Systematic Separation and Purification of Alkaloids from Euchresta tubulosa Dunn. by Various Chromatographic Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
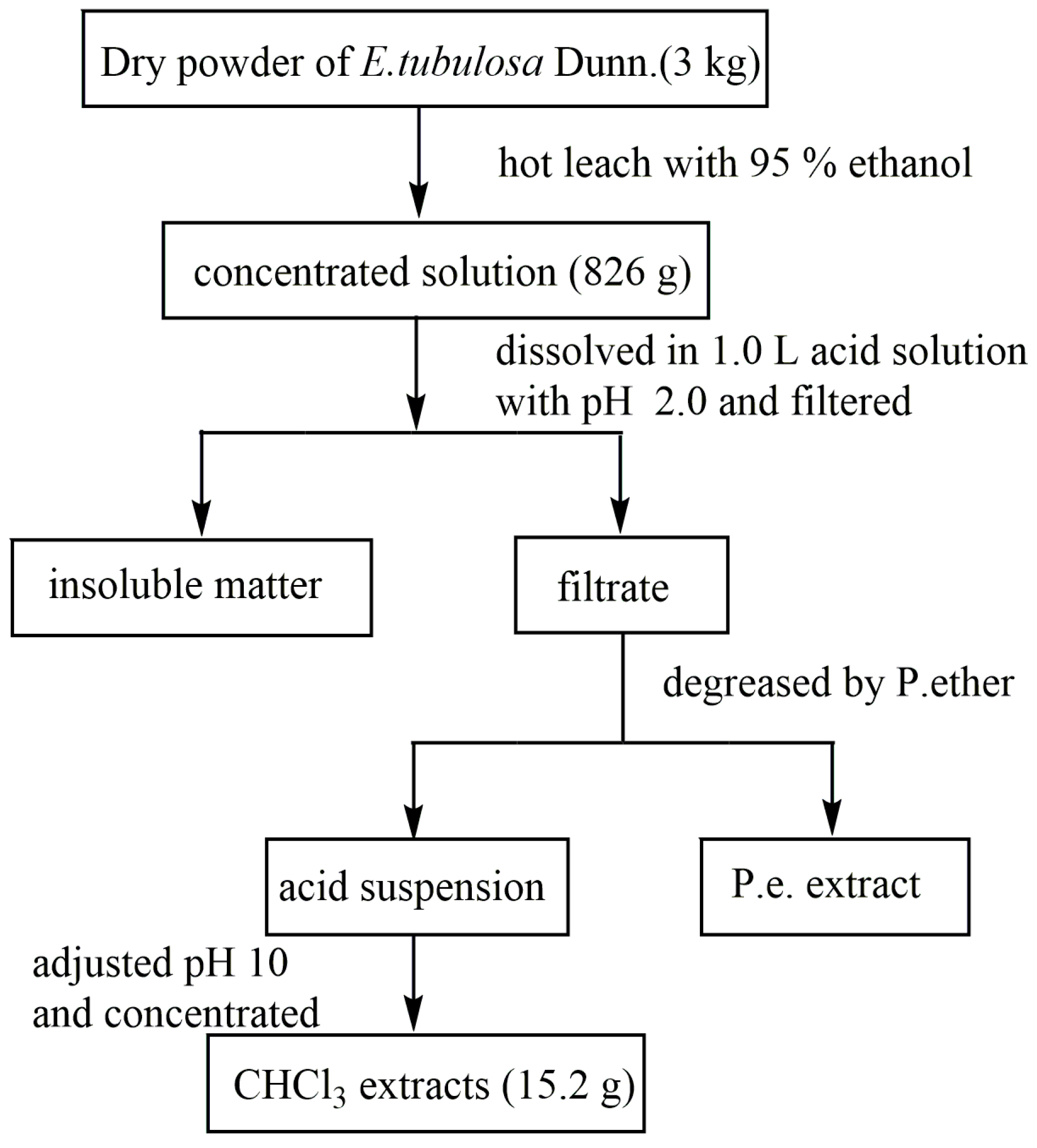
2.2. Preparation of Crude Alkaloid Extract
2.3. Preliminary Separation of Crude Alkaloid Extract
2.4. Partition Coefficient Measurement
2.5. Selection of Two-Phase Solvent System
2.6. HSCCC Separation
2.7. HPLC-UV Analysis and Structural Identification of HSCCC Peak Fractions
3. Results
3.1. Selection of Two-Phase Solvent System
3.2. Systematic Separation
3.3. HPLC-UV Analysis
3.4. The Structural Identification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matsuura, N.; Iinuma, M.; Tanaka, T.; Mizuno, M. Flavonoids and a benzofuran in roots of Euchresta tubulosa. Phytochemistry 1993, 33, 701–705. [Google Scholar] [CrossRef]
- Ohmiya, S.; Otomasu, H.; Haginiwa, J.; Murakoshi, I. The alkaloid constituents of Euchresta japonica and the stereochemical assignment of two isomeric sophoridine N-oxides. Chem. Pharm. Bull. 1980, 28, 546–551. [Google Scholar] [CrossRef]
- Ohno, H.; Inuki, S. Recent progress in palladium-catalyzed cascade cyclizations for natural product synthesis. Synthesis 2018, 50, 700–710. [Google Scholar] [CrossRef]
- Wu, D.; Du, J.; Zhang, Y.; Su, Y.; Zhang, H. Anti-tumor effects of phenolic alkaloids of menispermum dauricum on gastric cancer in vivo and In Vitro. J. Cancer Res. Ther. 2018, 14, 505–511. [Google Scholar]
- Zheng, S.; Gu, Y.; Zhu, R.; Li, L.; Bai, H.; Zhang, J. Synthesis and Antibacterial Activity of Calycanthaceous Alkaloid Derivatives. Chem. Nat. Compd. 2018, 54, 127–130. [Google Scholar] [CrossRef]
- Metrouh-Amir, H.; Amir, N. Evaluation in vivo of anti-inflammatory and analgesic properties of Matricaria pubescens alkaloids. S. Afr. J. Bot. 2018, 116, 168–174. [Google Scholar] [CrossRef]
- Aggrey, M.O.; Li, H.H.; Wang, W.Q.; Wang, Y.; Xuan, L.J. Indole alkaloid from Nauclea latifolia promotes LDL uptake in HepG2 cells by inhibiting PCSK9. Phytomedicine 2019, 55, 264–268. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E.; Shawer, D.M. Facile synthesis of silver nanoparticles using harmala alkaloids and their insecticidal and growth inhibitory activities against the khapra beetle. J. Pest Sci. 2018, 91, 727–737. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef]
- Tutka, P.; Vinnikov, D.; Courtney, R.J.; Benowitz, N.L. Cytisine for nicotine addiction treatment: A review of pharmacology, therapeutics and an update of clinical trial evidence for smoking cessation. Addiction 2019, 114, 1951–1969. [Google Scholar] [CrossRef]
- Bartusik, D.; Aebisher, D.; Tutka, P. A Review of the Organic Synthesis and Medicinal Applications of the Natural Product Cytisine. Mod. Org. Chem. Res. 2016, 1, 10–23. [Google Scholar] [CrossRef]
- Pan, Q.M.; Li, Y.H.; Hua, J.; Huang, F.P.; Wang, H.S.; Liang, D. Antiviral matrine-type alkaloids from the rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015, 78, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.M.; Zhang, G.J.; Huang, R.Z.; Pan, Y.M.; Wang, H.S.; Liang, D. Cytisine-type alkaloids and flavonoids from the rhizomes of Sophora tonkinensis. J. Asian Nat. Prod. Res. 2016, 18, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, G.H.; Chen, L.; Tang, Y.Q.; Xu, Y.K.; Liu, B.; Yin, S. New pyridocarbazole alkaloids from Strychnos nitida. Nat. Prod. Res. 2018, 32, 1532–1536. [Google Scholar] [CrossRef]
- Ito, Y. Efficient preparative counter-current chromatography with a coil planet centrifuge. J. Chromatogr. A 1981, 214, 122–125. [Google Scholar] [CrossRef]
- Cai, X.; Yu, H.; Yu, Y.; Li, Q.; Chen, B.; Huang, Y.; Zou, X.W.; Huang, B.S.; Tang, J. Separation of five naphtho-γ-pyrones from Pleurotus ostreatus by high-speed countercurrent chromatography. J. Sep. Sci. 2018, 41, 4551–4558. [Google Scholar] [CrossRef]
- Fang, L.; Liu, Y.; Yang, B.; Wang, X.; Huang, L. Separation of alkaloids from herbs using high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 2545–2558. [Google Scholar] [CrossRef]
- Cao, X.L. High-Speed Counter-Current Chromatography Technology and Its Application; China Chemical Industry Press: Beijing, China, 2005; p. 44. [Google Scholar]
- Khakimova, T.V.; Pukhlyakova, O.A.; Shavaleeva, G.A.; Fatykhov, A.A.; Vasil’eva, E.V.; Spirikhin, L.V. Synthesis and stereochemistry of new n-substituted cytisine derivatives. Chem. Nat. Compd. 2001, 37, 356–360. [Google Scholar] [CrossRef]
- Turdybekov, K.M.; Kulakov, I.V.; Turdybekov, D.M.; Mahmutova, A.S. Conformational states and crystal structure of n-formylcytisine. Russ. J. Gen. Chem. 2017, 87, 2493–2496. [Google Scholar] [CrossRef]
- Ling, J.Y.; Zhang, G.Y.; Cui, Z.J.; Zhang, C.K. Supercritical fluid extraction of quinolizidine alkaloids from sophora flavescens ait. and purification by high-speed counter-current chromatography. J. Chromatogr. A 2007, 1145, 123–127. [Google Scholar] [CrossRef]
- Ohmiya, S.; Otomas, H.; Murakoshi, I.; Haginiwa, J. N-formylcytisine: A new alkaloid from thermopsis chinensis. Phytochemistry 1974, 13, 643–644. [Google Scholar] [CrossRef]
- Abdel-Halim, O.B.; Fattah, H.A.; Halim, A.F.; Murakoshi, I. (+)-Sparteine N-16-oxide, a lupin alkaloid from Lygos raetam var. sarcocarpa. Acta Pharm. Hung. 1997, 67, 9–12. [Google Scholar] [PubMed]
- Sagen, A.L.; Gertsch, J.; Becker, R.; Heilmann, J.; Sticher, O. Quinolizidine alkaloids from the curare adjuvant Clathrotropis glaucophylla. Phytochemistry 2002, 61, 975–978. [Google Scholar] [CrossRef]





| Solvent System | K Values | ||
|---|---|---|---|
| Carbon Tetrachloride/Dichloromethane/Methanol/Water (3:7:6:4, v/v) | Dichloromethane/Methanol/Water (4:3:2, v/v) | Dichloromethane/Methanol/Water (5:2:3, v/v) | |
| Matrine | 0.52 | − | − |
| Oxymatrine | 0.88 | − | − |
| N-formyl cytisine | − | 0.92 | − |
| Cytisine | − | − | 1.11 |
| NO. | Matrine | Oxymatrine | ||
|---|---|---|---|---|
| 13C | 1H | 13C | 1H | |
| 1 | / | / | / | / |
| 2 | 53.17 | 2.81 (t, 1H, 13.1 Hz) 1.96 (s, 1H) | 68.02 | 3.36–3.29 (m, 1H) 3.07 (ddd, 1H, 7.7, 3.8, 1.9 Hz) |
| 3 | 20.73 | 1.83–1.54 (m,1H) 1.52–1.37 (m, 1H) | 16.85 | 2.64–2.46 (m, 2H) |
| 4 | 27.12 | 1.83–1.54 (m, 1H) 1.52–1.37 (m, 1H) | 25.37 | 1.91–1.80 (m, 1H) 1.79–1.55 (m, 1H) |
| 5 | 36.39 | 1.83–1.54 (m, 1H) | 34.33 | 1.91–1.80 (m, 1H) |
| 6 | 63.76 | 2.29–2.18 (m, 1H) | 68.50 | 3.36–3.29 (m, 1H) |
| 7 | 41.45 | 1.52–1.37 (m,1H) | 41.84 | 1.79–1.55 (m, 1H) |
| 8 | 26.42 | 1.83–1.54 (m, 1H) 1.52–1.37 (m, 1H) | 23.75 | 1.91–1.80 (m, 1H) 1.79–1.55 (m, 1H) |
| 9 | 21.14 | 2.10–2.02 (m, 1H) 1.52–1.37 (m, 1H) | 18.21 | 2.08 (d, 1H, 12.8 Hz) 1.79–1.55 (m, 1H) |
| 10 | 57.25 | 2.77 (d, 1H, 11.3 Hz) 2.10–2.02 (m, 1H) | 66.42 | 3.36–3.29 (m, 1H) 3.07 (ddd, 1H, 7.7, 3.8, 1.9 Hz) |
| 11 | 57.19 | 3.80 (td,1H, 9.7, 6.0 Hz) | 53.56 | 3.36–3.29 (m, 1H) |
| 12 | 27.73 | 1.83–1.54 (m, 1H) 1.89–1.80 (m, 1H) | 27.88 | 1.42–1.33 (m, 1H) 2.33–2.23 (m, 1H) |
| 13 | 18.93 | 1.94–1.90(m,1H) 1.83–1.54 (m, 1H) | 16.73 | 1.79–1.55 (m, 2H) |
| 14 | 32.79 | 2.40 (dt, 1H, 17.2, 3.7 Hz) 2.29–2.18 (m, 1H) | 32.18 | 2.40 (d, 1H, 17.4 Hz) 2.33–2.23 (m, 1H) |
| 15 | 169.46 | / | 171.40 | / |
| 17 | 43.30 | 4.37 (dd, 1H, 12.9, 4.5 Hz) 3.03 (t, 1H, 12.7 Hz) | 42.19 | 4.32 (d, 1H, 5.1 Hz) 4.09 (t, 1H, 12.4 Hz) |
| NO. | N-Formyl Cytisine | Cytisine | ||
|---|---|---|---|---|
| 13C | 1H | 13C | 1H | |
| 1 | / | / | / | 2.37(s, 1H, N-H) |
| 2 | 51.90/45.84 | 3.44/3.42 (dd, 1H, 12.8, 2.5 Hz) 3.56/3.53 (d, 1H, 1.8 Hz) | 51.76 | 3.07 (d, 1H, 12.0 Hz) 2.99–2.97 (d, 1H, 13.2 Hz) |
| 3 | 26.89/26.52 | 2.55 (s, 1H) | 27.51 | 2.39–2.35(m,1H) |
| 4 | 48.65/48.42 | 3.85/3.88 (dd, 1H, 15.7, 6.0 Hz) 4.10/4.08 (d, 1H, 15.6, 3.6 Hz) | 52.80 | 4.08 (d, 1H, 15.5 Hz) 3.93 (dd, 1H, 15.5, 6.8 Hz) |
| 5 | / | / | / | / |
| 6 | 163.14/163.0 | / | 164.4 | / |
| 7 | 117.56/117.26 | 6.45/6.44 (d, 1H, 9.0 Hz) | 115.38 | 6.45 (d, 1H, 9.0 Hz) |
| 8 | 138.87/138.50 | 7.27/7.19 (m, 1H) | 139.9 | 7.49 (dd, 1H, 9.0, 7.0 Hz) |
| 9 | 104.96/105.70 | 6.08/6.02 (d, 1H, 6.8 Hz) | 106.7 | 6.30 (d, 1H, 7.0 Hz) |
| 10 | 147.93/147.88 | / | 151.7 | / |
| 11 | 33.65/34.38 | 3.10 (m, 1H) | 35.11 | 3.03 (m, 1H) |
| 12 | 53.20/46.89 | 4.46/4.36 (d, 1H, 13.6 Hz) 2.88 (m, 1H) | 49.75 | 3.46 (m, 1H, 11.4 Hz) 2.99–2.97 (d, 1H, 12.0 Hz) |
| 13 | 26.13/26.07 | 2.08/2.09 (dd, 2H, 8.3, 4.8 Hz) | 25.32 | 2.10–1.93 (m, 2H) |
| 14 | 160.90/161.01 | 7.83/7.60 (s, 1H) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-X.; Wang, H.; Dong, A.-W. Systematic Separation and Purification of Alkaloids from Euchresta tubulosa Dunn. by Various Chromatographic Methods. Processes 2019, 7, 924. https://doi.org/10.3390/pr7120924
Li W-X, Wang H, Dong A-W. Systematic Separation and Purification of Alkaloids from Euchresta tubulosa Dunn. by Various Chromatographic Methods. Processes. 2019; 7(12):924. https://doi.org/10.3390/pr7120924
Chicago/Turabian StyleLi, Wei-Xin, Huan Wang, and Ai-Wen Dong. 2019. "Systematic Separation and Purification of Alkaloids from Euchresta tubulosa Dunn. by Various Chromatographic Methods" Processes 7, no. 12: 924. https://doi.org/10.3390/pr7120924
APA StyleLi, W.-X., Wang, H., & Dong, A.-W. (2019). Systematic Separation and Purification of Alkaloids from Euchresta tubulosa Dunn. by Various Chromatographic Methods. Processes, 7(12), 924. https://doi.org/10.3390/pr7120924





