with Coomassie Blue. Lane M1—molecular mass marker proteins (100, 70, 50, 40, 30, 25, and 14 kDa), Lane 1—crude extract, Lane 2—purified oleate hydratase enzyme.
3.2. Identification of Products by GC-MS Analysis
Figure 3 represents the GC-MS spectra of the silylated hydroxy fatty acid product obtained from oleic acid hydration by the oleate hydratase from L. garvieae. A characteristic peak appeared at m/z 215.20 by the loss of C12H25O2Si moiety from the silylated HFA. Similarly, the elimination of C8H17 and C12H27OSi from the silylated product results in the formation of a peak at m/z 331. These characteristic fragmentation peaks clearly indicate the HFA as 10-hydroxystearic acid (Figure S1).
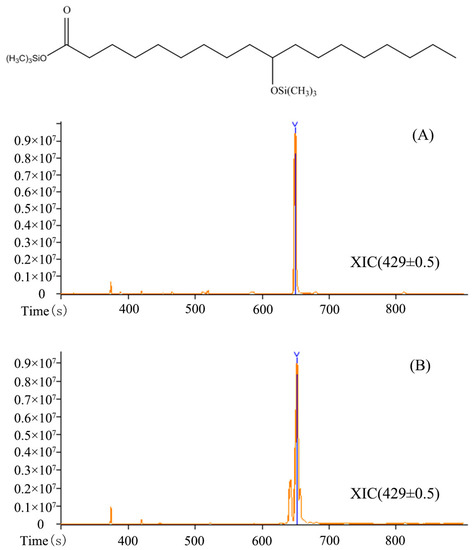
Figure 3.
Gas chromatography spectrum. (A) Standard, and (B) silylated hydroxy fatty acid product (10-hydroxystearic acid) obtained from oleic acid by the oleate hydratase from L. garvieae.
3.3. Optimization of Reaction Conditions
3.3.1. Influence of Temperature
The thermal influence of varying reaction temperatures ranging from 20 to 45 °C on the relative activity of oleate hydratase was performed. The results obtained are portrayed in Figure 4A. The time-dependent thermal stability was investigated by incubating the optimally active fraction of oleate hydratase for various times (0 to 120 h) at the temperature range 20 to 45 °C (Figure 4B). In an earlier case (activity profile), percent relative activity of oleate hydratase was increased gradually with increasing temperature and found to be maximum at 30 °C. Beyond this temperature, the enzyme activity was dramatically decreased at 35 °C (up to 71% loss) presumably due to enzyme denaturation and thereafter remains negligibly unchanged up to 45 °C. This sudden reduction in relative activity at ≥30 °C has also been found in another oleate hydratase [11], regardless of the enzyme source and fermentation strategy. In the latter case (stability profile), the optimal fraction incubated for 90 h at 30 °C retained the highest stability (more than 50%) among all tested fractions at lower/higher temperatures. The stability trend was followed by fractions incubated at 25 °C and 35 °C for the same period (90 h) and retained up to 40% relative activity. At the end of stability analysis (120 h), the fractions incubated at 20 °C, 40 °C and 45 °C showed the highest activity loss (up to 90%). However, the optimal fraction incubated at 30 °C retained up to 25% of its activity with an overall loss of 75% at that incubation period. Relatively high-thermostability of an enzyme is a desirable and fascinating characteristic for industrial applications [15]. Earlier, Takeuchi et al. [16] reported that the enzyme CLA-HY (hydratase/dehydratase) retained higher than 80% of its original activity at temperatures up to 32 °C and 28 °C in the presence and absence of FAD, respectively.
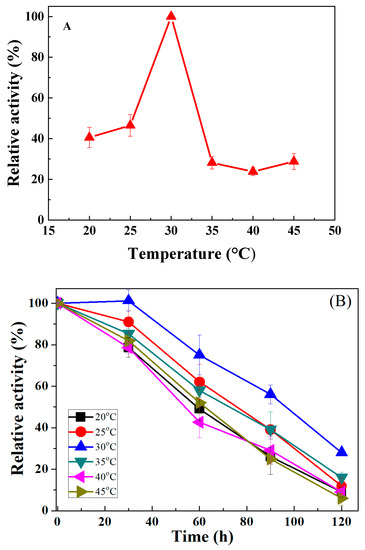
Figure 4.
Influence of varying temperature on the relative activity (A) and thermal stability (B) of oleate hydratase for oleic acid biotransformation to 10-hydroxystearic acid.
3.3.2. Influence of pH
In order to assess the effect of pH on enzyme activity and stability, the reaction was carried out at varying pH ranging from pH 5.5 to 8.0. The time-dependent stability analysis was performed by incubating the optimally active fraction of oleate hydratase for various times (0 to 90 min) at the same pH range used for an activity profile. Results in Figure 5A revealed that the relative activity of oleate hydratase was gradually increased with increasing pH values. The optimal pH of oleate hydratase was found to be pH 7.5, and above this pH, the enzyme activity was decreased. The stability curves revealed that oleate hydratase incubated at pH 7.5 for 30 min was the most stable fraction at that period and retained up to 75% of its original relative activity, as shown in Figure 5B. The fraction incubated at pH 5.5 at the same period was the least stable and retained only up to 25% (loss up to 75%). At the end of 90 min incubation, almost all fractions were not stable and showed maximum activity loss, that is, up to 100% at pH 5.5, 6 and 6.5, up to 95% at pH 7 and 8, and up to 90% at pH 7.5 (optimal fraction). The current optimal pH finding is well aligned with earlier reported literature. Kim et al. [11] reported that the conversion of oleic acid to 10-hydroxystearic acid by whole cells of S. nitritireducens was maximal at 35 °C and pH 7.5. Nocardia cholesterolicum NRRL 5767 gave a good yield with optimum conditions at 40 °C and pH 6.5, while the conversion rate was maximal at 35 °C and pH 7.5 by the whole cells of Nocardia paraffinae [7,8].
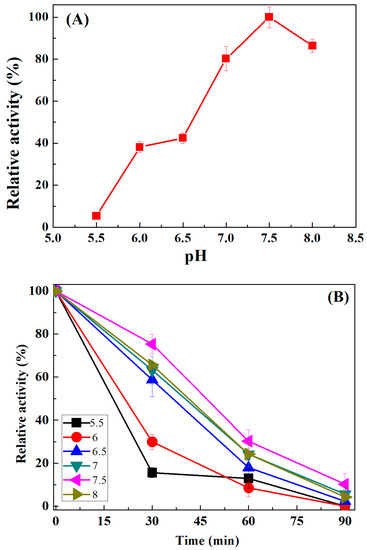
Figure 5.
Influence of varying pH levels on the relative activity (A) and stability (B) of oleate hydratase for oleic acid bioconversion to 10-hydroxystearic acid.
3.3.3. Influence of Metal Ions
The stimulatory or inhibitory influence of various monovalent, divalent, and trivalent metal ions including K+, Na+, Ca2+, Mg2+, Fe2+, Zn2+, Cu2+, Mn2+, and Fe3+, respectively were tested against the freshly obtained oleate hydratase (Figure 6). As compared to the control, the oleate hydratase activity was significantly influenced and induced by monovalent and divalent metal ions, except Fe2+. The stimulatory potential order of these metal ions was as: Mg2+ > Ca2+ > Cu2+ > Mn2+ > Zn2+ > K+ > Na+. Both Fe2+ and Fe3+ inhibited the oleate hydratase activity. The inhibitory potential of Fe3+ was more evident. The inhibitory activity may be due to the insoluble complex formation with these metals [16,17]. In a previous study, Joo et al. [18] reported up to 25–44% reduction in the ethylenediaminetetraacetic acid (EDTA)-treated oleate hydratase activity upon the addition of divalent metal ions. As compared to earlier studies, the activity profile of oleate hydratase showed its high stability against different metal ions.
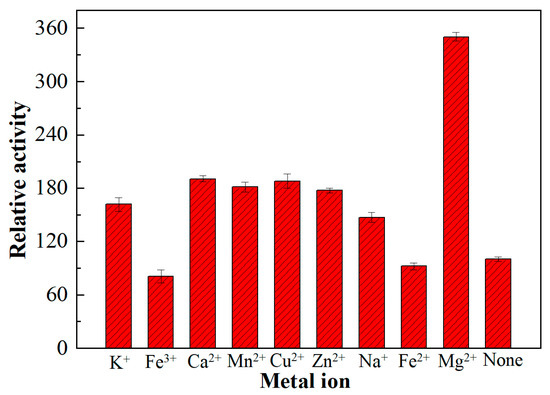
Figure 6.
Influence of various monovalent, divalent, and trivalent metal ions on the activity of freshly obtained oleate hydratase from Lactococcus garvieae.
3.3.4. Influence of Substrate Concentration
To investigate the substrate influence on the oleate hydratase activity, varying concentrations, that is, 10–70 g/L of oleic acid, as a substrate, were trialed. The results obtained are displayed in Figure 7. The maximal enzyme activity was found at the substrate concentration of 30 g/L. The substrate-dependent activity curve in Figure 7 confirms that the recorded activity trend was proportional to the substrate concentration. This is because the enzyme activity increases initially with increasing substrate concentration. However, beyond the optimal substrate level (30 g/L), the enzyme activity starts decreasing. This observed increasing/decreasing activity phenomenon is according to the enzyme-substrate interfaces which follow the law of mass-action [19]. Further addition of substrate beyond the optimal level (30 g/L) causes reduction in the relative activity, which could be due to the substrate hindrance around the catalytic site, and substrate or product inhibition phenomena.
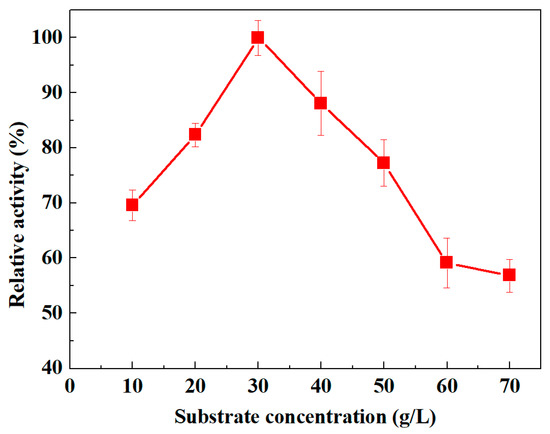
Figure 7.
Influence of varying substrate (oleic acid) concentrations on the activity of freshly obtained oleate hydratase from Lactococcus garvieae.
3.3.5. Influence of Reaction Time on Oleic Acid Biotransformation to 10-HSA
Time-course reactions for biotransformation of oleic acid to 10-HSA by oleate hydratase was carried out for 0.5 to 8 h using the optimal conditions of pH 7.5, and 30 °C. The results obtained are shown in Figure 8. The biotransformation rate of oleic acid to 10-hydroxystearic acid by oleate hydratase was quite slow during initial reaction hours. At the end of 3 h reaction period, up to 92 µg/mL, 10-hydroxystearic acid was recorded. However, by the end of 7th h, the biotransformation of oleic acid to 10-HSA was increased up to 250 µg/mL, which is double of the first 3 h reaction. Further, with an increase in the reaction time from 7 h up to 8 h, the product conversion was not significant, and the maximum yield was 258 µg/mL. As reported earlier by Kim et al. [11], only 19.9 g/L 10-hydroxystearic acid was produced within 2.7 h with a yield of 63% (mol/mol) under aerobic conditions. Koritala et al. [8] reported that anaerobic conditions are more favorable for a stimulated conversion of oleic acid to 10-hydroxystearic acid. Comparatively, under anaerobic conditions, around 98% hydroxy acid and 2% keto acid were obtained, whereas, under aerobic conditions, only 88% hydroxy acid and 12% keto acid were obtained [8].
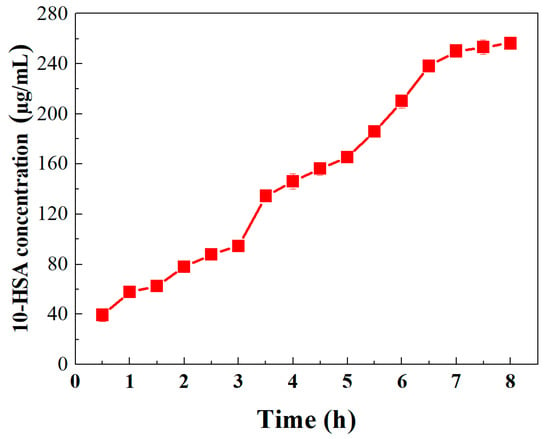
Figure 8.
Time-course reactions for oleic acid transformation to 10-hydroxystearic acid by oleate hydratase under the optimal conditions of pH 7.5 and 30 °C. Data represent the means of three experiments and error bars represent standard deviation.
4. Conclusions
In conclusion, the production of 10-HSA by recombinant oleic acid hydratase has more advantages than microbial biotransformation. The use of recombinant technology to obtain a purified enzyme is a simple, low-cost operation and results in 10-HSA with high yield. Genes encoding oleic acid hydratase could be improved by genetic manipulation, error-prone PCR, DNA shuffling and modifying determinant residues on or near the active site. The characterization profiles revealed that the pH and thermal tolerance of freshly obtained and recombinant oleate hydratase from Lactococcus garvieae was higher compared with the previous reports. The effect of monovalent metal ions was also positive and stimulated the enzyme activity up to a certain extent. Moreover, a maximal of 258 µg/mL 10-hydroxystearic acid was also recorded during the biotransformation of oleic acid by oleate hydratase. This research could be helpful for the industrial-scale synthesis of 10-HAS and contributes to the promotion of the flavor and fragrance industries.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/7/6/326/s1. Figure S1. Gas chromatography/mass spectroscopy (GC/MS) spectrum of the silylated hydroxy fatty acid product (10-hydroxystearic acid) obtained from oleic acid by the oleate hydratase from L. garvieae.
Author Contributions
Study design, J.Z. (Jing Zhang) and Y.Z.; Data collection, J.Z. (Jiaheng Zhang); Data analysis, S.L. and H.L.; Data interpretation, C.L. and H.L.; Literature search, J.Z. (Jing Zhang) and H.S.; Writing-Original Draft Preparation, M.B., and H.M.N.I.; Revisions & Final editing, J.Z. (Jing Zhang), M.B., H.M.N.I. and Y.Z.; Project & Grant acquisition, Y.Z.
Funding
This work was financially supported by a study on highly efficient biotransformation of oleic acid and linoleic acid to γ-decalactone in Yarrowia lipolytica based on synthetic biology (21606097). The authors also acknowledge the support from Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX17_0700), and Young academic leaders in Jiangsu Province, Six-talent peaks project in Jiangsu Province (2015-SWYY-026).
Conflicts of Interest
The authors report no conflicting interests in any capacity; competing or financial.
References
- Chen, B.S.; Otten, L.G.; Hanefeld, U. Stereochemistry of enzymatic water addition to c=c bonds. Biotechnol. Adv. 2015, 33, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Lee, G.H.; Kim, D.W.; Jang, E.H.; Kwon, H.T.; Chi, Y.M. Crystal structure of oleate hydratase from, Stenotrophomonas, sp. kctc 12332 reveals conformational plasticity surrounding the fad-binding site. Biochem. Biophys. Res. Commun. 2018, 499, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Hernández-Santoyo, A. Functional characterization of a fatty acid double-bond hydratase from lactobacillus plantarum and its interaction with biosynthetic membranes. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Chance, D.L.; Gerhardt, K.O.; Mawhinney, T.P. Gas-liquid chromatography-mass spectrometry of hydroxy fatty acids as their methyl esters tert.-butyldimethylsilyl ethers. J. Chromatogr. A 1998, 793, 91–98. [Google Scholar] [CrossRef]
- Naughton, F.C. Production, chemistry and commercial applica-tions of various chemicals from castor oil. J. Am. Oil Chem. Soc. 1974, 51, 65–71. [Google Scholar] [CrossRef]
- Kim, K.R.; Oh, D.K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Latrasse, A.; Paitier, S.; Lachot, B.; Bonnarme, P.; Feron, G.; Durand, A.; Le, Q.J. Conversion of oleic acid to 10-hydroxystearic acid by Nocardia paraffinae. Biotechnol. Lett. 1997, 19, 715–718. [Google Scholar] [CrossRef]
- Koritala, S.; Hou, C.; Hesseltine, C.; Bagby, M. Microbial conversion of oleic acid to 10-hydroxystearic acid. Appl. Microbiol. Biotechnol. 1989, 32, 299–304. [Google Scholar] [CrossRef]
- Hudson, J.; MacKenzie, C.; Joblin, K. Conversion of oleic acid to 10-hydroxystearic acid by two species of ruminal bacteria. Appl. Microbiol. Biotechnol. 1995, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Kim, S.U.; Song, J.W.; Lee, J.H.; Kang, W.R.; Jo, Y.S. Biotransformation of linoleic acid into hydroxy fatty acids and carboxylic acids using a linoleate double bond hydratase as key enzyme. Adv. Synth. Catal. 2015, 357, 408–416. [Google Scholar] [CrossRef]
- Kim, B.N.; Yeom, S.J.; Oh, D.K. Conversion of oleic acid to 10-hydroxystearic acid by whole cells of Stenotrophomonas nitritireducens. Biotechnol. Lett. 2011, 33, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Kitazume, T.; Yamazaki, Y.; Matsuyama, S.; Shoun, H.; Takaya, N. Production of hydroxy-fatty acid derivatives from waste oil by Escherichia coli cells producing fungal cytochrome P450foxy. Appl. Microbiol. Biotechnol. 2008, 79, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.B.; Zhang, Z.; Doherty, W.O.; O’Hara, I.M. A multi-criteria analysis approach for ranking and selection of microorganisms for the production of oils for biodiesel production. Bioresour. Technol. 2015, 190, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; An, J.U.; Lee, S.H.; Oh, D.K. Selective production of 9R-hydroxy-10E,12Z,15Z-octadecatrienoic acid from alpha-linolenic acid in perilla seed oilhydrolyzate by a lipoxygenase from Nostoc Sp. SAG 25.82. PLoS ONE 2015, 10, e0137785. [Google Scholar]
- Fadel, M. Production physiology of cellulases and β-glucosidase enzymes of Aspergillus niger grown under solid state fermentation conditions. Online J. Biol. Sci. 2000, 1, 401–411. [Google Scholar]
- Takeuchi, M.; Kishino, S.; Hirata, A.; Park, S.B.; Kitamura, N.; Ogawa, J. Characterization of the linoleic acid Δ9 hydratase catalyzing the first step of polyunsaturated fatty acid saturation metabolism in Lactobacillus plantarum AKU 1009a. J. Biosci. Bioeng. 2015, 119, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, H.; Jiang, L.; Wang, S. Fatty-Acid–Metal-Ion Complexes as Multicolor Superhydrophobic Coating Materials. Chem. Asian J. 2011, 6, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.C.; Jeong, K.W.; Yeom, S.J.; Kim, Y.S.; Kim, Y.; Oh, D.K. Biochemical characterization and FAD-binding analysis of oleate hydratase from Macrococcus caseolyticus. Biochimie 2012, 94, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Ha, C.E. Enzymes and Enzyme Regulation. In Essentials of Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2011; pp. 47–58. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).