A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization
Abstract
1. Introduction
2. Method
2.1. Theoretical
2.2. Experimental
2.2.1. Material
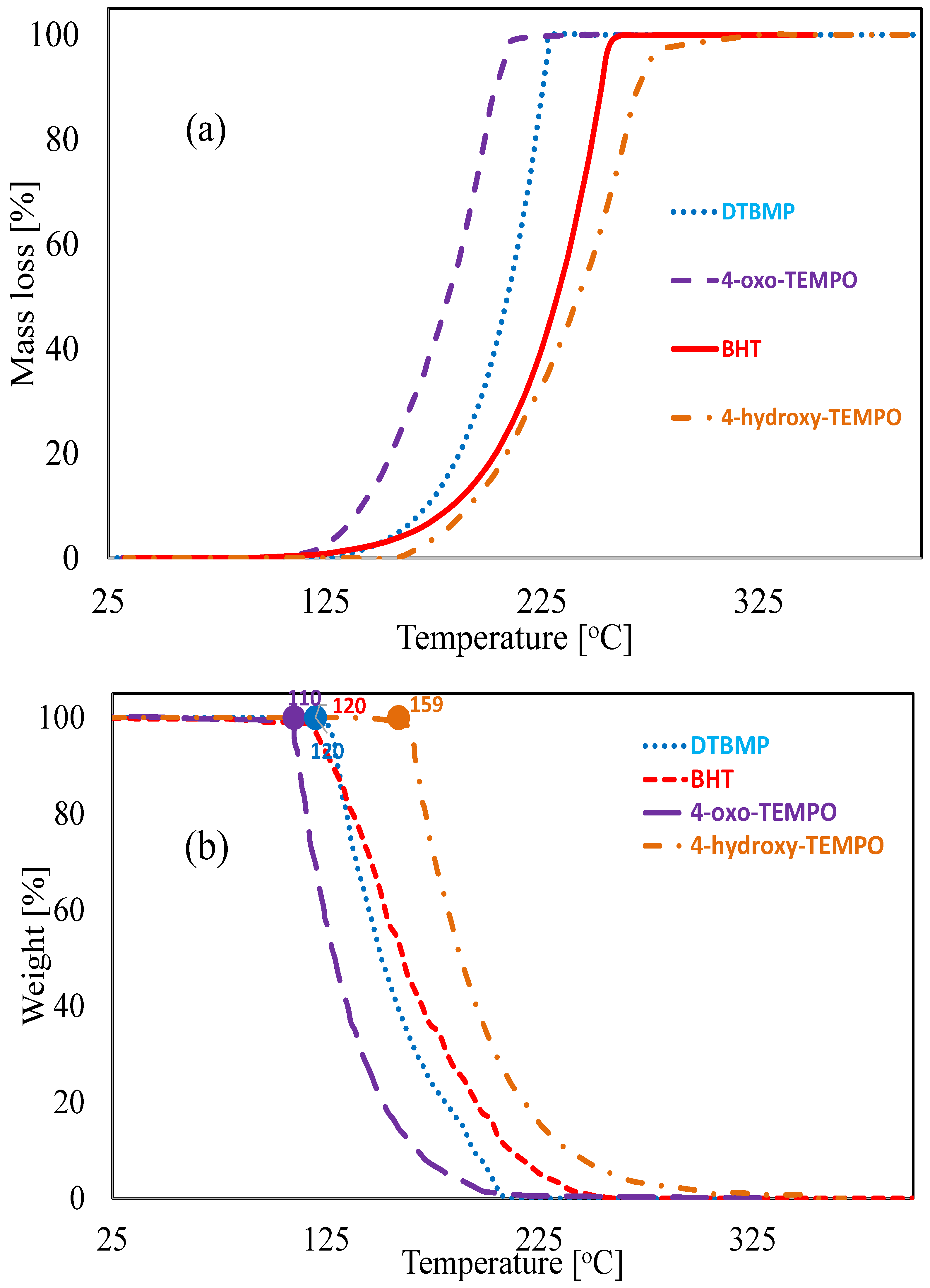
2.2.2. Thermogravimetric Analysis
2.2.3. Analysis of Product (Styrene, Dimer Styrene, and Trimer Styrene)
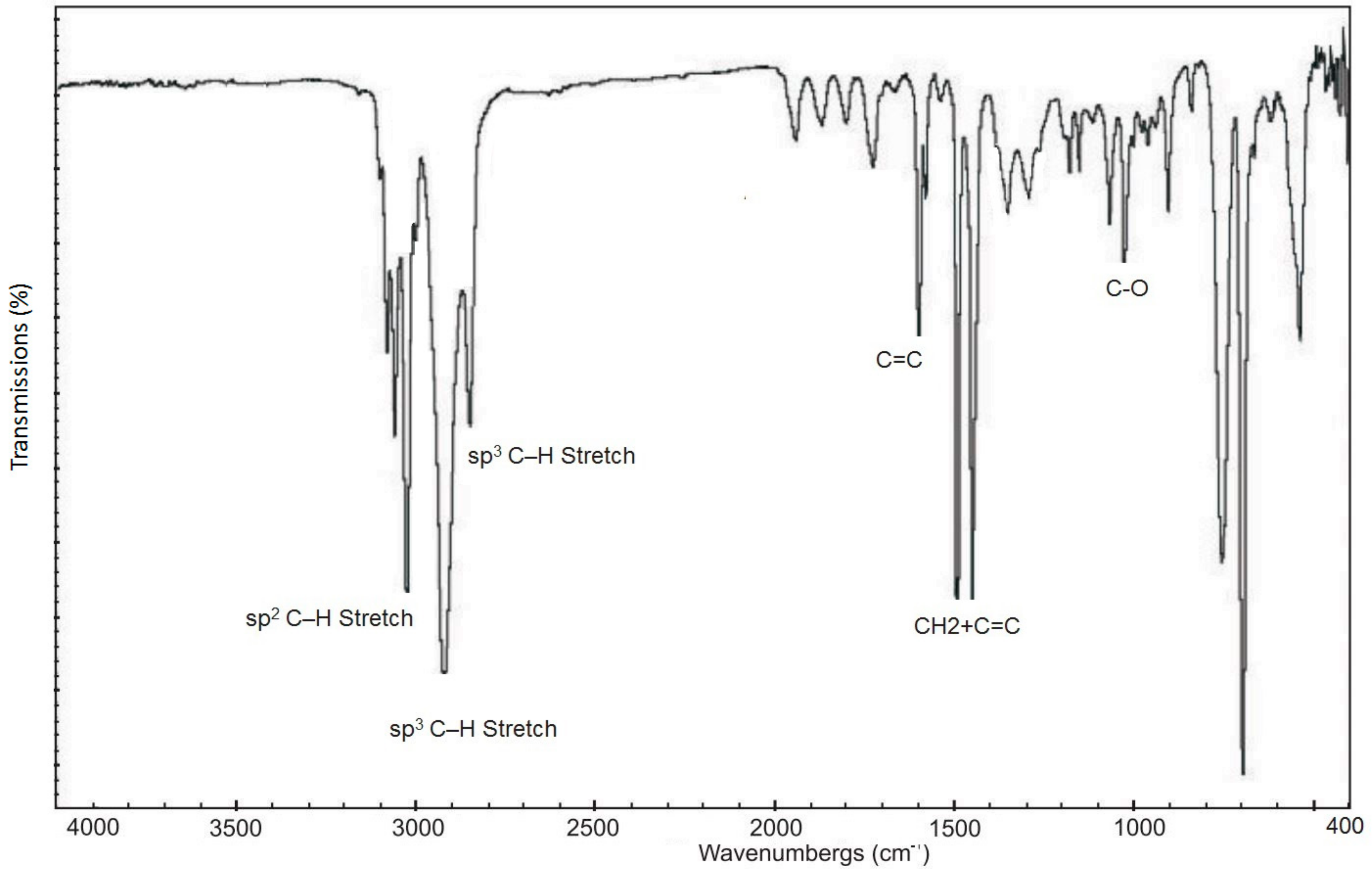
2.2.4. FTIR
2.2.5. Inhibition Test
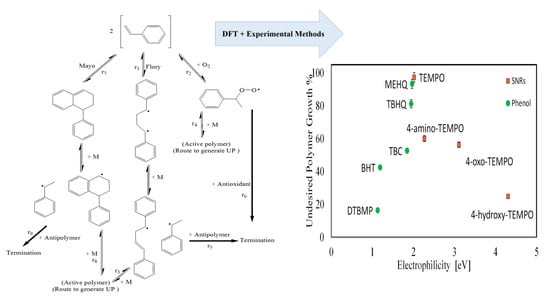
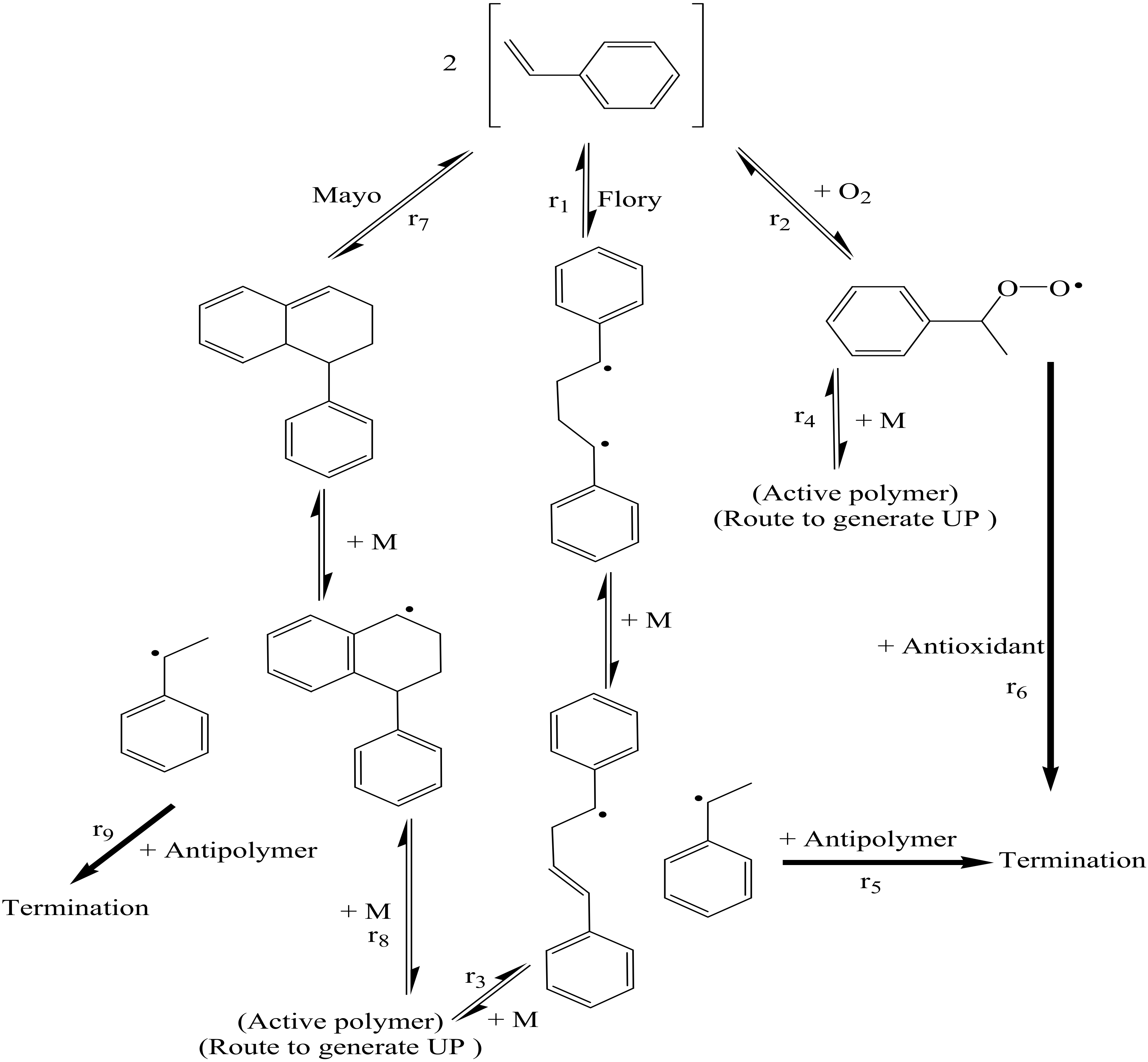
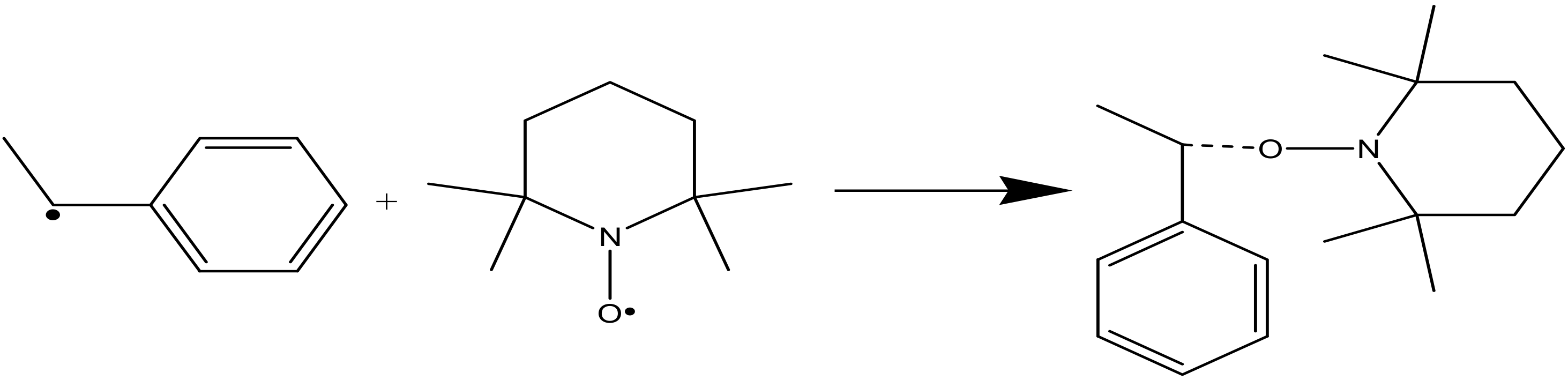
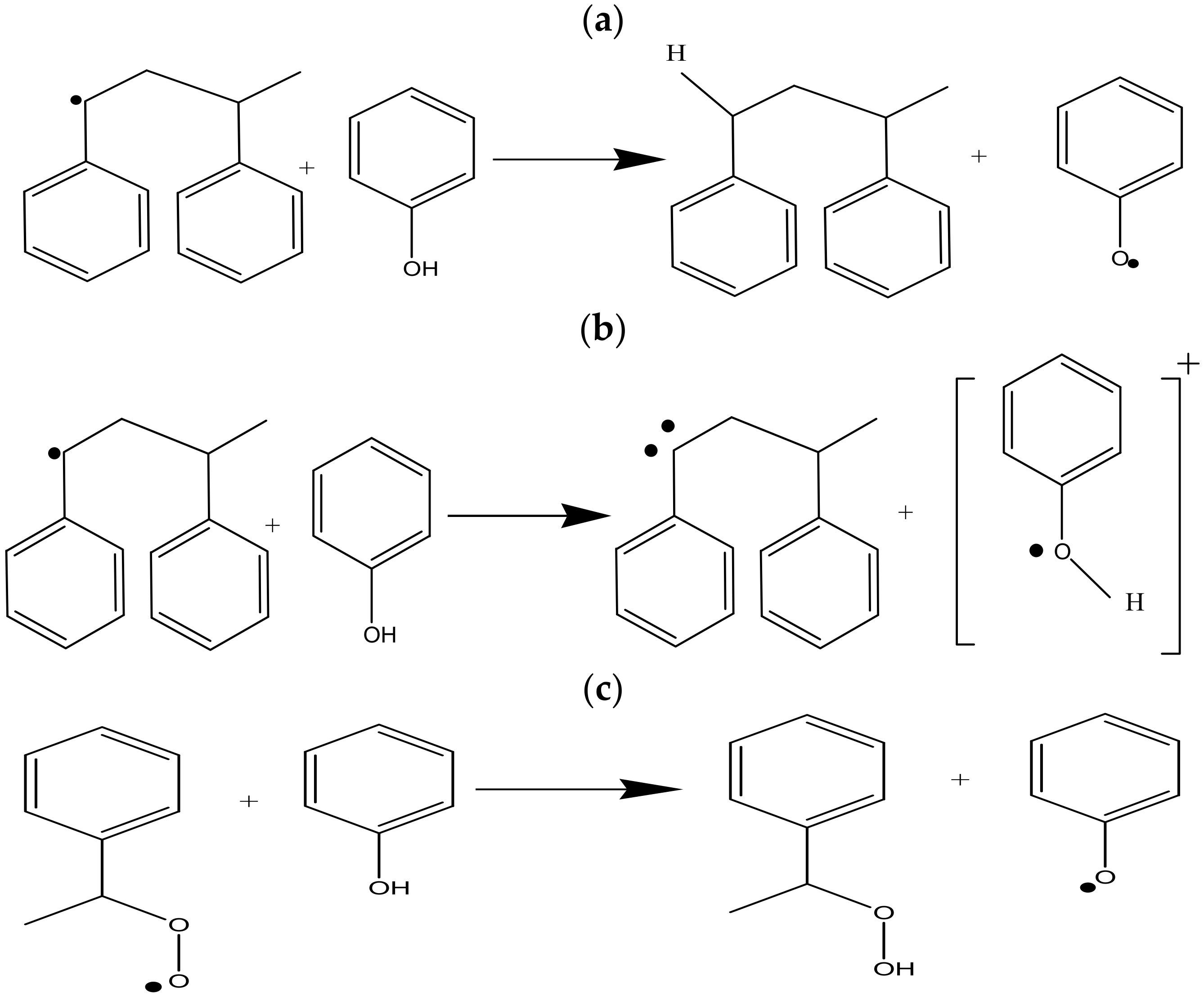
3. Result
3.1. DFT Calculation Results
3.2. Experimental Results
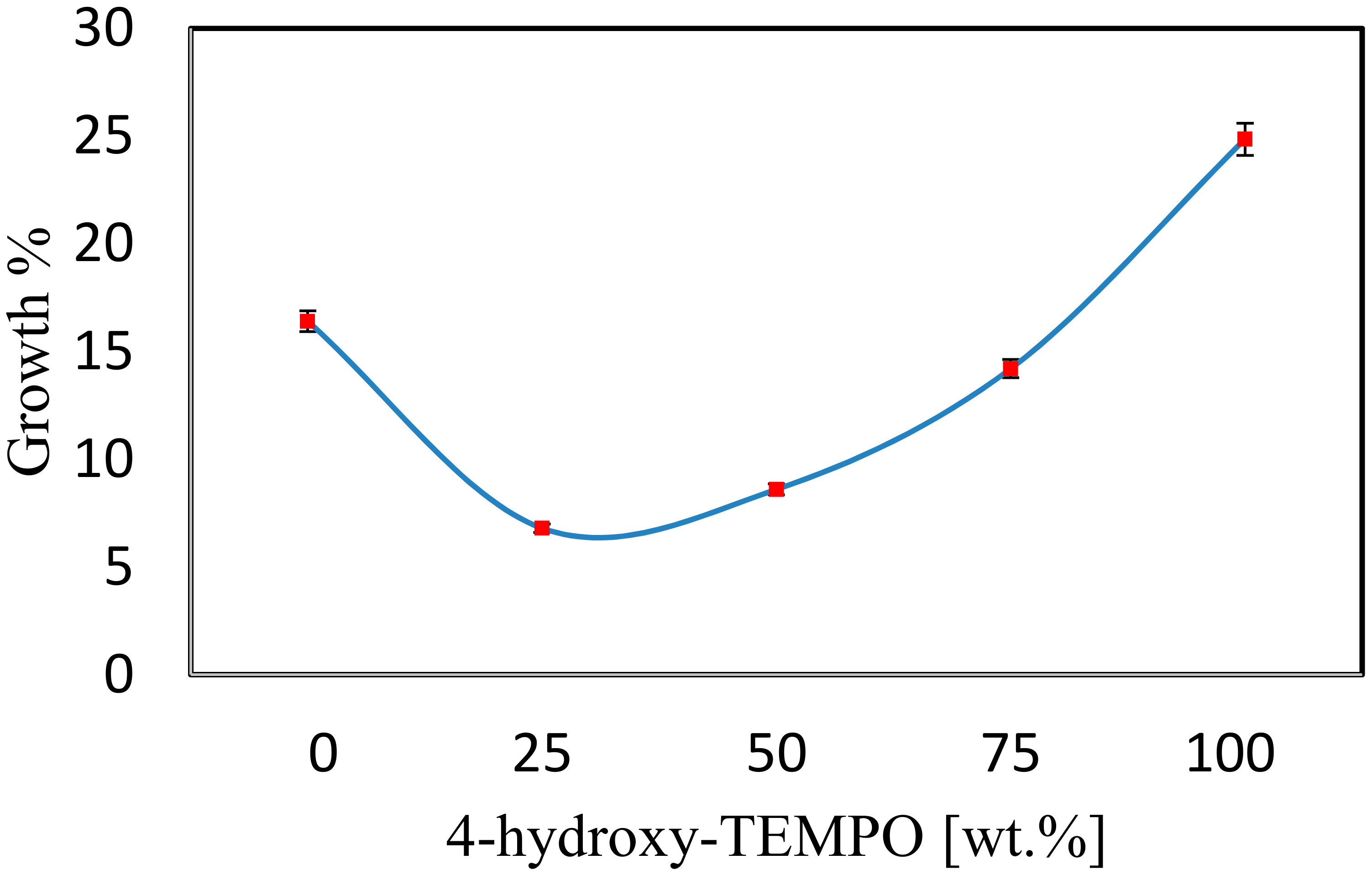
3.3. Synergic effect of Antioxidant and Antipolymer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, S.; Zhang, B.; Cremaschi, L. Review of moisture behavior and thermal performance of polystyrene insulation in building applications. Build. Environ. 2017, 123, 50–65. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghorai, S.; Bhunia, S.; Roy, M.; De, D. The role of devulcanizing agent for mechanochemical devulcanization of styrene butadiene rubber vulcanizate. Polym. Eng. Sci. 2018, 58, 74–85. [Google Scholar] [CrossRef]
- Kamelian, F.S.; Saljoughi, E.; Nasirabadi, P.S.; Mousavi, S.M. Modifications and research potentials of acrylonitrile/butadiene/styrene (ABS) membranes: A review. Polym. Compos. 2018, 39, 2835–2846. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S. Energy Efficient Styrene Process: Design and Plantwide Control. Ind. Eng. Chem. Res. 2019, 58, 4890–4905. [Google Scholar] [CrossRef]
- Ovejero, G.; Romero, M.D.; Díaz, I.; Mestanza, M.; Díez, E. Bentonite as an Alternative Adsorbent for the Purification of Styrene Monomer: Adsorption Kinetics, Equilibrium and Process Design. Adsorpt. Sci. Technol. 2010, 28, 101–123. [Google Scholar] [CrossRef]
- Díaz, I.; Langston, P.; Ovejero, G.; Romero, M.D.; Díez, E. Purification process design in the production of styrene monomer. Chem. Eng. Process Process Intensif. 2010, 49, 367–375. [Google Scholar] [CrossRef]
- Miller, G.H.; Perizzolo, A.F. Styrene popcorn polymers. J. Polym. Sci. 1955, 18, 411–416. [Google Scholar] [CrossRef]
- Hsieh, H.; Quirk, R.P. Anionic Polymerization: Principles and Practical Applications; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Liao, C.-C.; Wu, S.-H.; Su, T.-S.; Shyu, M.-L.; Shu, C.-M. Thermokinetics evaluation and simulations for the polymerization of styrene in the presence of various inhibitor concentrations. J. Therm. Anal. Calorim. 2006, 85, 65–71. [Google Scholar] [CrossRef]
- Chen, C.-C.; Duh, Y.-S.; Shu, C.-M. Thermal polymerization of uninhibited styrene investigated by using microcalorimetry. J. Hazard. Mater. 2009, 163, 1385–1390. [Google Scholar] [CrossRef]
- Nanda, A.; Kishore, K. Autocatalytic oxidative polymerization of indene by cobalt porphyrin complex and kinetic investigation of the polymerization of styrene. Macromolecules 2001, 34, 1600–1605. [Google Scholar] [CrossRef]
- Cui, J.; Ni, L.; Jiang, J.; Pan, Y.; Wu, H.; Chen, Q. Computational Fluid Dynamics Simulation of Thermal Runaway Reaction of Styrene Polymerization. Org. Process Res. Dev. 2019, 23, 389–396. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Pakdel, A.S.; Saeb, M.R.; Boodhoo, K. Monte Carlo simulation of free radical polymerization of styrene in a spinning disc reactor. Chem. Eng. J. 2014, 247, 231–240. [Google Scholar] [CrossRef]
- Sobani, M.; Haddadi-Asl, V.; Mirshafiei-Langari, S.-A.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Khezri, K. A kinetics study on the in situ reversible addition–fragmentation chain transfer and free radical polymerization of styrene in presence of silica aerogel nanoporous particles. Des. Monomers Polym. 2014, 17, 245–254. [Google Scholar] [CrossRef][Green Version]
- Sütekin, S.D.; Atıcı, A.B.; Güven, O.; Hoffman, A.S. Controlling of free radical copolymerization of styrene and maleic anhydride via RAFT process for the preparation of acetaminophen drug conjugates. Radiat. Phys. Chem. 2018, 148, 5–12. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chiefari, J.; Chong, Y.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G. Living free-radical polymerization by reversible addition—Fragmentation chain transfer: the RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Encinas, M.V.; Lissi, E.A.; Norambuena, E. Inhibition of Styrene Polymerization by β-Nitrostyrene. A Novel Inhibition Mechanism. Macromolecules 1998, 31, 5171–5174. [Google Scholar] [CrossRef]
- Harper, C.A.; Petrie, E.M. Plastics Materials and Processes: A Concise Encyclopedia; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Luo, K.; Zheng, W.; Zhao, X.; Wang, X.; Wu, S. Effects of antioxidant functionalized silica on reinforcement and anti-aging for solution-polymerized styrene butadiene rubber: Experimental and molecular simulation study. Mater. Des. 2018, 154, 312–325. [Google Scholar] [CrossRef]
- Gomes, G.d.P.; Loginova, Y.; Vatsadze, S.Z.; Alabugin, I.V. Isonitriles as Stereoelectronic Chameleons: The Donor–Acceptor Dichotomy in Radical Additions. J. Am. Chem. Soc. 2018, 140, 14272–14288. [Google Scholar] [CrossRef]
- Kolthoff, I.; Bovey, F. Studies of Retarders and Inhibitors in the Emulsion Polymerization of Styrene. I. Retarders1a. J. Am. Chem. Soc. 1948, 70, 791–799. [Google Scholar] [CrossRef]
- Bevington, J.; Ebdon, J.; Huckerby, T. An appraisal of NMR methods for study of end-groups derived from initiators in radical polymerizations. Eur. Polym. J. 1985, 21, 685–694. [Google Scholar] [CrossRef]
- Yokota, H.; Kawakatsu, T. Modeling induction period of polymer crystallization. Polymer 2017, 129, 189–200. [Google Scholar] [CrossRef]
- Cohen, S.G. Inhibition and Retardation of the Peroxide Initiated Polymerization of Styrene. J. Am. Chem. Soc. 1947, 69, 1057–1064. [Google Scholar] [CrossRef]
- Wright, J.S.; Carpenter, D.J.; McKay, D.J.; Ingold, K. Theoretical calculation of substituent effects on the O−H bond strength of phenolic antioxidants related to vitamin E. J. Am. Chem. Soc. 1997, 119, 4245–4252. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, I.; Roginsky, V.; Pliss, E. The chain-breaking antioxidant activity of phenolic compounds with different numbers of O-H groups as determined during the oxidation of styrene. Int. J. Chem. Kinet. 2009, 41, 92–100. [Google Scholar] [CrossRef]
- Mardare, D.; Matyjaszewski, K. Thermal Polymerization of Styrene in the Presence of Stable Radicals and Inhibitors; Carnegie-Mellon Univ. Pittsburgh Pa. Dept of Chemistry: Pittsburgh, PA, USA, 1994. [Google Scholar]
- Kemmere, M.; Mayer, M.; Meuldijk, J.; Drinkenburg, A. The influence of 4-tert-butylcatechol on the emulsion polymerization of styrene. J. Appl. Polym. Sci. 1999, 71, 2419–2422. [Google Scholar] [CrossRef]
- Finson, S.; Jury, J.M.; Crivello, J.V. Azodioxides as Inhibitors and Retarders in Photoinitiated Cationic Polymerization. Macromol. Chem. Phys. 2013, 214, 1806–1816. [Google Scholar] [CrossRef]
- Moghadam, N.; Liu, S.; Srinivasan, S.; Grady, M.C.; Soroush, M.; Rappe, A.M. Computational Study of Chain Transfer to Monomer Reactions in High-Temperature Polymerization of Alkyl Acrylates. J. Phys. Chem. A 2013, 117, 2605–2618. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Kwart, H. Some Inhibitors and Retarders in the Polymerization of Liquid Vinyl Acetate. II. 1, 3, 5-Trinitrobenzene and Sulfur1. J. Am. Chem. Soc. 1952, 74, 3969–3973. [Google Scholar] [CrossRef]
- Anbazhakan, K.; Sadasivam, K.; Praveena, R.; Dhandapani, M. Target prediction and antioxidant analysis on isoflavones of demethyltexasin: A DFT study. J. Mol. Model. 2019, 25, 169. [Google Scholar] [CrossRef]
- Sharma, V.; Arora, E.K.; Cardoza, S. 4-Hydroxy-benzoic acid (4-diethylamino-2-hydroxy-benzylidene) hydrazide: DFT, antioxidant, spectroscopic and molecular docking studies with BSA. Luminescence 2016, 31, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Tüdös, F.; Kende, I.; Azori, M. Inhibition kinetics of the polymerization of styrene. II. Investigations on the effect of s-trinitrobenzene. J. Polym. Sci. Part A Gen. Pap. 1963, 1, 1353–1368. [Google Scholar]
- Tüdös, F.; Kende, I.; Azori, M. Inhibition kinetics of polymerization of styrene. III. Investigations on the effect of substituted trinitrobenzenes. J. Polym. Sci. Part A Gen. Pap. 1963, 1, 1369–1381. [Google Scholar]
- Pellecchia, C.; Grassi, A. Syndiotactic-specific polymerization of styrene: Catalyst structure and polymerization mechanism. Top. Catal. 1999, 7, 125–132. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. Global and local reactivity indices for electrophilic/nucleophilic free radicals. Org. Biomol. Chem. 2013, 11, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschouwer, F.; van Speybroeck, V.; Waroquier, M.; Geerlings, P.; de Proft, F. Electrophilicity and nucleophilicity index for radicals. Org. Lett. 2007, 9, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Zhou, Y.; Liang, Q.; Chen, D.-F.; Guo, R.; Xiong, C.-L.; Xu, X.J.; Zhang, Z.N.; Huang, Z.J.; Xu, X.J.; et al. Solvent effects on the intramolecular hydrogen-bond and anti-oxidative properties of apigenin: A DFT approach. Dyes Pigments 2017, 141, 179–187. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Chen, D.-F.; Deng, G.; Guo, R.; Fu, Z.-M. The antioxidative activity of piceatannol and its different derivatives: Antioxidative mechanism analysis. Phytochemistry 2018, 156, 184–192. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Chen, D.-F.; Deng, G.; Guo, R. The Substituent Effect on the Radical Scavenging Activity of Apigenin. Molecules 2018, 23, 1989. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Chen, D.-F.; Liang, Q.; Guo, R.; Fu, Z.-M. Theoretical studies on the antioxidant activity of pinobanksin and its ester derivatives: Effects of the chain length and solvent. Food Chem. 2018, 240, 323–329. [Google Scholar] [CrossRef]
- Wang, X.; Lin, F.; Qu, J.; Hou, Z.; Luo, Y. DFT Studies on Styrene Polymerization Catalyzed by Cationic Rare-Earth-Metal Complexes: Origin of Ligand-Dependent Activities. Organometallics 2016, 35, 3205–3214. [Google Scholar] [CrossRef]
- Coote, M.L.; Henry, D.J. Computer-Aided Design of a Destabilized RAFT Adduct Radical: Toward Improved RAFT Agents for Styrene-block-Vinyl Acetate Copolymers. Macromolecules 2005, 38, 5774–5779. [Google Scholar] [CrossRef]
- Kauffman, G. The Cost of Clean Water in the Delaware River Basin (USA). Water 2018, 10, 95. [Google Scholar] [CrossRef]
- Cinar, Z. The role of molecular modeling in TiO2 photocatalysis. Molecules 2017, 22, 556. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, R.M.; Salcedo, R.; Martínez, A.; Ramos, E.; Sansores, L.E. Electronic Peculiarities of a Self-Assembled M12L24 Nanoball (M = Pd+ 2, Cr, or Mo). Molecules 2019, 24, 771. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.E.; Gil, D.M.; Lanús, H.E.; Altabef, A.B. Theoretical study on the molecular structure and vibrational properties, NBO and HOMO–LUMO analysis of the POX3 (X = F, Cl, Br, I) series of molecules. J. Mol. Struct. 2015, 1081, 536–542. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Duhaidahawi, D.; Al-Azawi, K.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Coumarins as potential antioxidant agents complemented with suggested mechanisms and approved by molecular modeling studies. Molecules 2016, 21, 135. [Google Scholar] [CrossRef]
- Rao, P.S.; Puyad, A.L.; Bhosale, S.V.; Bhosale, S.V. Triphenylamine-Merocyanine-Based D1-A1-π-A2/A3-D2 Chromophore System: Synthesis, Optoelectronic, and Theoretical Studies. Int. J. Mol. Sci. 2019, 20, 1621. [Google Scholar]
- Ichikawa, K.; Sasada, R.; Chiba, K.; Gotoh, H. Effect of Side Chain Functional Groups on the DPPH Radical Scavenging Activity of Bisabolane-Type Phenols. Antioxidants 2019, 8, 65. [Google Scholar] [CrossRef]
- Murayama, K.; Yoshioka, T. Studies on stable free radicals. IV. Decomposition of stable Nitroxide radicals. Bull. Chem. Soc. Jpn. 1969, 42, 2307–2309. [Google Scholar] [CrossRef]
- Ma, Y.; Loyns, C.; Price, P.; Chechik, V. Thermal decay of TEMPO in acidic media via an N-oxoammonium salt intermediate. Org. Biomol. Chem. 2011, 9, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Boutevin, B.; Bertin, D. Controlled free radical polymerization of styrene in the presence of nitroxide radicals I. Thermal initiation. Eur. Polym. J. 1999, 35, 815–825. [Google Scholar] [CrossRef]
- Conte, M.; Ma, Y.; Loyns, C.; Price, P.; Rippon, D.; Chechik, V. Mechanistic insight into TEMPO-inhibited polymerisation: Simultaneous determination of oxygen and inhibitor concentrations by EPR. Org. Biomol. Chem. 2009, 7, 2685–2687. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Nitroxides in Mechanistic Studies: Ageing of Gold Nanoparticles and Nitroxide Transformation in Acids. Ph.D. Thesis, University of York, York, UK, 2010. [Google Scholar]
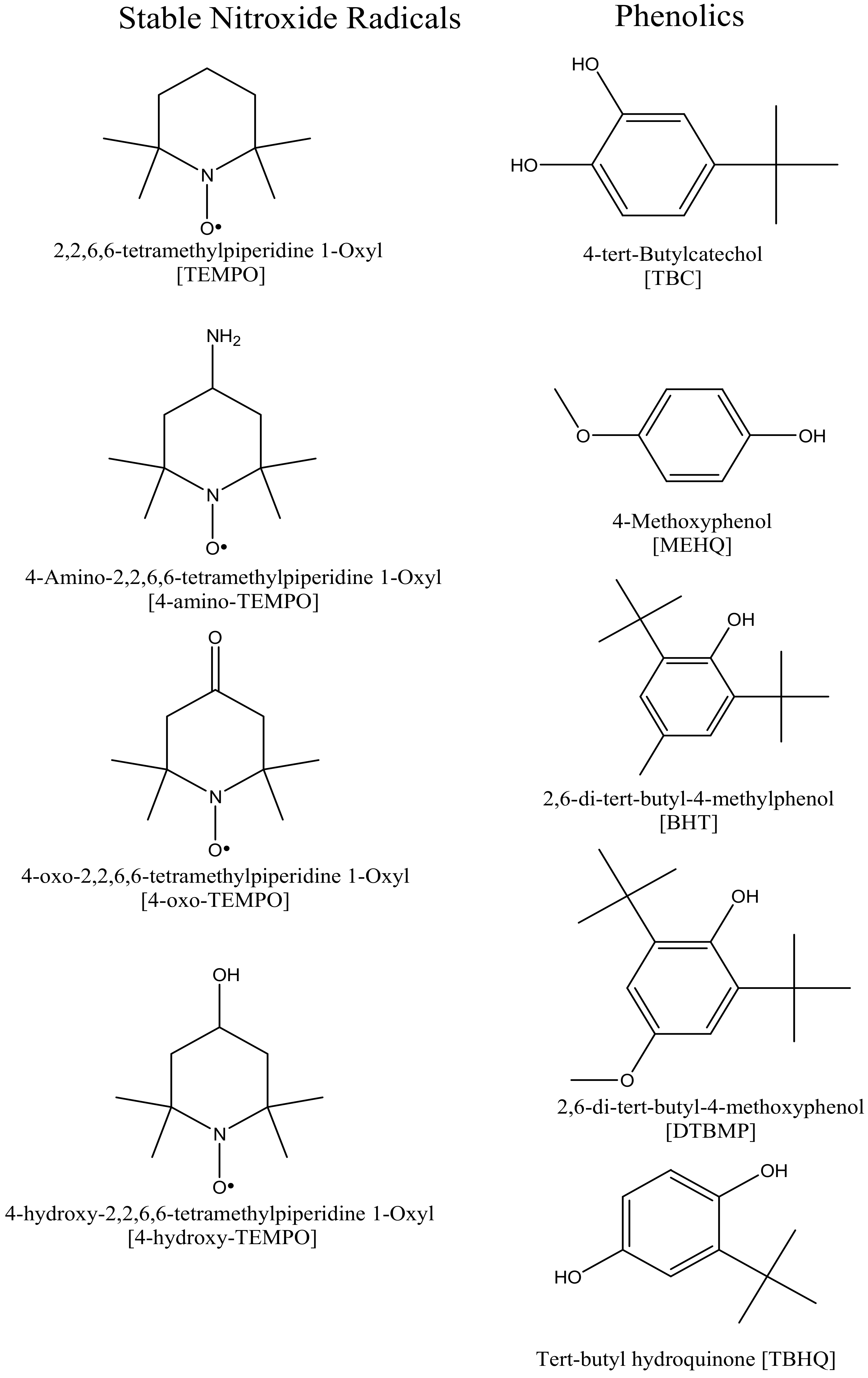


| Properties | Value | Unit |
|---|---|---|
| Temperature | 115 | °C |
| Pressure | 1 | bar |
| Inhibitor dosing | 50 | ppm |
| Monomer volume | 50 | mL |
| Water dosing | 2 | mL |
| Initial weight of ST polymer | 0.2 | g |
| Sample ID | Component | |||
|---|---|---|---|---|
| SNRs | ||||
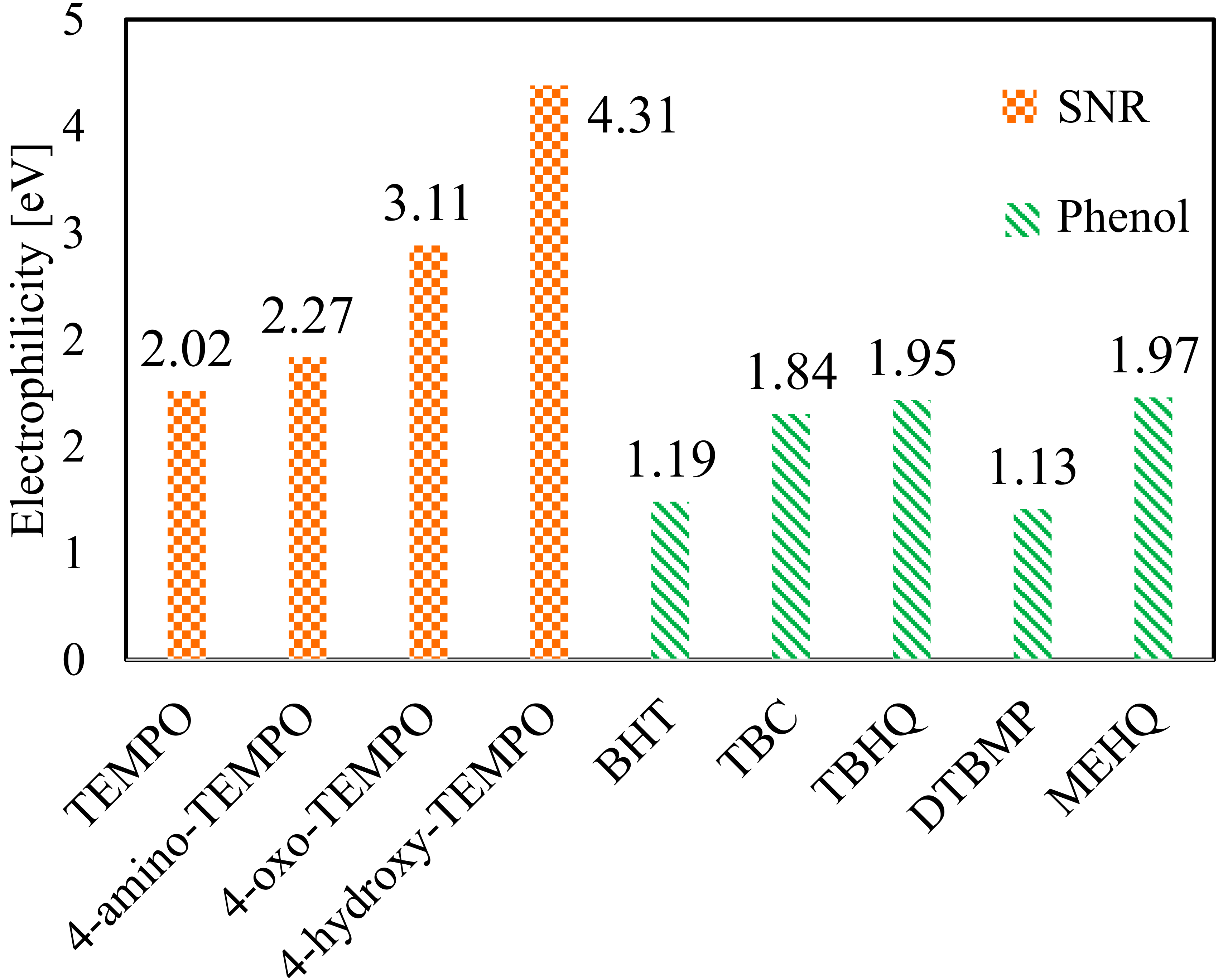
| S1 | TEMPO | −3.1688 | 2.4450 | 2.0170 |
| S2 | 4-amino-TEMPO | −3.4344 | 2.5718 | 2.2675 |
| S3 | 4-oxo-TEMPO | −3.6544 | 2.1485 | 3.1079 |
| S4 | 4-hydroxy-TEMPO | −3.8239 | 1.6973 | 4.3075 |
| Phenolics | ||||
| S5 | BHT | −2.6654 | 2.9900 | 1.1880 |
| S6 | TBC | −3.2072 | 2.7878 | 1.8448 |
| S7 | TBHQ | −3.2376 | 2.6937 | 1.9457 |
| S8 | DTBMP | −2.5161 | 2.8021 | 1.1297 |
| S9 | MEHQ | −3.2282 | 2.6478 | 1.9680 |
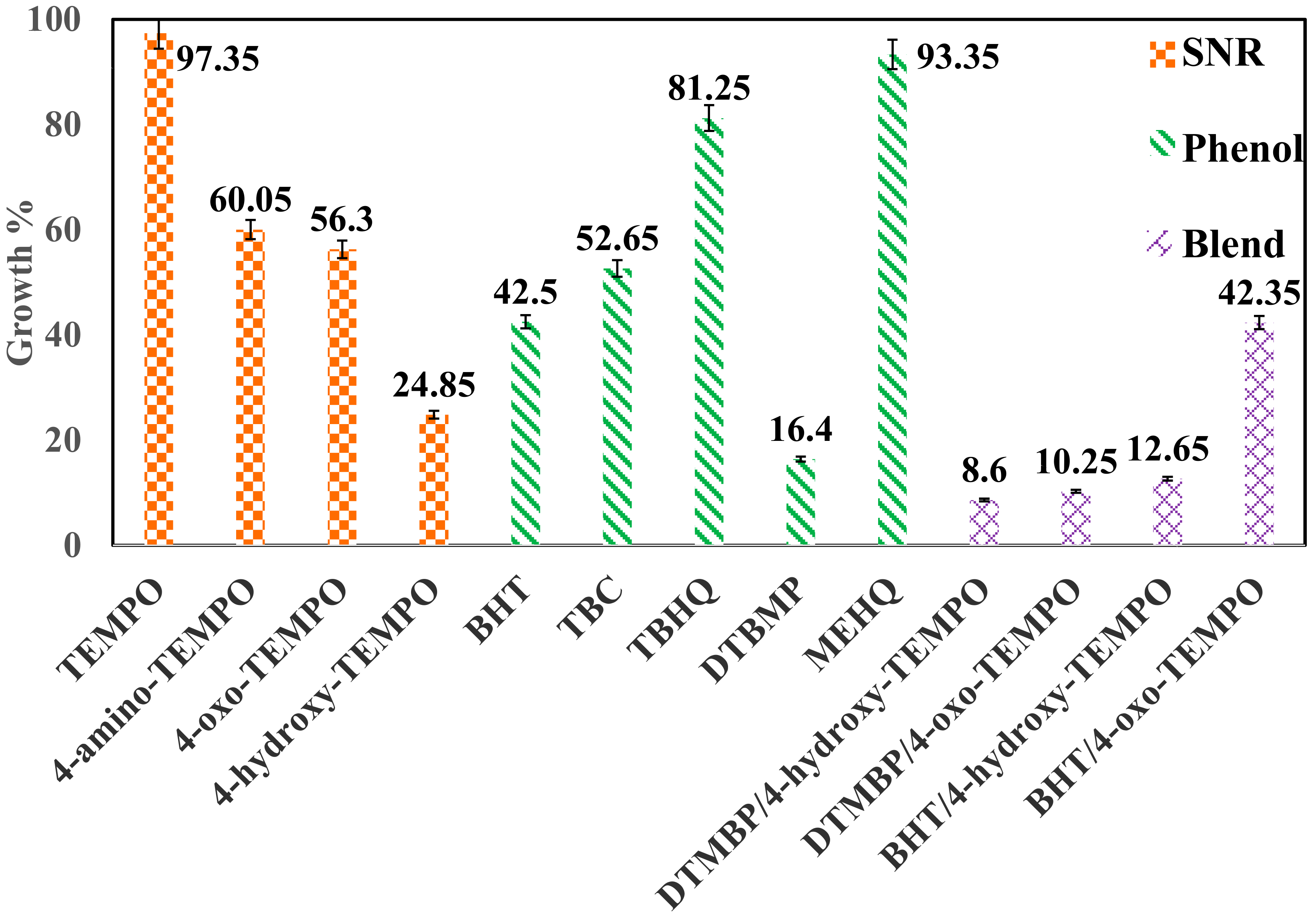
| Sample ID | Component | Weight [g] | Growth Percentage | Outlet Mass Fraction [wt.%] | Conversion [%] | ||
|---|---|---|---|---|---|---|---|
| SNRs | Styrene | Dimer | Trimer | ||||
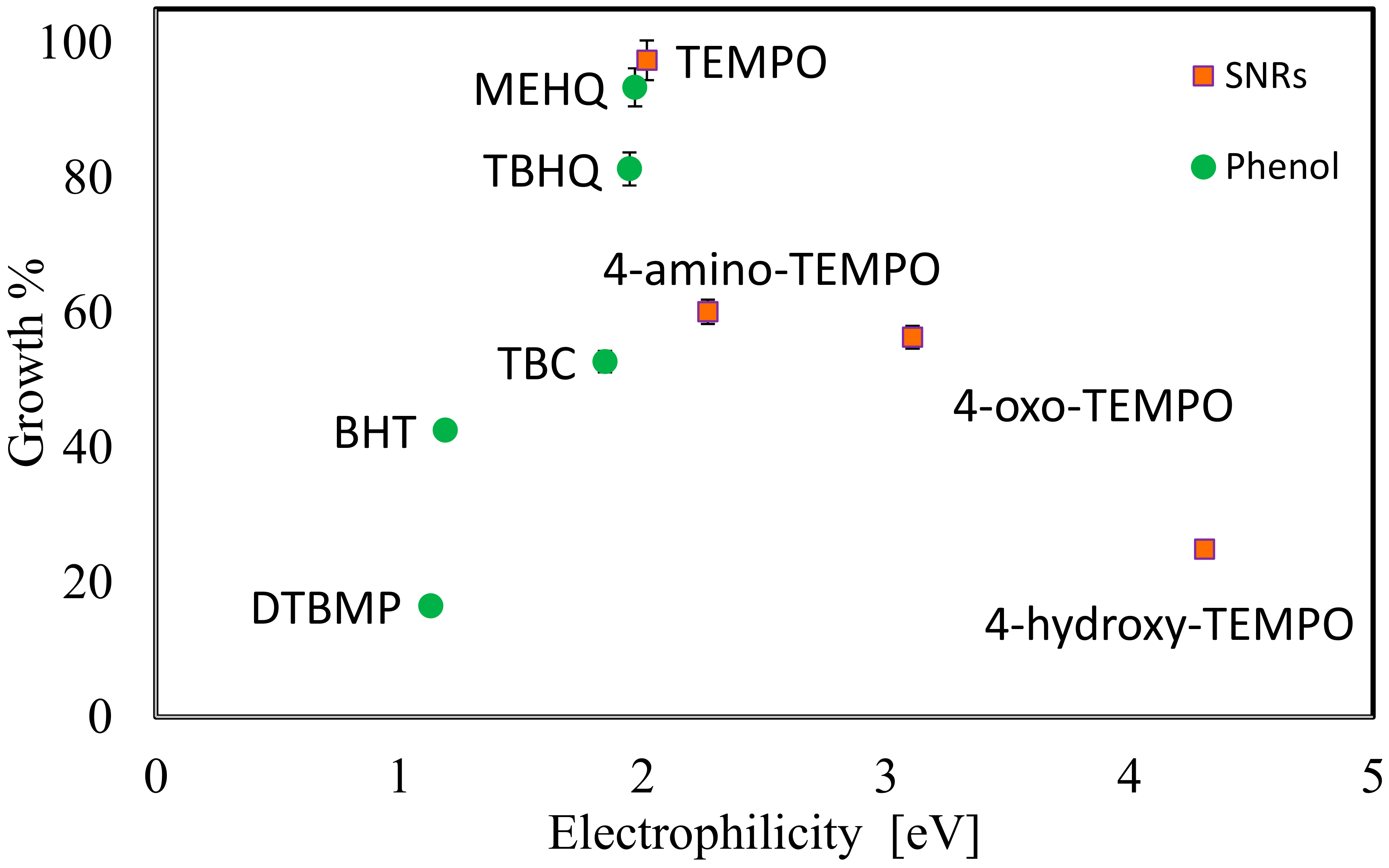
| S1 | TEMPO | 0.395 | 97.35 | 99.719 | 0.034 | 0.015 | 0.231 |
| S2 | 4-amino-TEMPO | 0.320 | 60.05 | 99.808 | 0.021 | 0.009 | 0.142 |
| S3 | 4-oxo-TEMPO | 0.313 | 56.30 | 99.811 | 0.020 | 0.009 | 0.134 |
| S4 | 4-hydroxy-TEMPO | 0.250 | 24.85 | 99.885 | 0.013 | 0.006 | 0.065 |
| Phenolics | Styrene | Dimer | Trimer | ||||
| S5 | BHT | 0.285 | 42.50 | 99.839 | 0.022 | 0.010 | 0.111 |
| S6 | Commercial TBC | 0.305 | 52.65 | 99.811 | 0.028 | 0.012 | 0.139 |
| S7 | TBHQ | 0.363 | 81.25 | 99.749 | 0.034 | 0.015 | 0.201 |
| S8 | DTBMP | 0.233 | 16.40 | 99.902 | 0.012 | 0.005 | 0.048 |
| S9 | MEHQ | 0.387 | 93.35 | 99.730 | 0.053 | 0.023 | 0.251 |
| Sample ID | Component | Weight [g] | Growth Percentage | Outlet Mass Fraction [wt.%] | Conversion [%] | ||
|---|---|---|---|---|---|---|---|
| SNRs | Styrene | Dimer | Trimer | ||||
| S1 | TEMPO | 0.474 | 136.82 | 99.630 | 0.045 | 0.019 | 0.320 |
| S2 | 4-amino-TEMPO | 0.464 | 132.07 | 99.654 | 0.034 | 0.015 | 0.296 |
| S3 | 4-oxo-TEMPO | 0.422 | 111.00 | 99.703 | 0.035 | 0.015 | 0.257 |
| S4 | 4-hydroxy-TEMPO | 0.350 | 74.79 | 99.783 | 0.019 | 0.008 | 0.167 |
| Phenolics | Styrene | Dimer | Trimer | ||||
| S5 | BHT | 0.399 | 99.50 | 99.713 | 0.036 | 0.015 | 0.237 |
| S6 | Commercial TBC | 0.470 | 135.08 | 99.643 | 0.038 | 0.016 | 0.307 |
| S7 | TBHQ | 0.526 | 162.81 | 99.568 | 0.054 | 0.023 | 0.382 |
| S8 | DTBMP | 0.319 | 59.47 | 99.812 | 0.019 | 0.008 | 0.138 |
| S9 | MEHQ | 0.630 | 215.16 | 99.493 | 0.062 | 0.027 | 0.491 |
| Sample ID | Component | Weight [g] | Growth Percentage |
|---|---|---|---|
| S6 | Commercial TBC | 0.3053 | 52.65 |
| S10 | DTMBP/4-hydroxy-TEMPO | 0.2042 | 8.60 |
| S11 | DTMBP/4-oxo-TEMPO | 0.2185 | 10.25 |
| S12 | BHT/4-hydroxy-TEMPO | 0.2093 | 12.65 |
| S13 | BHT/4-oxo-TEMPO | 0.2847 | 42.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darvishi, A.; Rahimpour, M.R.; Raeissi, S. A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization. Processes 2019, 7, 677. https://doi.org/10.3390/pr7100677
Darvishi A, Rahimpour MR, Raeissi S. A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization. Processes. 2019; 7(10):677. https://doi.org/10.3390/pr7100677
Chicago/Turabian StyleDarvishi, Ali, Mohammad Reza Rahimpour, and Sona Raeissi. 2019. "A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization" Processes 7, no. 10: 677. https://doi.org/10.3390/pr7100677
APA StyleDarvishi, A., Rahimpour, M. R., & Raeissi, S. (2019). A Theoretical and Experimental Study for Screening Inhibitors for Styrene Polymerization. Processes, 7(10), 677. https://doi.org/10.3390/pr7100677