Simulation of Ion Exchange Resin with Finite Difference Methods
Abstract
1. Introduction
2. Modeling
2.1. Modeling Assumptions
2.2. Equations
2.3. Initial Condition
2.4. Numerical Method
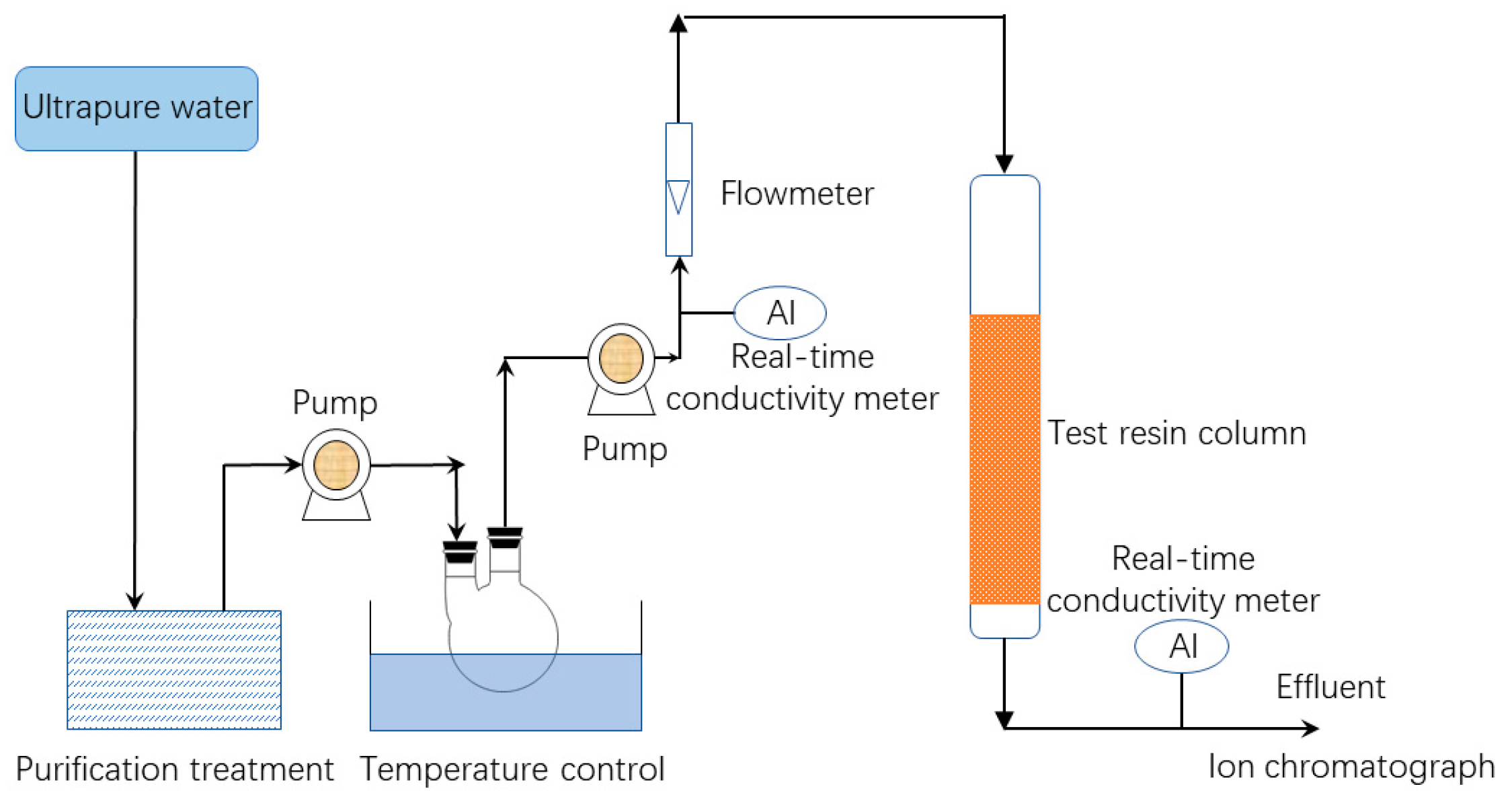
3. Experiment
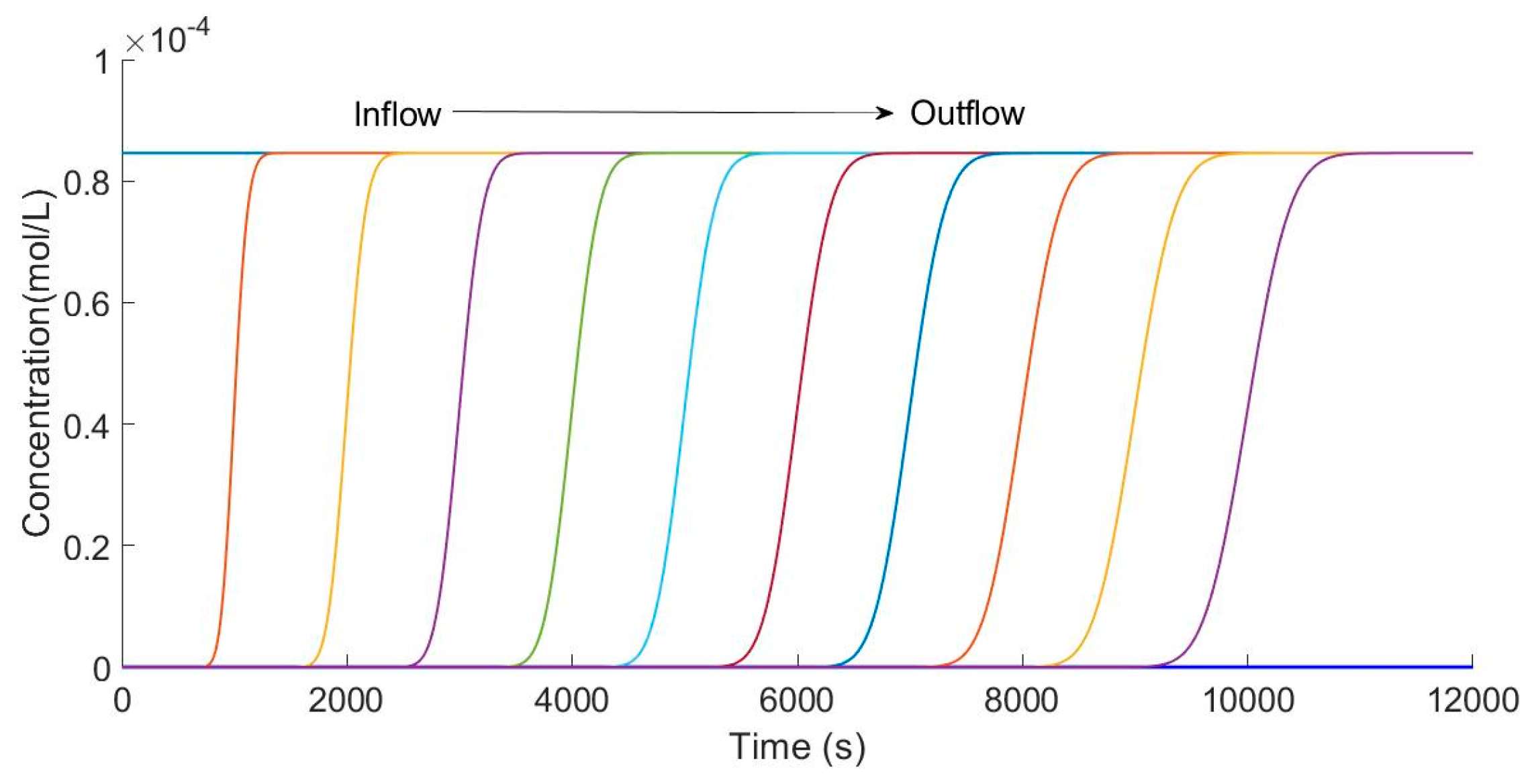
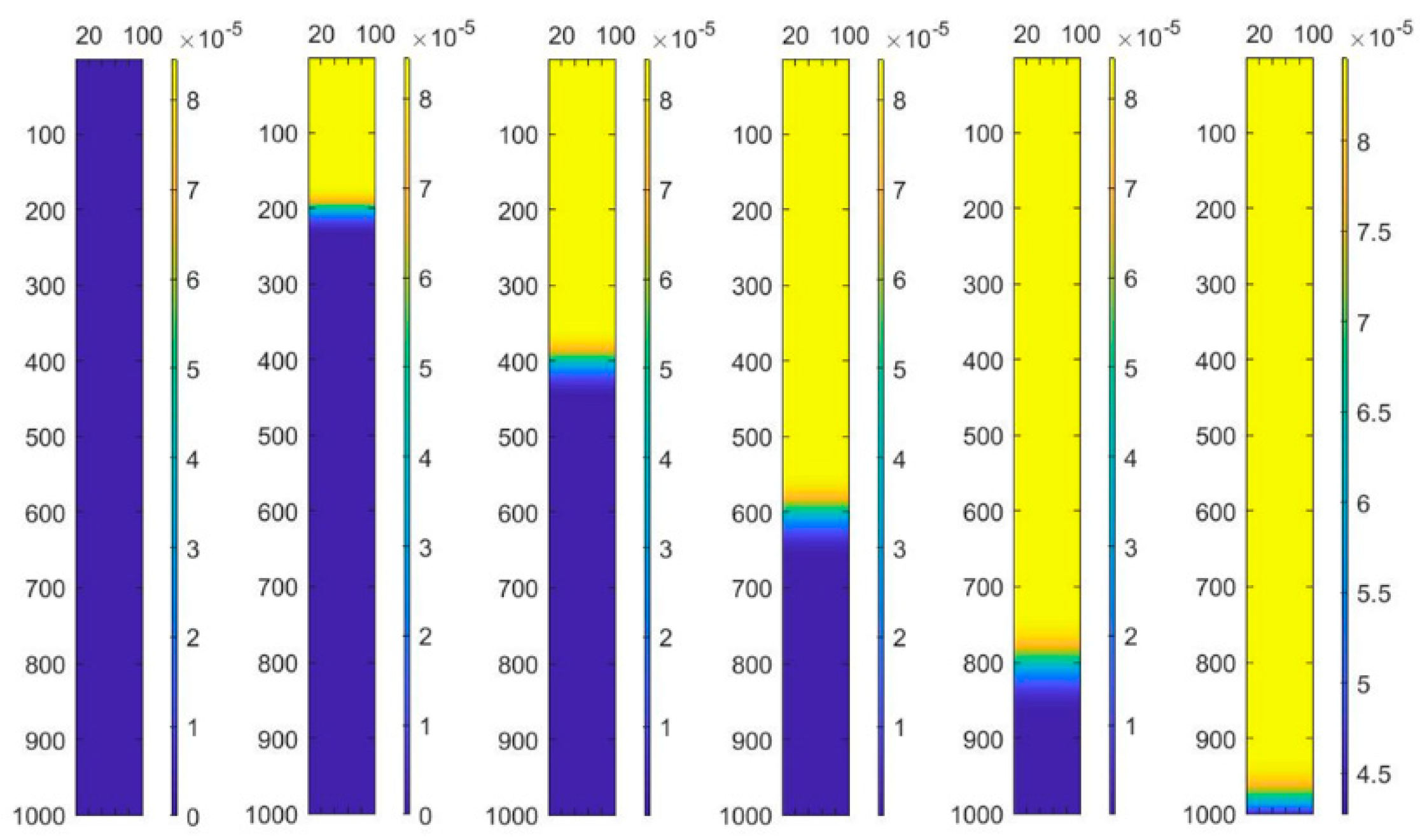
4. Results and Discussion
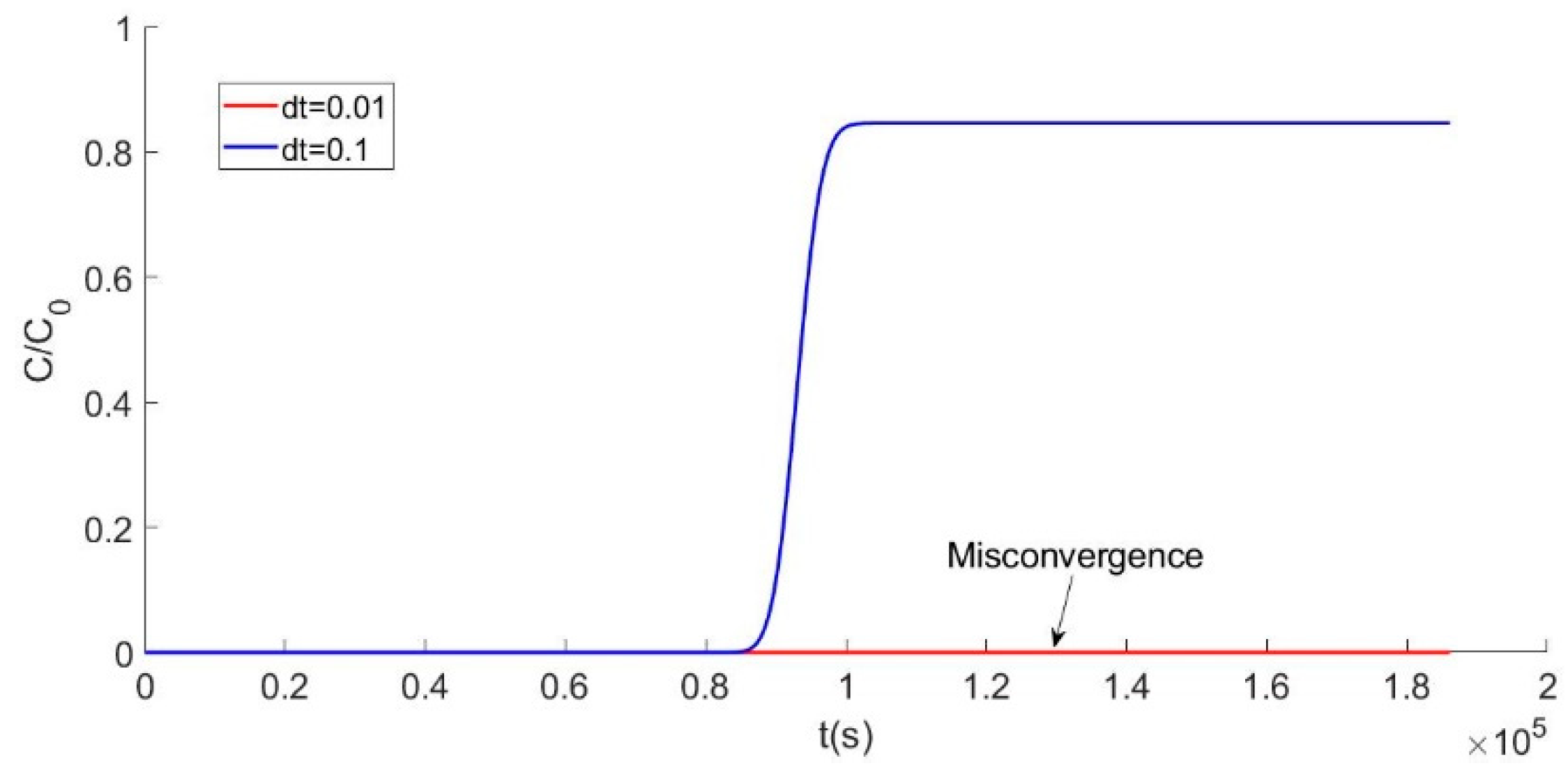
4.1. Convergence of the Model
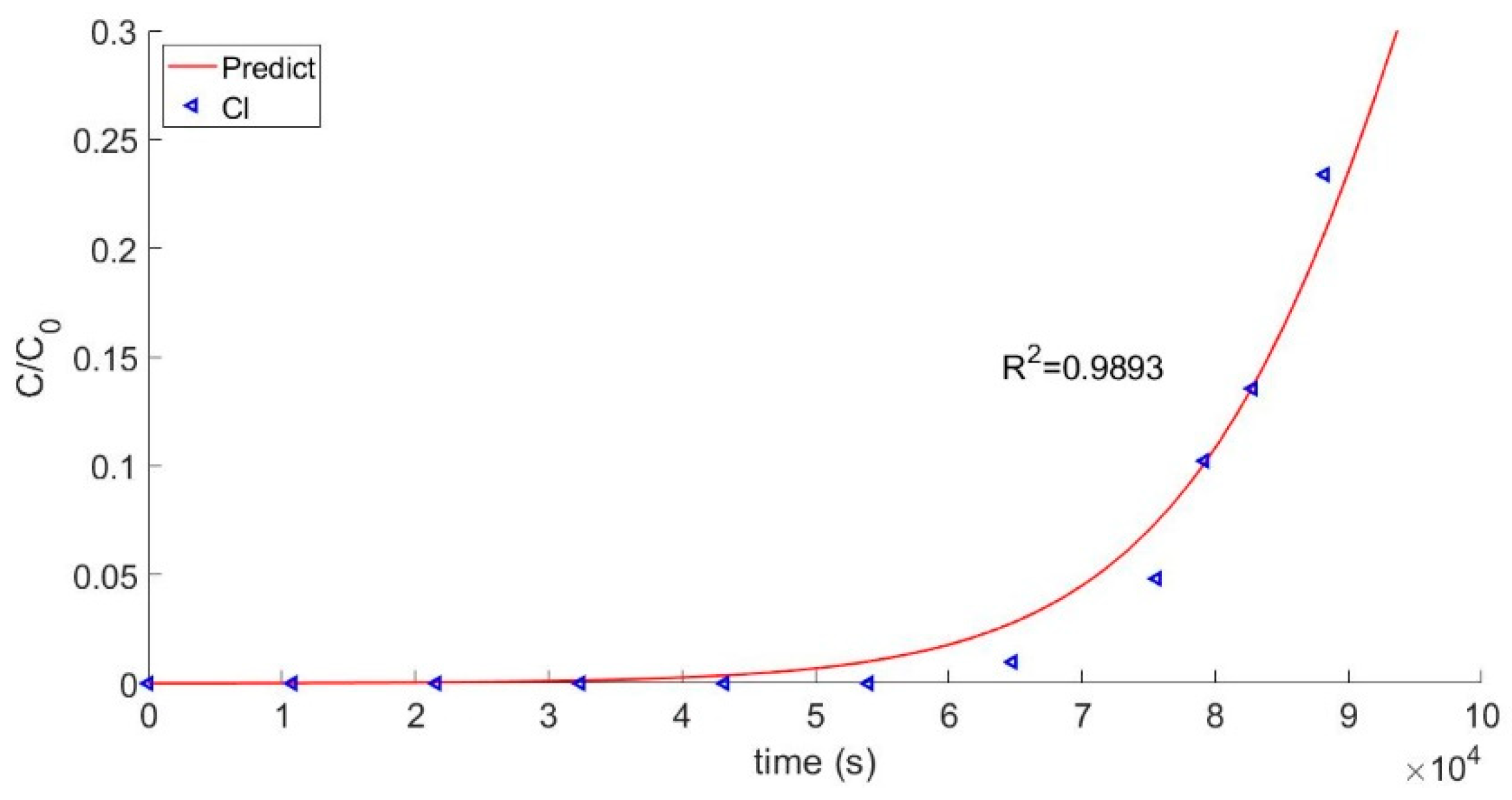
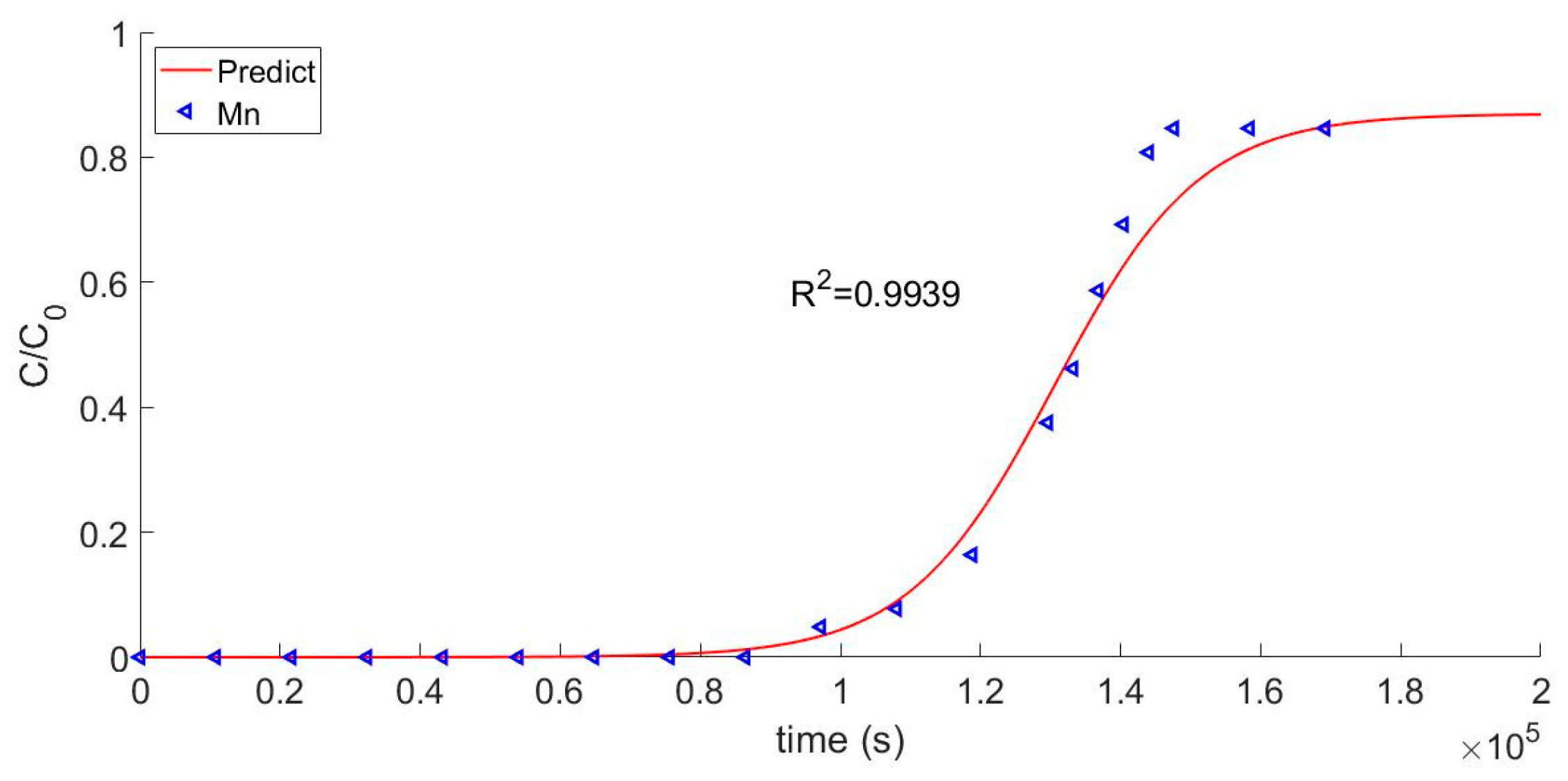
4.2. Analysis of Theoretical Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Parameter of ion i | |
| Parameter of ion i | |
| Total equivalent concentration (keq/m3) | |
| Concentration of ion i (kmol/m3) | |
| C | Liquid phase concentration (mol/L) |
| C0 | Initial liquid phase concentration (mol/L) |
| dt | Time step (s) |
| dp | Diameter of resin particle (m) |
| Individual diffusivity of ion i (m2/s) | |
| Representative diffusivity (m2/s) | |
| E | Back mixing coefficient (dimensionless) |
| F | Faraday’s constant |
| H | Height of column (m) |
| J | Ion flux (mol/m2·s) |
| t | Time (s) |
| q | Adsorption capacity (mg/g) |
| S | Cross-section area (m2) |
| u | Linear velocity (m/s) |
| UL | Superficial velocity (m/s) |
| Void fraction (dimensionless) | |
| k | Representative mass transfer coefficient (m/s) |
| m | Number of co-ions |
| Relative valence | |
| Р | Exponent |
| Loading of ion i (mol/g) | |
| R | Gas law constant |
| T | Temperature |
| Variable for the numerical solution | |
| Equivalent fraction of ion i in the solution | |
| Equivalent fraction of ion i in the resin phase | |
| Electrochemical valence (negative for anions) | |
| Nernst film thickness (m) | |
| Resin particle density(kg/m3) | |
| of the counterions |
Appendix A
- is the inflow minus outflow in the interface
- is the change of ions in the microelement, and
- is the ion change in the resin.
References
- Smith, R.P.; Woodburn, E.T. Prediction of Multicomponent Ion Exchange Equilibria for the Ternary System SO-NO-Cl from Data of Binary Systems. AIChE J. 1978, 24, 577–587. [Google Scholar] [CrossRef]
- Haub, C.E.; Foutch, G.L. Mixed-bed ion exchange at concentrations approaching the dissociation of water. 2. Column model applications. Ind. Eng. Chem. Res. 1986, 25, 381–385. [Google Scholar] [CrossRef]
- Zecchini, E.J.; Foutch, G.L.; Yoon, T. Multi-Component Mixed-Bed Ion Exchange Modeling in Aminated Waters. In Ion Exchange. Advances; Springer: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Yang, J.E.; Skogley, E.O. Diffusion Kinetics of Multinutrient Accumulation by Mixed-Bed Ion-Exchange Resin. Soil Sci. Soc. Am. J. 1992, 56, 408. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind. Eng. Chem. Fundam. 1966, 5, 587–594. [Google Scholar] [CrossRef]
- Dwivedi, P.N.; Upadhyay, S.N. Particle-Fluid Mass Transfer in Fixed and Fluidized Beds. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 157–165. [Google Scholar] [CrossRef]
- Shams, K. Sorption dynamics of a fixed-bed system of thin-film-coated monodisperse spherical particles/hollow spheres. Chem. Eng. Sci. 2001, 56, 5383–5390. [Google Scholar] [CrossRef]
- Chowdiah, V.N.; Foutch, G.L.; Lee, G.C. Binary Liquid-Phase Mass Transport in Mixed-Bed Ion Exchange at Low Solute Concentration. Ind. Eng. Chem. Res. 2003, 42, 1485–1494. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. Continuous fixed-bed column study and adsorption modeling: Removal of cadmium (II) and lead (II) ions in aqueous solution by dead calcareous skeletons. Biochem. Eng. J. 2014, 87, 50–61. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, N.H.L. Computer simulations of the dynamics of multicomponent ion exchange and adsorption in fixed beds—Gradient-directed moving finite element method. Comput. Chem. Eng. 1989, 13, 915–926. [Google Scholar] [CrossRef]
- Carta, G.; Cincotti, A.; Cao, G. Film Model Approximation for Particle-Diffusion-Controlled Binary Ion Exchange. Sep. Sci. Technol. 1999, 34, 1–16. [Google Scholar] [CrossRef]
- Spalding, G.E. Predictive Theory of Coion Transport Accompanying Particle-Diffusion-Controlled Ion Exchange. J. Chem. Phys. 1971, 55, 4991–4995. [Google Scholar] [CrossRef]
- Rahmant, K.; Streat, M. Mass transfer in liquid fluidized beds of ion exchange particles. Chem. Eng. Sci. 1981, 36, 293–300. [Google Scholar] [CrossRef]
- Zecchini, E.J.; Foutch, G.L. Mixed-bed ion-exchange modeling with amine form cation resins. Ind. Eng. Chem. Res. 1991, 30, 1886–1892. [Google Scholar] [CrossRef]
- Janvion, P.; Motellier, S.; Pitsch, H. Ion-exchange mechanisms of some transition metals on a mixed-bed resin with a complexing eluent. J. Chromatogr. A 1995, 715, 105–115. [Google Scholar] [CrossRef]
- Fenn, M.E.; Poth, M.A.; Arbaugh, M.J. A throughfall Collection Method Using Mixed Bed Ion Exchange Resin Columns. Sci. World J. 2002, 2, 122–130. [Google Scholar] [CrossRef]
- Korak, J.A.; Huggins, R.; Arias-Paic, M. Regeneration of pilot-scale ion exchange columns for hexavalent chromium removal. Water Res. 2017, 118, 141–151. [Google Scholar] [CrossRef]
- Franzreb, M.; Hoell, W.H.; Eberle, S.H. Liquid-Phase Mass Transfer in Multicomponent Ion Exchange. 2. Systems with Irreversible Chemical Reactions in the Film. Ind. Eng. Chem. Res. 1995, 34, 2670–2675. [Google Scholar] [CrossRef]
- FREY, D.D. Prediction of liquid-phase mass-transfer coefficients in multicomponent ion exchange: Comparison of matrix, film-model, and effective-diffusivity methods. Chem. Eng. Commun. 1986, 47, 273–293. [Google Scholar] [CrossRef]
- Chatterjee, A.; Schiewer, S. Multi-resistance kinetic models for biosorption of Cd by raw and immobilized citrus peels in batch and packed-bed columns. Chem. Eng. J. 2014, 244, 105–116. [Google Scholar] [CrossRef]
- Rosen, J.B. Kinetics of a Fixed Bed System for Solid Diffusion into Spherical Particles. J. Chem. Phys. 1952, 20, 387–394. [Google Scholar] [CrossRef]
- Jia, Yi.; Foutch, G.L. True multi-component mixed-bed ion-exchange modeling. React. Funct. Polym. 2004, 60, 121–135. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Roberts, D.J. Mathematical modelling and reactor design for multi-cycle bioregeneration of nitrate exhausted ion exchange resin. Water Res. 2016, 88, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Xiu, G.H.; Li, P. Prediction of breakthrough curves for adsorption of lead(II) on activated carbon fibers in a fixed bed. Carbon 2000, 38, 975–981. [Google Scholar] [CrossRef]
- Wu, J.; Jia, L.; Wu, L.; Long, C.; Deng, W.; Zhang, Q. Prediction of the breakthrough curves of VOC isothermal adsorption on hypercrosslinked polymeric adsorbents in a fixed bed. RSC Adv. 2016, 6, 28986–28993. [Google Scholar] [CrossRef]
- Yoshida, H.; Kataoka, T. Intraparticle ion-exchange mass transfer in a ternary system. Ind. Eng. Chem. Res. 1987, 26, 1179–1184. [Google Scholar] [CrossRef]
- Chern, J.M.; Chien, Y.W. Adsorption of nitrophenol onto activated carbon: Isotherms and breakthrough curves. Water Res. 2002, 36, 647–655. [Google Scholar] [CrossRef]
- Faizal, A.M.; Kutty, S.R.M.; Ezechi, E.H. Modelling of Adams-Bohart and Yoon-Nelson on the Removal of Oil from Water Using Microwave Incinerated Rice Husk Ash (MIRHA). Appl. Mech. Mater. 2014, 625, 788–791. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 189, 669. [Google Scholar]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Kataoka, T.; Sato, N.; Ueyama, K. Effective liquid phase diffusivity in ion exchange. Chem. Eng. Jpn. 1968, 1, 38–42. [Google Scholar] [CrossRef][Green Version]
- Kataoka, T.; Yoshida, H.; Uemura, T. Liquid-side ion exchange mass transfer in a ternary system. AIChE J. 1987, 33, 202–210. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Wen, Q.; Wu, J.; Fan, J.; Jin, X.; Qian, W.; Liu, D.; Chen, X.; Chen, Y.; et al. Experimental and modeling studies on the sorption breakthrough behaviors of butanol from aqueous solution in a fixed-bed of KA-I resin. Biotechnol. Bioprocess Eng. 2013, 18, 223–233. [Google Scholar] [CrossRef]
- Mabrouk, A.; Lagneau, V.; De Dieuleveult, C.; Bachet, M.; Schneider, H.; Coquelet, C. Experiments and Modeling of Ion Exchange Resins for Nuclear Power Plants. Int. J. Eng. Appl. Sci. 2012, 6, 130–134. [Google Scholar]
- Yang, J.; Renken, A. Intensification of mass transfer in liquid fluidized beds with inert particles. Chem. Eng. Process. Process Intensif. 1998, 37, 537–544. [Google Scholar] [CrossRef]
- Murillo, R.; Garcıa, T.; Aylón, E.; Callén, M.S.; Navarro, M.V.; López, J.M.; Mastral, A.M. Adsorption of phenanthrene on activated carbons: Breakthrough curve modeling. Carbon 2004, 42, 2009–2017. [Google Scholar] [CrossRef]
- Pan, B.C.; Meng, F.W.; Chen, X.Q.; Pan, B.J.; Li, X.T.; Zhang, W.M.; Zhang, X.; Chen, J.L.; Zhang, Q.X.; Sun, Y. Application of an effective method in predicting breakthrough curves of fixed-bed adsorption onto resin adsorbent. J. Hazard. Mater. 2005, 124, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Franzreb, M.; Höll, W.H.; Sontheimer, H. Liquid-phase mass transfer in multi-component ion exchange I. Systems without chemical reactions in the film. React. Polym. 1993, 21, 117–133. [Google Scholar] [CrossRef]
- Bachet, M.; Jauberty, L.; De Windt, L.; Tevissen, E.; De Dieuleveult, C.; Schneider, H. Comparison of Mass Transfer Coefficient Approach and Nernst–Planck Formulation in the Reactive Transport Modeling of Co, Ni, and Ag Removal by Mixed-Bed Ion-Exchange Resins. Ind. Eng. Chem. Res. 2014, 53, 11096–11106. [Google Scholar] [CrossRef][Green Version]
- Tedesco, M.; Hamelers, H.V.M.; Biesheuvel, P.M. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
| Name of Index | Cationic Resin | Anionic Resin |
|---|---|---|
| Classification form | gel-type | gel-type |
| functional group | -SO3 | -N(CH3)3 |
| Ion form | H+ | OH- |
| Volume full exchange capacity (mmol/mL) | ≥1.80 | ≥1.10 |
| Ratio of anion resin to anion resin (V/V) | 1.0 | 2.0 |
| particle size range mm | 0.40–1.20 | 0.40–1.20 |
| Wet apparent density (g/mL) | 0.68–0.78 | 0.68–0.78 |
| Maximum operating temperature (°C) | 60.0 | 60.0 |
| Ratio of H+ | ≥99.9% | - |
| Ratio of OH− | - | ≥95.0% |
| Target Ion (104 s) | Experimental Value | Calculate Value |
|---|---|---|
| K+ | 16.21 | 11.14 |
| Cl− | 6.15 | 6.16 |
| Mn2+ | 8.64 | 8.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Liu, B.; Peng, R.; Luo, Y.; Yu, P. Simulation of Ion Exchange Resin with Finite Difference Methods. Processes 2019, 7, 675. https://doi.org/10.3390/pr7100675
Zhu Y, Liu B, Peng R, Luo Y, Yu P. Simulation of Ion Exchange Resin with Finite Difference Methods. Processes. 2019; 7(10):675. https://doi.org/10.3390/pr7100675
Chicago/Turabian StyleZhu, Yawen, Bobo Liu, Ruichao Peng, Yunbai Luo, and Ping Yu. 2019. "Simulation of Ion Exchange Resin with Finite Difference Methods" Processes 7, no. 10: 675. https://doi.org/10.3390/pr7100675
APA StyleZhu, Y., Liu, B., Peng, R., Luo, Y., & Yu, P. (2019). Simulation of Ion Exchange Resin with Finite Difference Methods. Processes, 7(10), 675. https://doi.org/10.3390/pr7100675






