1. Introduction
Continuous improvement of people’s living standards is associated with intensive catering industry development, which results in a large amount of restaurant wastewater contaminated by various amounts of vegetable and animal oils. Untreated direct discharge of catering wastewater can reach more than 100 million tons each year [
1]; however, untreated restaurant wastewater is not allowed to be discharged directly into the municipal sewerage network [
1]. Domestic wastewater commonly contains average oil and grease contents in the range from 50 to 150 mg/L; however, local contents may be significantly higher (over 400 ppm) [
2]. Several international conventions regulate the concentration of oil, which can be discharged to the environment from 15 to 42 ppm [
3].There are several analytical techniques, such as measuring total organic content, spectroscopy, and chromatography, to measure contamination of water by oil. However, these techniques require using costly and non-compact instruments. Hence, a cheap, simple, and portable tool to detect oil contamination would be useful.
One approach to detect oil contamination is based on using sensors for liquids and gases based on electrically conductive polymer composites (ECPCs) designed from various kinds of polymeric matrices and electrically conductive fillers [
4]. The principle of sensing is that the ECPC resistivity increases once the material is exposed to organic solvent vapor or liquid due to wetting of the ECPC by the organic solvent and diffusion into the composite. This results in swelling of the polymer matrix, thereby increasing the space between conductive fillers, which increases the resistivity. In addition to the solubility of the solvent in the polymer, the sensing capability depends on the thickness of the sensing element, the content of the conductive filler, and the organic vapor saturated pressure [
5]. Typically, for ECPC preparation, either the solution or hot melting mixing is used to incorporate conductive nanofillers into the polymer matrix. This is followed by casing, hot melt pressing, or electrospinning [
6]. The distribution, size, and geometrical parameters (e.g., aspect ratio) of the nanoparticles are crucial for reaching the percolation threshold, which is known as the critical volume content of conductive fillers at the transition of the polymer from being an insulator to being semi-conductive. For this reason, conductive nanofillers such as CNTs, graphite, and carbon black are often preferred because they can form a continuous conductive network with a matrix at low concentrations [
7]. Among other applications, ECPCs have also found applicability in oil detection [
8]. The principle is the selective absorption of oil components from a water/oil dispersion.
Herein, for the first time, the preparation of a simple, easy to use, and portable sensor for the detection of dispersed vegetable oil within water using electrically conductive nanocomposites based on styrene-isoprene-styrene copolymer and multiwall carbon nanotubes fabricated by electrospinning is reported. CNT fillers were used in this study in order to increase electrical conductivity of SIS electrospun mats. CNTs are relatively cheap, well-known fillers that are frequently used to reinforce various properties of polymeric composites.
In order to prepare the active layer of a sensor, the electrospinning approach possess several advantages such as an ability to tailor the porosity of the layers and the size of individual electrospun fibers. This opens the possibility of easily optimizing swelling characteristics of the sensor.
2. Materials and Methods
2.1. Materials
The following materials were used in this study: Styrene-isoprene-styrene (SIS) tri-block copolymer, 30 wt.% polystyrene (KRATON D1165 PT, KRATON POLYMERS, Belpre, OH, USA), multiwall carbon nanotubes (CNTs) with an outer diameter of 20–30 nm and a length of 10–30 µm (cheaptubes.com), dimethyl formamide (DMF) (Sigma Aldrich, St. Louis, MO, USA), tetrahydrofuran (THF) (Sigma Aldrich, USA), and sunflower vegetable oil (Adams Group, Arbuckle, CA, USA).
2.2. Preparation of Solutions for Electrospinning
SIS was diluted in a mixed solvent of THF and DMF 80:20 (v/v) at room temperature for 20 h and subsequently used for electrospinning.
In the case of a solution of SIS and CNTs, CNTs were first added to a DMF/THF solution followed by two drops of Triton X, and were then sonicated using an internal probe for 30 min. Then, SIS was added to a mixture of CNTs and solvent and stirred for 5 h at room temperature. This procedure led to the formation of a homogeneous, long-term stable dispersion. Based on the optimization protocol, the SIS content in the solution was 13 wt.% and the concentration of CNTs was 10 wt.% relative to the polymer concentration.
2.3. Electrospinning
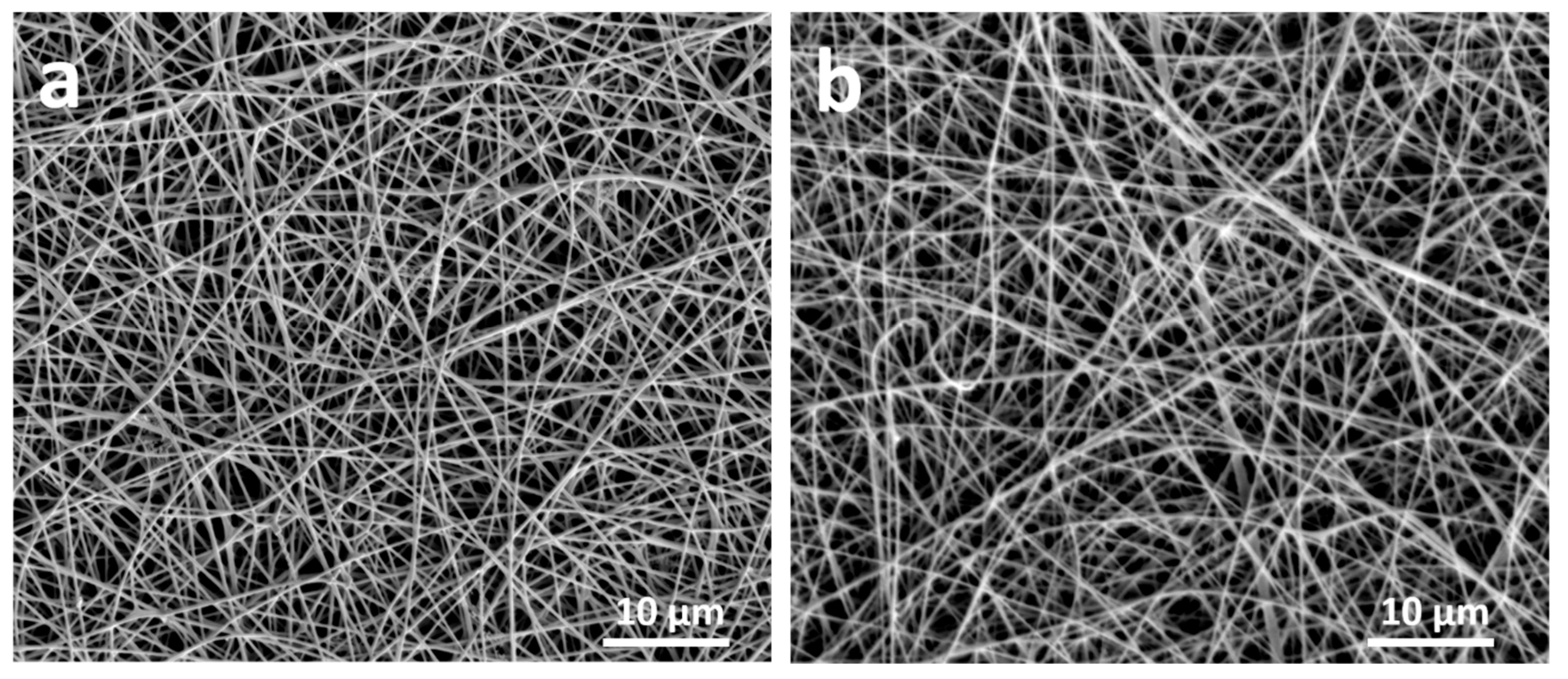
Electrospun nanofibers were fabricated using a NaBond (Shenzhen, China) electrospinning device. Typically, 5 mL of SIS/CNT dispersion was added into a 10 mL syringe with a blunt-end, stainless steel needle type 8. The needle was connected to the emitting electrode of a high voltage supply capable of generating DC voltages. Aluminum foil was used as the collection screen, which connected to the ground electrode of the power supply. The distance between the screen and the needle tip was 15 cm. The electrospinning process was carried out at room temperature at a voltage of 15 kV for 20 min at a flow rate of 1.0 mL/h.
2.4. Fabrication of the Sensor
Gold interdigitated electrodes were used for fabrication of the sensors as shown in
Figure 1. The electrodes were placed onto aluminum foil in an electrospinning device. The electrospinning process was performed under the conditions described in part 2.3 using solutions composed of 13 wt.% SIS and 10 wt.% CNT for 20 min under 20 kV and a flow rate of 2 mL/h. The samples were dried in the oven for 24 h at 60 °C. The average weight of the electrospun layer was 10 mg ± 2 mg, and the average thickness was approximately 100 ± 10 μm.
2.5. Oil Sensing Measurements
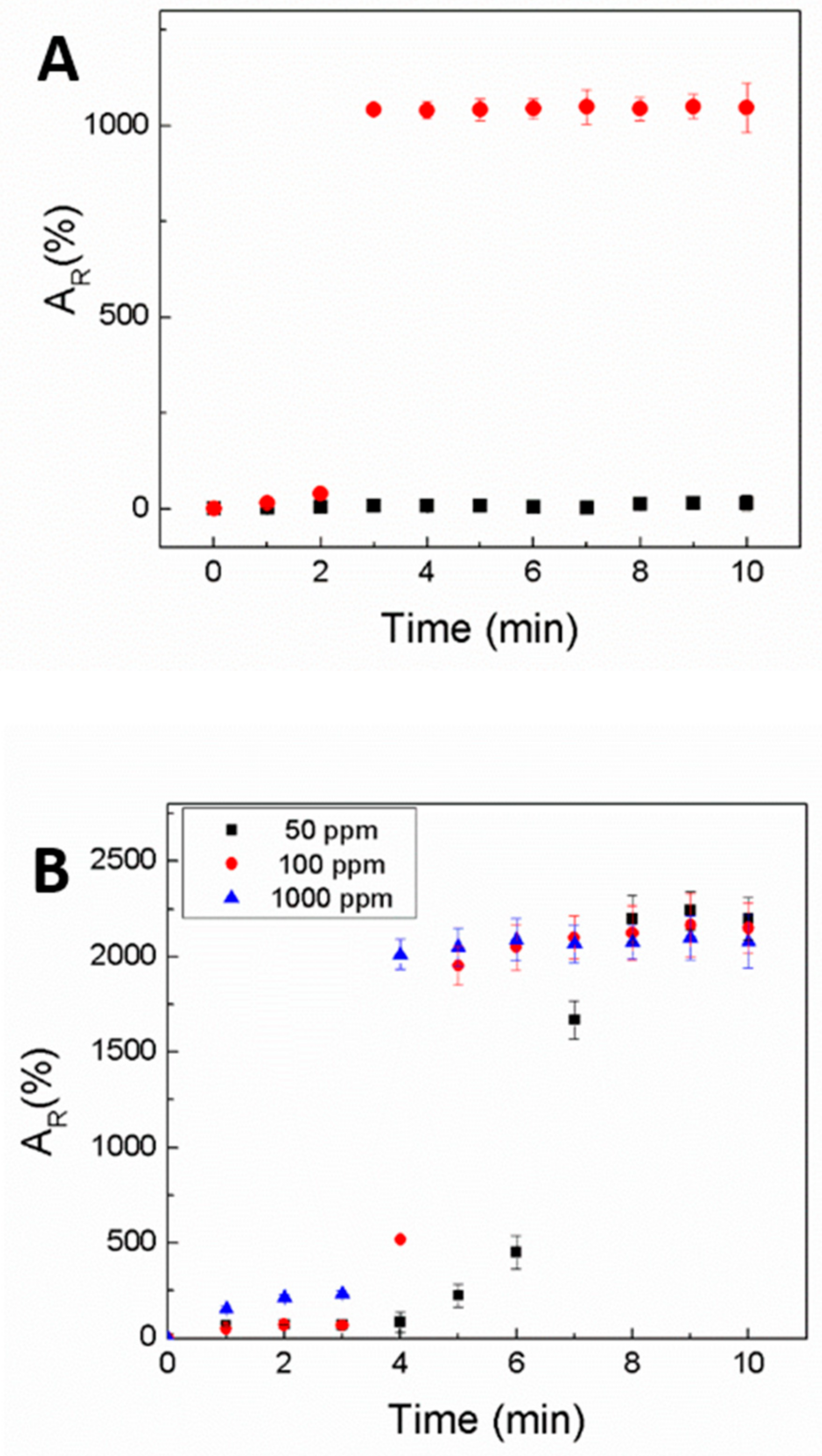
Water, vegetable oil, and their mixtures with concentrations of 100 and 1000 ppm of vegetable oil were prepared by mixing water and vegetable oil with internal probe sonication for 20 min and then stirring with a magnetic stirrer overnight. A drop of emulsion was applied onto a prepared sensor to ensure full coverage of the active area of the sensor, and resistivity was subsequently measured.
Resistivity changes were measured by using the KEITHLEY 2635A system source meter upon exposure to drops of different oil mixtures over time.
Terminal-coated electrodes were connected to a source meter system (KEITHLEY 2635A, Austin, TX, USA) under 10 mA to measure the resistivity of the samples. The relative resistivity (A
R) for each sensor was calculated using Equation (1):
where R is the resistance at time t and R
0 is the initial resistance of the specimen. Each measurement was repeated five times.
4. Conclusions
The preparation of electrospun mats from styrene-isoprene-styrene copolymer (SIS) and multiwall carbon nanotubes was optimized. The most uniform mats without beads were prepared using THF/DMF 80:20 (v/v) as the solvent and 13 wt.% of SIS. The CNT content was 10 wt.% because that amount resulted in the most pronounced changes in electrical resistivity upon sorption of the oil component.
The sensors for detection of vegetable oil in water were designed by deposition of SIS/CNT mats onto gold electrodes through electrospinning.
Sensors were successfully applied for detection of oil dispersed in water in the range from 50 to 1000 ppm. The detection of oil impurities below 50 ppm was complicated and ambiguous due to low changes in the electrical resistivity. Further research will be focused on the investigation of an influence of common water-soluble components occurring in waste water (e.g., salts) on oil detection ability. These impurities may interfere with the sensitivity of measurements; however, this is outside the scope of this paper.