Abstract
The sintering process is significantly important for the ironmaking in China because of the large amount of sinter consumed. Al2O3 is an important element determining the quality and quantity of sinter. However, different conclusions have been made regarding the effects of Al2O3 on the amount and fluidity of the liquid phase formed in the sinter phase. Therefore, it is necessary to examine the effects of Al2O3 content on the amount and fluidity of the liquid phase. The present work investigated the effects of different Al2O3 contents of iron ore fines on the liquid phase formation, mineral composition, and consolidation strength. The results showed that a small amount of Al2O3 increased the amount of calcium ferrite, making the liquid phase formation easier. As the Al2O3 content in iron ore fines increased, the liquidity index decreased continuously, while the fluidity and the consolidation strength of the sintered body were directly related to the content squared. The quality of the sinter is optimal when the Al2O3 content of the iron ore fines is about 2 wt % (the SiO2 content is 4 wt %).
1. Introduction
The blast furnace still plays a dominant position in the ironmaking process, and will for the foreseeable future [1]. In China, blast furnaces consumed about 940 million t of sinter in 2018, which is a very large quantity. The sintering machine is the main apparatus that produces sinter. In the sintering process, iron ore fines and coke breezes are mixed together and charged on the surface of sinter machine, and then heated to higher than 1200 °C to produce liquid-like calcium ferrite to form a sinter cake that is cooled, crushed, and screened to sinter [2]. The constituents of iron ore fines, such as SO2, CaO, Al2O3, MgO, and additional oxides, determine the quality and quantity of sinter [2,3,4,5,6]. Among them, Al2O3 is an important element in the formation of complex calcium ferrite [7]. Al2O3 affects the formation of complex calcium ferrite phase (SFCA), and has an important effect on the structure of calcium ferrite. It is believed that SFCA cannot be produced in the absence of Al2O3, and Al2O3 is beneficial to the stable presence of calcium ferrite in the liquid phase [8,9]. Scarlett et al., studied the crystal structure, chemical composition, and formation sequence of composite calcium ferrite by high-temperature in situ X-ray detection [10]. The research showed that there are only two kinds of crystalline ferrites of SFCA and SFCA-I in the actual sintered ore: the solid solution formed by SFCA and SFCA-I is located near the line composed of CaO∙3Fe2O3–4CaO–3SiO2; and Al3+ exists only in the form of substituting Fe3+, resulting in different crystal forms of the two composite calcium ferrites. The effect of aluminum dissolved in hematite on the formation of calcium ferrites at 1473 K was studied. The results revealed that the formation of calcium ferrite containing aluminum (CFA) was promoted by both granular Al2O3 wrapped in Fe2O3 and aluminum dissolved in the Fe2O3 solid solution [11]. Machida et al., evaluated the fluidity of the melts by measuring the viscosity of the melts formed in the sintering reaction. The viscosity of low SiO2 content increased with the addition of Al2O3 and SiO2. With melt compositions generated from actual ores, the viscosity depended on the contents of Al2O3 and SiO2 [12]. Wu et al., studied the influencing factors and effects of the assimilation characteristics of iron ores in the sintering process, where iron ores’ assimilation has a large effect on the fluidity of the liquid phase and the bonding strength of the sintered body. The results indicated that low Al2O3 (<1.5 mass %) was good for the assimilation, but high Al2O3 (≥1.5 mass %) was bad [13].
The presented research focuses on the formation of calcium ferrite (CF), CFA, and SFCA in the presence of Al2O3 and its effects on the assimilation and viscosity behaviors. However, the Al2O3 content also affects the amount and the fluidity of the liquid phase, and further affects the sinter quality.
Kasai et al., studied the effect of iron ore properties on the flow of melt formed in the sintering process and found that an increase of the Al2O3 content in ores led to decreased fluidity of the melt formed and increased melt-down temperature [14]. Zhang et al., showed that the liquidity index decreased with increased Al2O3 content [15]. Dong et al., indicated that the high alumina content was bad for the melt formation and fluidity properties of iron ores, and had an adverse effect on sinter quality [16]. Jeon et al., studied the assimilation behavior of quasi-particle-comprised, high alumina pisolitic ore. The results showed that the quasi-particle comprised high Al2O3 pisolitic ore and ultra-fine magnetite ore obtained high sinter quality [17]. Umadevi et al., revealed that with increasing alumina content in the sinter of both the conventional and selective granulation processes, the fractions of hematite and of SFCA, and the pore phase increased, whereas the magnetite and silicate phases decreased [18]. In addition, Xiao et al., showed that the Al2O3 content has little effect on the liquidity of the liquid phase [19].
Thus, earlier studies have resulted in different conclusions concerning the effects of Al2O3 on the amount and fluidity of the liquid phase. With the deterioration of the quality of iron ore fines, the content of Al2O3 has been increasing significantly in recent years. Therefore, it is necessary to investigate the effects of Al2O3 content on the amount and fluidity of the liquid phase and reveal the influence mechanism of Al2O3 on sinter quality.
2. Experimental
2.1. Experimental Content
Firstly, the liquid isotherm of the CaO–Fe2O3–Al2O3 ternary system with different Al2O3 contents (fixed SiO2 content) was analyzed by FactSage thermodynamics software, and the liquid phase formation under equilibrium conditions was studied. Secondly, XRD was used. Scanning electron microscopy analysis were used to study the formation of the binder phase with different Al2O3 contents (fixed SiO2 content) under non-equilibrium conditions, and to analyze the influence mechanism of Al2O3 content on fluidity. Lastly, the effects of different Al2O3 contents (fixed SiO2 content) on the consolidation strength of mineral powder was examined.
Referring to the actual mixed mineral composition of Shanghai Meishan Iron and Steel Company, the analytical pure Fe2O3, SiO2, and Al2O3 chemical reagents were used to prepare the mixture, as shown in Table 1. The weighed reagents were placed in an agate mortar and added with absolute ethanol for 20 min. After drying in an oven at 70 °C, the mixture was stirred for another 20 min to uniformly mix the raw materials, and the mixture was pressed into a block under a pressure of 20 MPa. Considering that SiO2 and Al2O3 are mainly present in iron ore in the form of gangue, the mixture was calcined at 1300 °C for 4 h with a muffle furnace before the test to simulate natural ore. The mixed ore was then taken out and broken to −0.05 mm pieces.

Table 1.
The influence of Al2O3 content on liquid phase fluidity (wt %).
2.2. Experimental Methods
2.2.1. Liquid Phase Fluidity Test of Iron Ore Fines with Different Gangue Contents
The conditions of the actual sintering raw materials were simulated, and this work investigated the liquid phase fluidity changes when the Al2O3 content was changed from 1 to 5 wt % under the conditions of sintering temperature of 1300 °C and 4 wt % of SiO2.
2.2.2. XRD Diffraction, Mineral Phase Analysis, and Scanning Electron Microscopy Analysis
A part of the sintered liquid phase fluid sample, after cooling, was ground and matched with a certain proportion of the reference phase, and uniformly mixed. Then, it was subjected to XRD detection, for which the diffraction angle was in the range of 10° to 90°, and the scanning speed was 2°/min. The samples were then identified against the International Diffraction Data Center Data (ICDD) standard card.
Another part of the sample was analyzed by scanning electron microscopy. The sample was longitudinally cut in the direction of the center line; then, the surface was cut and polished. Afterwards, the mineral phase analysis and scanning electron microscope analysis were performed.
2.2.3. Consolidation Strength Test of the Sintered Body with Different Gangue Content Samples
The consolidation strengths of iron ore fine powders with different Al2O3 contents were determined by the micro-sintering consolidation strength test method.
3. The Influence Mechanism of Al2O3 Content on the Liquid Phase Fluidity of Iron Ore Fines
The change of the liquid phase fluidity when the Al2O3 content changed from 1 to 5 wt % is shown in Figure 1.
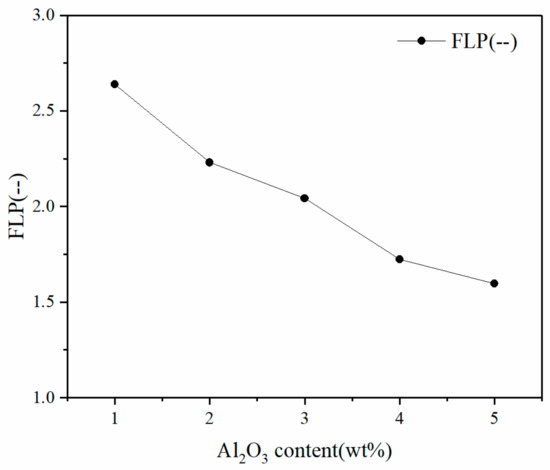
Figure 1.
The effect of Al2O3 content on the liquidity index of the liquid phase.
Fluidity of the liquid phase (FLP) is the index from our previous work to evaluate the fluidity of the liquid phase [13,15]. It can be calculated by
where So and Sa are the projected areas of original sintered cake and the projected area of the sintered cake after the liquid phase fluidity test, respectively. Figure 1 shows that as the content of Al2O3 in the iron ore fines increased, the liquidity index of the liquid phase continuously decreased. According to the fitting relationship between the Al2O3 content and the liquidity index of the liquid phase, the fluidity index decreased with the increase of Al2O3 content as long as the Al2O3 concentration was no higher than 5%.
3.1. Thermodynamic Theoretical Analysis of the Liquid Phase Formation of Iron Ore Powders with Different Al2O3 Contents
The isothermal liquids at 1200–1350 °C with different Al2O3 content were calculated by FactSage 7.0 software. The partial pressure of oxygen was fixed at 5 × 10−3 atm and the content of SiO2 was fixed at 3.40 wt %. The calculation results are shown in Figure 2. The chemical compositions of the samples according to the different Al2O3 contents in Table 1 are indicated by black dots in the figure.
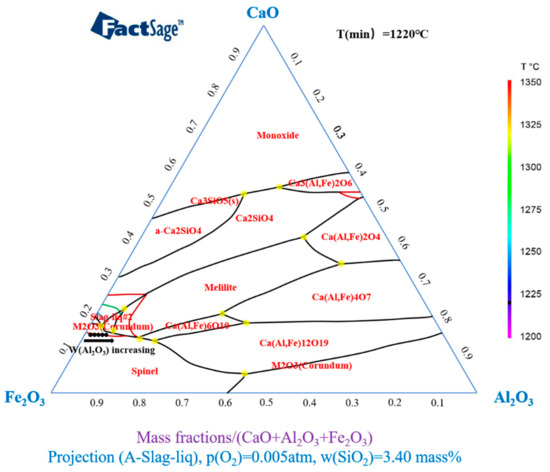
Figure 2.
Isothermal liquids and phase compositions of iron ore fines with different Al2O3 contents from 1200 °C to 1350 °C.
According to the phase diagram calculated by Factsage, the melting temperature of the samples with different Al2O3 content was near the 1350 °C isotherm, and the melting temperature of the sample decreased slightly as Al2O3 content in the sample increased. The results show that the 1350 °C isotherm consists of two parts; namely, the Fe-rich zone and the Al-rich zone. Therefore, during the cooling process, the CaO–Fe2O3–Al2O3–3.40 wt % SiO2 system may precipitate the primary phase except for the melt slag phase, corundum phase M2O3 (Corundum), spinel (Spinel) phase, and potentially the feldspar phase. Melilite and Ca2SiO4 are present in the phase of Ca3(Al,Fe)2O6. The “Equilib” module was used to calculate the liquid phase formation of samples with differing Al2O3 contents at 1300°C and P (O2) = 5 × 10−3 atm, as shown in Figure 3.
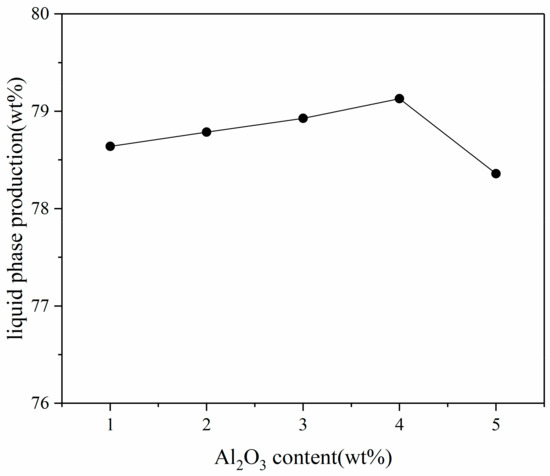
Figure 3.
Relationship between Al2O3 content of iron ore fines and liquid phase production.
The calculation results show that as the Al2O3 content in iron ore fines increased, the amount of the liquid phase formation increased slightly, which is consistent with the conclusions of other researchers [10,20,21]. However, once the Al2O3 content in the iron ore fines reached 4.0 wt %, as the Al2O3 content continued to increase, the liquid phase production began to decrease. Combined with the phase diagram analysis, it was observed that when the Al2O3 content reached 5.0 wt %, the possibility of the presence of spinel in the sample was greatly increased, resulting in a decreased amount of the liquid phase. In general, the change in Al2O3 content has little effect on the amount of the liquid phase formation based on the thermodynamic liquid phase results.
3.2. The Effect of Al2O3 Content on Mineral Composition under Non-Steady-State Conditions
The X-ray diffraction pattern of the samples with different Al2O3 concentrations of iron ore fines with 15 wt % CaO at 1300 °C are shown in Figure 4. The peak of the main phase is indicated by the dotted line.

Figure 4.
XRD diffraction pattern of sintered samples with different Al2O3 contents.
Figure 4 shows that as the Al2O3 content in iron ore fines increased, the intensity of hematite diffraction peak (marking 1) was weakened, indicating that the hematite mineral content in the sample decreased with the increased of Al2O3 content.
According to the analysis of the ore phase, as the content of Al2O3 in the iron ore fines increased, the content of hematite decreased; the hematite gradually changed from coarser scorpion, to fish ridge, and to smaller and discontinuous blocks. According to the analysis, the coarse Fe2O3 grains were mainly composed of secondary oxidation of magnetite without mineralization, while the smaller Fe2O3 grains were oxidized by the SFCA melt at a high temperature. As a result, as the Al2O3 content increased, the possibility of Fe2O3 participating in the reaction increased during the heating phase, because Al3+ with a smaller ionic radius can dissolve the Fe3+ with a larger ionic radius, making Fe2O3 unstable and more soluble into the melt [22]. In addition, the results of Webster et al. [23] showed that increasing the Al2O3 concentration in the sample inhibited the conversion of Fe3O4 to Fe2O3 during cooling.
According to Figure 4, the intensity of the diffraction peak of magnetite (mark 2) increases with increased Al2O3 content, and the intensity of the diffraction peak of magnetite changed little when the content of Al2O3 was 2–4 wt %. When the Al2O3 content reached 5 wt %, the strength of the magnetite peak increased significantly, indicating that when the Al2O3 content is high, the residual magnetite minerals concentration increases. This is consistent with the thermodynamic analysis.
According to the type of composite calcium ferrite mineral formed by different Al2O3 content samples, as shown in Figure 4, when the content of Al2O3 is low, the composite calcium ferrite is mainly SFCA-I type, and as the concentration of Al2O3 increases, the formed composite calcium ferrite peak shifts to the right, forming a chemical formula of the SFCA phase (indicator 4). Webster et al., studied the effects of different Al2O3 contents on the formation and thermal stability of SFCA and SFCA-I phases [24]. The formation temperature of the calcium phase was lowered, and the results show that increasing the Al2O3 concentration seems to be more favorable for the formation of the SFCA phase than the formation of the SFCA-I phase.
The K-valued XRD (Rigaku, Tokyo, Japan) was used to quantitatively analyze the trend of calcium ferrite mineral content under different Al2O3 contents, and the results are shown in Figure 5.
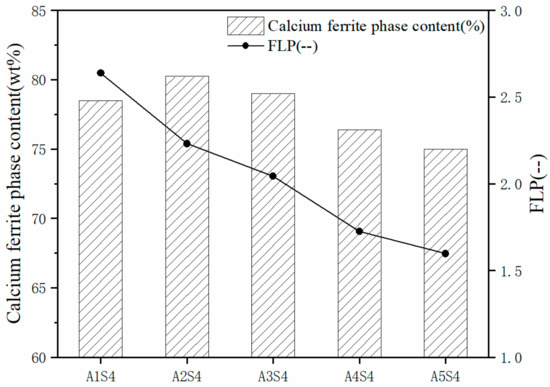
Figure 5.
Calcium ferrite phase formation and liquid phase fluidity index of different Al2O3 content samples.
As shown in Figure 5, as the content of Al2O3 in the sample increased, the content of composite calcium ferrite minerals increased slightly. For example, when the Al2O3 content in the sample was 1.70 wt % (A2S4 sample), the maximum value was reached, along with Al2O3. As the content continued to increase, the calcium ferrite mineral content began to decrease, especially when the Al2O3 content in the iron ore fines exceeded 4.0 wt %. Ding studied the effect of Al2O3 content on SFC (precursor formed by SFCA) in the Fe2O3–CaO–SiO2 system at 1200 °C [25]. The results show that the composition of the sintered mixture sample was similar to the one in that paper. The results show that when the Al2O3 content in the sample was 0.86 wt %, the amount of SFC was much higher than the sample without Al2O3. As the Al2O3 content continued to increase, the rate of SFC formation was more rapid (when it reached 1.72 wt %), especially for promoting SFC production. When the Al2O3 content continued to increase to 2.58 wt %, the promotion of Al2O3 to the formation of SFC decreased. The analysis suggests that the Al2O3 content exceeds the SFC solid solubility limit formed, and the excess Al2O3 exists in the form of C2 (F1−xAx).
The A5S4 sample was taken as an example to study this phenomenon. Figure 6 shows the backscattered electron image of the A5S4 sample. The results of the energy spectrum analysis are shown in Table 2.
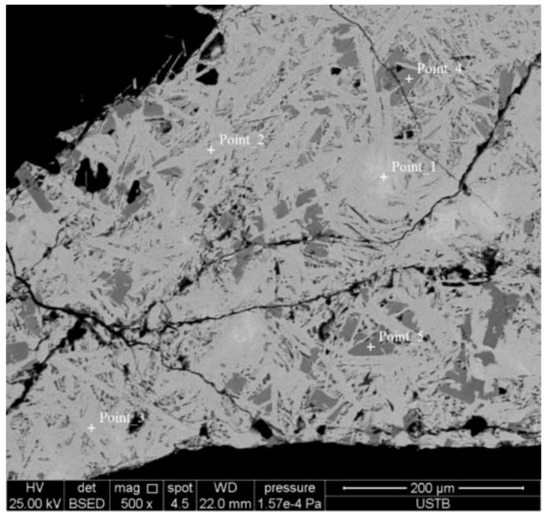
Figure 6.
A5S4 sample backscattered electron image.

Table 2.
Results of main phase energy spectrum analysis of A5S4 sample.
According to the results of energy spectrum analysis and XRD analysis, the minerals in the energy spectrum of Figure 6 are magnetite minerals with small amounts of Al3+ and Ca2+. The compositions of energy spectrum points 2 and 3 were similar, and only the solid solution of Si had the difference analyzed according to composition and morphology as SFCA-I type. The results show that the composition analysis of energy spectrum points 4 and 5 presented a kind of aluminate mineral that dissolved a certain amount of silicon, made from the Al3+ and Fe3+. The concentrations fluctuated within a certain range, but the total ion ratios of Al3+ and Fe3+ were 19%, equivalent to the ratio of the Ca ion. Thus, it was considered to be Ca2(AlxFe2-x)O5 mineral by XRD analysis.
The analysis shows that, in general, an appropriate amount of Al2O3 solid solution reduces the stability of the Fe2O3 crystal, making liquid phase formation easier. The dissolution of Al2O3 in the liquid phase leads to an increase in the viscosity of the liquid phase, and according to the study by Maeda et al. [26], Al2O3 promotes the dissolution of solid-phase Fe2O3 into the calcium ferrite melt, making the overall liquid phase more viscous and decreasing the liquid phase fluidity. In addition, when the Al2O3 content in the iron ore fines is too high (≥3 wt %), as the solid solubility of Al2O3 in the composite calcium ferrite exceeds the limit, the excess Al2O3 is an aluminate mineral (Ca2(AlxFe2-x)O5) form that precipitates at the leading edge of the liquid phase spreading, thereby further suppressing its fluidity.
In summary, in terms of liquid phase fluidity, an increase in the Al2O3 content of iron ore fines results in a more pronounced decrease in liquid phase fluidity, and when the Al2O3 content in the iron ore fines exceeds 3 wt %, there is a clear formation of aluminate minerals.
4. The Effect of Al2O3 Content on the Consolidation Strength of a Sintered Body
During the sintering process, as the temperature increases, the adherent powder in the sintered quasiparticles melted to form a liquid phase and flows, expands, and envelops the unmelted particles, and fills the voids between the quasiparticles. These binder phases are then in a cold, solidified knot. After the sintered body is obtained with sufficiently high strength, the liquid phase bonding in the sintering process and the strength of the binder phase have an important effect on the strength of the sintered ore. In particular, the degree of liquid phase bonding between the quasiparticles directly affects the consolidation strength of the sintered body, thereby affecting the sinter yield index.
The results of the determination of the consolidation strength of the sintered body of the different Al2O3 content iron ore fines as the adhesion powders are shown in Table 3, and the liquid phase fluidity relationship with the corresponding sample is shown in Figure 7.

Table 3.
Determination of consolidation strengths of sintered bodies of different Al2O3 content samples (%).
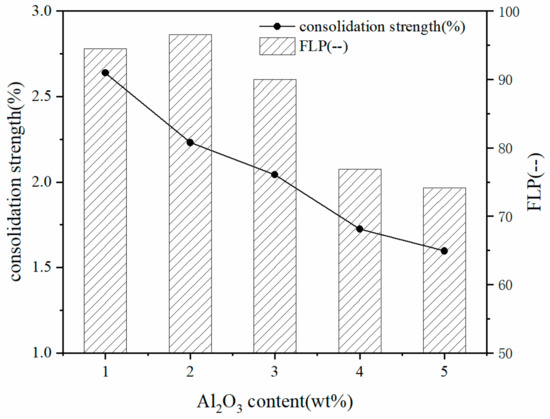
Figure 7.
Relationship between liquid phase fluidity and consolidation strength of samples with different Al2O3 content.
According to Figure 7, the consolidation strength of the sintered body composed of different Al2O3 content adhesion powders increased slightly initially; when the Al2O3 content exceeded 2 wt %, the consolidation strength of the sintered body began to decrease [1,7]. The results of the liquid phase fluidity index of different Al2O3 content adhesive powders show that with the increased Al2O3 content, the liquidity of the sample liquid phase decreased continuously. This rule is consistent with the case where the Al2O3 content is greater than 2 wt %. In the same manner, this is also caused by insufficient adhesion between the quasiparticles after the liquidity of the liquid phase is reduced. For the A1S4 sample, our analysis is that the liquid phase fluidity was too high, causing the microporous, thin-wall structure of the binder phase. Figure 8 shows the pore structure of the different Al2O3 content adhesion powders.
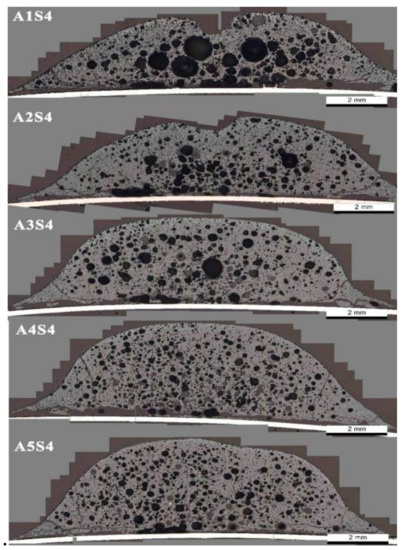
Figure 8.
Microstructure of pore structure of different Al2O3 content adhesive powders.
Figure 8 shows that as the content of Al2O3 in the sample increased, the pore diameter inside the sample significantly decreased. The pores of the A1S4 sample were larger, and the pore walls between the atmospheric pores were thinner, forming a typical, “large pore thin wall” structure. Studies have shown that the self-strength of this structural binder phase is low, which is not conducive to improving the consolidation strength of the sintered body [27].
To investigate the relationship between the liquid phase fluidity of the adherent powder and the consolidation strength of the sintered body, the liquid phase fluidity indexes of the iron ore fines of different gangue contents were fitted to the measurement results of the consolidation strength, as shown in Figure 9.
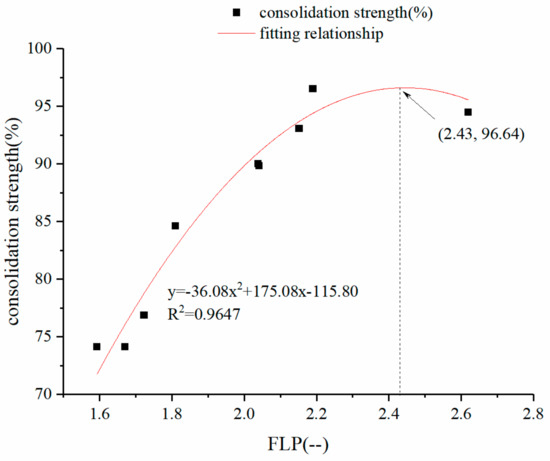
Figure 9.
Fitting relationship between liquid phase fluidity and consolidation strength of samples with different gangue contents.
According to Figure 9, the liquid phase fluidity of the adherent powder has a quadratic relationship with the consolidation strength of the sintered body. As the liquidity index increases, the consolidation strength of the sintered body begins to significantly increase. This increase is because, as the fluidity of the liquid phase is improved, the bonding effect between the quasiparticles is improved, and the consolidation strength is improved; when the liquid phase fluidity index of the adherent powder reaches a high level (FLP ≥ 2.43), the consolidation strength decreases, which is because that the liquid phase fluidity is large and the bonding between the quasiparticles is relatively sufficient. At this time, the adhesion between the quasiparticles is no longer a limiting link, and the liquid phase fluidity is excessive. In contrast, the binder phase produces a fragile structure with a “large pore thin wall,” so the consolidation strength of the sintered body is lowered.
In summary, the differences in the consolidation strength of the quasiparticles of iron ore fines, with different gangue contents as their adhesion powders, are mainly affected by the liquid phase fluidity of the iron ore adhering powder, and the suitable liquid phase fluidity is improved. Under the conditions of this study, when the SiO2 content of iron ore fines is about 4 wt % and the Al2O3 content is about 2 wt %, the consolidation strength of the sintered compact is optimal.
5. Conclusions
The mechanism of the influence of differing Al2O3 content on the liquidity of iron ore powder was studied by thermodynamic calculation, XRD, and scanning electron microscopy. Research indicates:
- (1)
- According to the results of the liquid phase fluidity index of iron ore fines with different gangue contents, as the Al2O3 content in iron ore fines increases, the liquidity index of the liquid phase decreases continuously.
- (2)
- The phase diagram analysis of the CaO–Fe2O3–Al2O3 ternary system shows that the change of Al2O3 content has little effect on the liquid phase formation. The melting temperature of different Al2O3 samples is near the 1350 °C isothermal liquidus.
- (3)
- A small amount of Al2O3 will increase the formation of calcium ferrite. The solid solution of Al2O3 reduces the stability of Fe2O3 crystal, which makes the liquid phase formation easier. As the content of Al2O3 in the sample increases, Al2O3 dissolves. The liquid phase leads to an increase in the viscosity of the liquid phase, decrease in the fluidity of the liquid phase, and coarsening of the grain of the calcium ferrite phase, which progresses from a typical needle-like strip-like SFCA-I phase to a plate-like, sheet-like SFCA phase.
- (4)
- The effect of different gangue contents of adhesive powder on the consolidation strength of the sintered body shows that under the conditions of this study, when the SiO2 content of iron ore fines is about 4 wt %, the Al2O3 content is about 2 wt %. The liquid phase fluidity of the iron ore fines, the amount of calcium ferrite formed, and the consolidation strength of the sintered body are optimal.
- (5)
- Under the conditions of the present study, the consolidation strength of the sintered compact is optimal when the SiO2 content and the Al2O3 content of iron ore fines are about 4 wt % and 2 wt %, respectively.
Author Contributions
Conceptualization, S.W. and M.K.; methodology, H.Z. and Z.H.; Software, H.L. and H.Z.; validation, W.Z. and Z.H.; formal analysis, H.L. and Z.H.; investigation, H.L. and Z.H.; resources, H.Z. and S.W.; data curation, H.L. and Z.H.; writing, H.L. and Z.H.; supervision, S.W. and M.K.; funding acquisition, H.Z. and M.K.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 51804027 and 51904023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X. Ferrous Metallurgy (Ironmaking Part), 3rd ed.; Metallurgical Industry Press: Beijing, China, 2013. [Google Scholar]
- Fu, J.Y.; Jiang, T.; Zhu, D.Q. Sintering and Pelleting, 1st ed.; Central South University Press: Changsha, China, 1996. [Google Scholar]
- Emamy, M.; Abbasi, R.; Kaboli, S.; Campbell, J. Fluidity of Al based metal matrix composites containing Al2O3 and SiC particles. Int. J. Cast Metal. Res. 2009, 22, 430–437. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; Mochón, J.; González-Gasca, C.; Verdeja, L.F. Iron ore sintering: Quality indices. Min. Process. Extract. Metall. Rev. 2017, 38, 254–264. [Google Scholar] [CrossRef]
- Kramer, S.; Yang, J.; Levi, C.G.; Johnson, C.A. Thermochemical interaction of thermal barrier coatings with molten CaO-MgO-Al2O3-SiO2 (CMAS) deposits. J. Am. Ceram. Soc. 2006, 89, 3167. [Google Scholar] [CrossRef]
- Muwanguzi, A.J.; Karasev, A.V.; Byaruhanga, J.K.; Jönsson, P.G. Characterization of chemical composition and microstructure of natural iron ore from Muko deposits. ISRN Mater. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Fan, X.H. Principle and Technology of Iron Ore Matching for Sintering; Metallurgical Industry Press: Beijing, China, 2013; p. 68. [Google Scholar]
- Park, J.H.; Cho, Y.J.; Yoon, S.S.; Huh, W.W.; Kim, H.S. Effect of Al2O3,SiO2 and MgO on the formation of calcium ferrites in sinter using X-ray diffraction method. J. Korean Inst. Met. Mater. 2002, 40, 811–817. [Google Scholar]
- Dawson, P.R.; Ostwald, J.; Haves, K.M. Influence of alumina on development of complex calcium ferrites in iron ore sinters. Trans. Inst. Min. Metall. Sect. C 1985, 94, 71–78. [Google Scholar]
- Scarlett, N.V.Y.; Pownceby, M.I.; Madsen, I.C.; Christensen, A.N. Reaction Sequences in the Formation of Silico-ferrites of Calcium and Aluminum in Iron Ore Sinter. Metall. Mater. Trans. B 2004, 35, 929–936. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X.M. Effect of Aluminum Dissolved in Hematite on Formation of Calcium Ferrites at 1473 K. Metall. Mater. Trans. B 2018, 49, 1974–1984. [Google Scholar] [CrossRef]
- Machida, S.; Nushiro, K.; Ichikawa, K.; Noda, H.; Sakai, H. Experimental evaluation of chemical composition and viscosity of melts during iron ore sintering. ISIJ Int. 2005, 45, 513–521. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, G.; Chen, S.; Su, B. Influencing factors and effects of assimilation characteristic of iron ores in sintering process. ISIJ Int. 2014, 54, 582–588. [Google Scholar] [CrossRef]
- Kasai, E.; Sakano, Y.; Nakamura, T. Influence of iron ore properties on the flow of melt formed in the sintering process. Tetsu-to-Hagané 2000, 86, 139–145. [Google Scholar] [CrossRef]
- Zhang, G.L.; Wu, S.L.; Chen, S.G.; Su, B.; Que, Z.G.; Hou, C.G. Influence of gangue existing states in iron ores on the formation and flow of liquid phase during sintering. Int. J. Miner. Metall. Mater. 2014, 21, 962–968. [Google Scholar] [CrossRef]
- Dong, J.J.; Wang, G.; Gong, Y.G.; Xue, Q.G.; Wang, J.S. Effect of high alumina iron ore of gibbsite type on sintering performance. Ironmak. Steelmak. 2015, 42, 34–40. [Google Scholar] [CrossRef]
- Jeon, J.W.; Kim, S.W.; Suh, I.K.; Jung, S.M. Assimilation behavior of quasi-particle comprising high alumina pisolitic ore. ISIJ Int. 2014, 54, 2713–2720. [Google Scholar] [CrossRef]
- Umadevi, T.; Deodar, A.V.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of alumina on iron ore sinter properties and productivity in the conventional and selective granulation sintering process. Steel Res. Int. 2009, 80, 686–692. [Google Scholar]
- Xiao, Z.X.; Chen, L.K.; Yang, Y.D.; Li, X.C.; Barati, M. Effect of Coarse-grain and Low-grade Iron Ores on Sinter Properties. ISIJ Int. 2017, 57, 795–804. [Google Scholar] [CrossRef]
- Lv, X.W.; Bai, C.G.; Deng, Q.Y.; Huang, X.B.; Qiu, G.B. Behavior of liquid phase formation during iron ores sintering. ISIJ Int. 2011, 51, 722–727. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, L.; Liu, L.X.; An, S.L. Relationship Between Liquid Fluidity of Iron Ore and Generated Liquid Content During Sintering. Metall. Mater. Trans. B 2017, 48, 538–544. [Google Scholar] [CrossRef]
- Nandy, B.; Chaudhury, M.K.; Paul, J.; Bhattacharjee, D. Sintering Characteristics of Indian Chrome Ore Fines. Metall. Mater. Trans. B 2009, 40, 662–675. [Google Scholar] [CrossRef]
- Ding, X. Study on the Mechanism on Formation of Calcium Ferrite in Then Fe2O3-CaO-SiO2 System; University of Science and Technology Beijing: Beijing, China, 2015. [Google Scholar]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of Calcium and Aluminum (SFCA) Iron Ore Sinter Bonding Phases: New Insights into Their Formation During Heating and Cooling. Metall. Mater. Trans. B 2012, 43, 1344–1357. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Madsen, I.C.; Pownceby, M.I.; Christensen, A.N. In situ X-ray diffraction analysis of iron ore sinter phases. J. Appl. Crystallogr. 2004, 37, 362–368. [Google Scholar] [CrossRef]
- Maeda, T.; Nishioka, K.; Nakashima, K.; Shimizu, M. Formation rate of calcium ferrite melt focusing on SiO2 and Al2O3 component. ISIJ Int. 2004, 44, 2046–2051. [Google Scholar] [CrossRef]
- Zhu, J. Study on Consolidation Mechanism and Strengthening Technology of Baosteel Ore Powder Sintering; University of Science and Technology Beijing: Beijing, China, 2014. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).