Experimental Studies on Corrosion Behavior of Ceramic Surface Coating using Different Deposition Techniques on 6082-T6 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
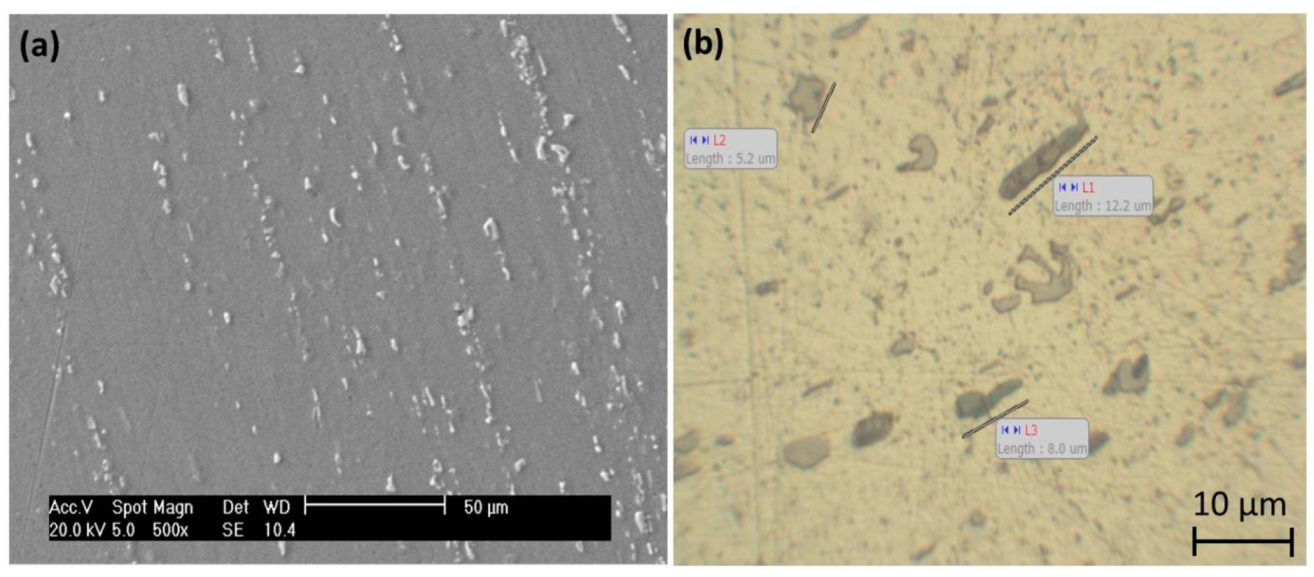
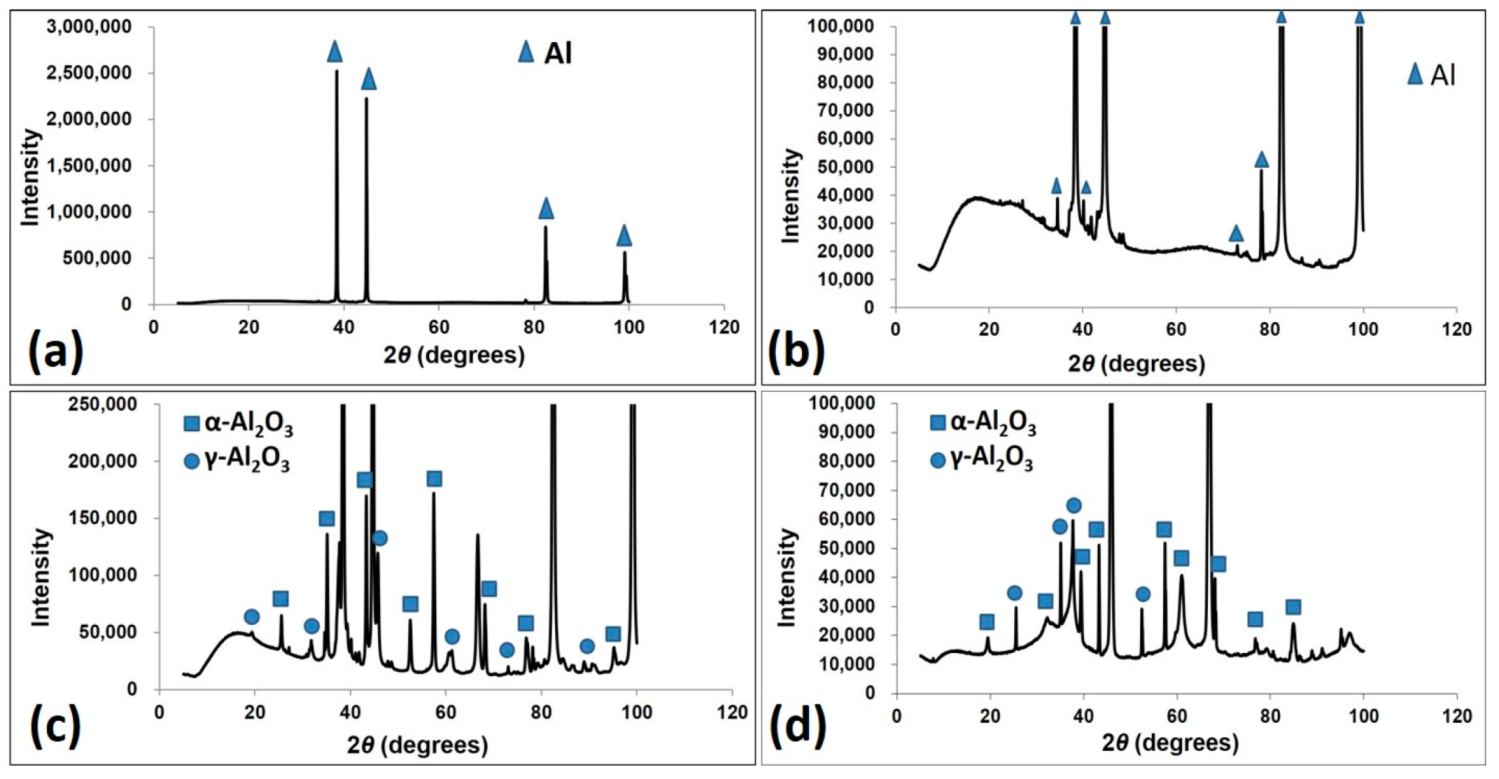
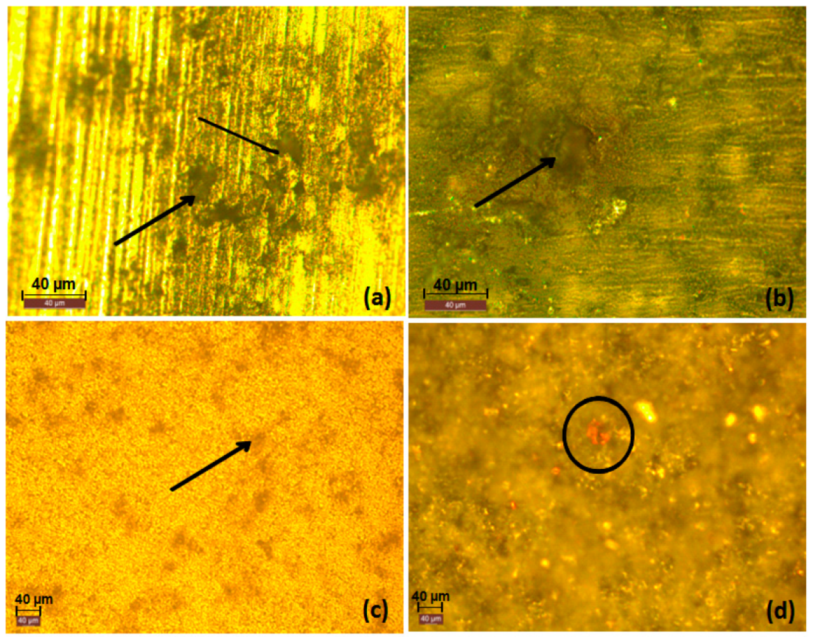
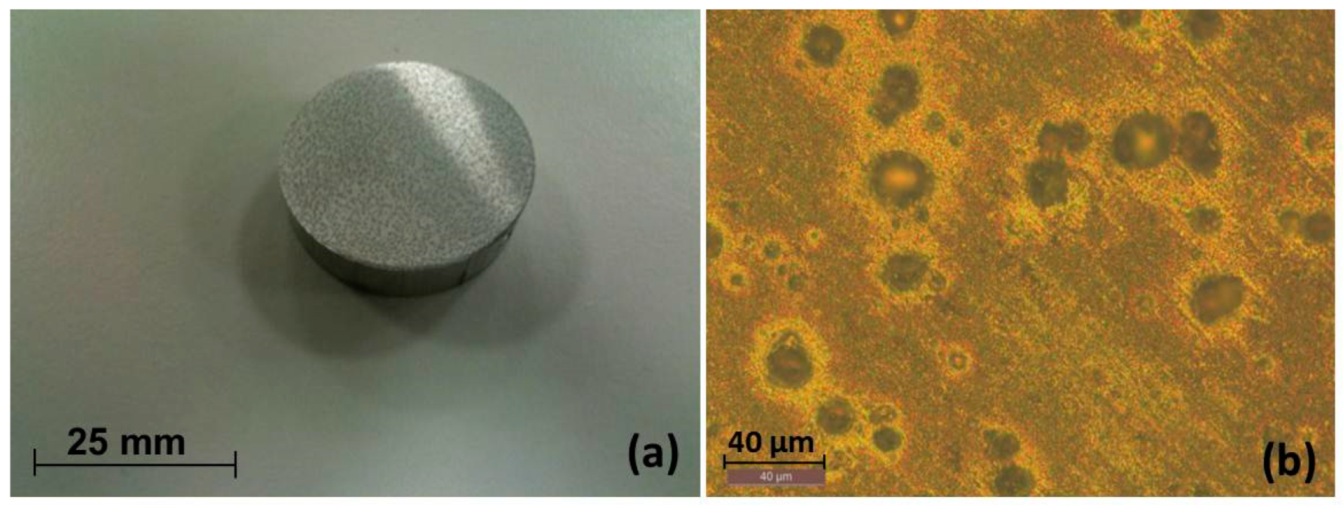
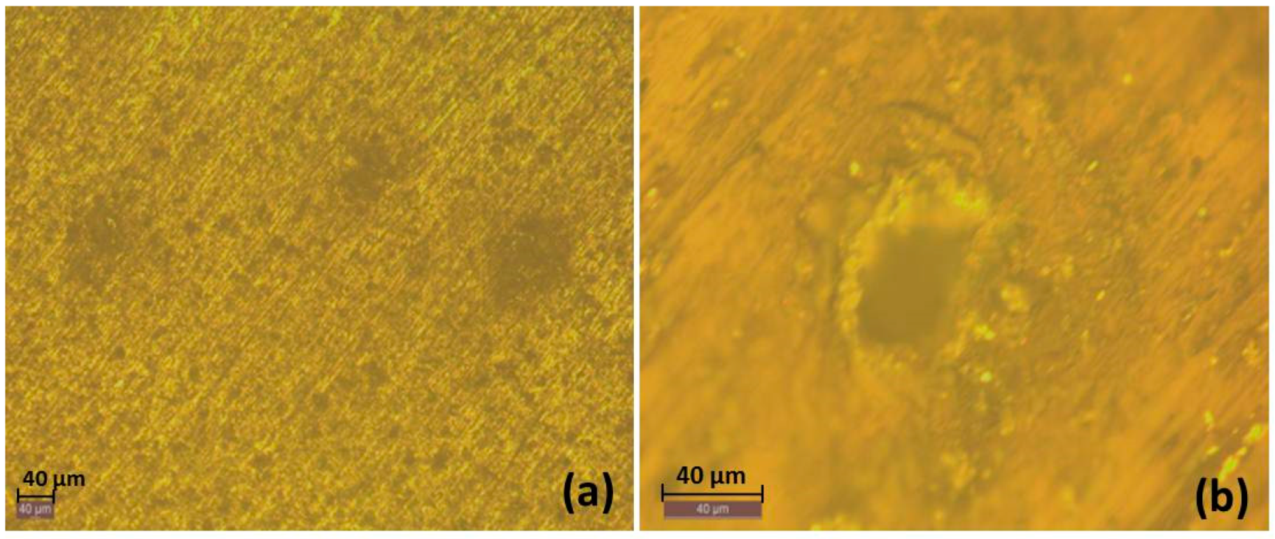
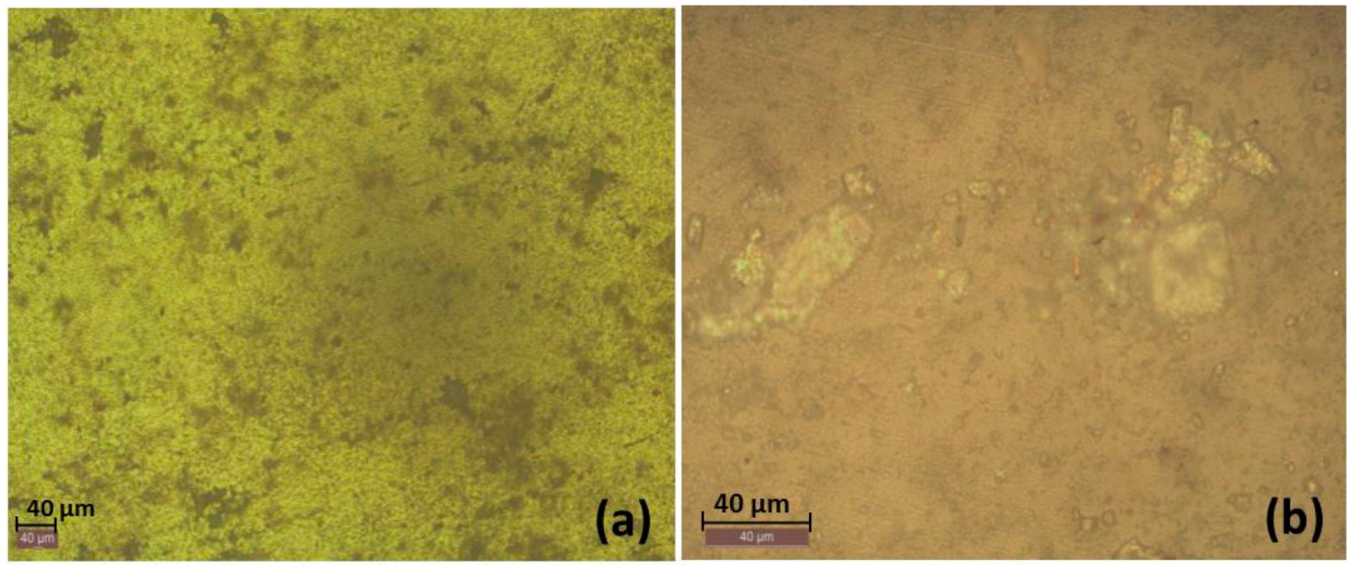
3.1. Macrol Microstructure Analysis
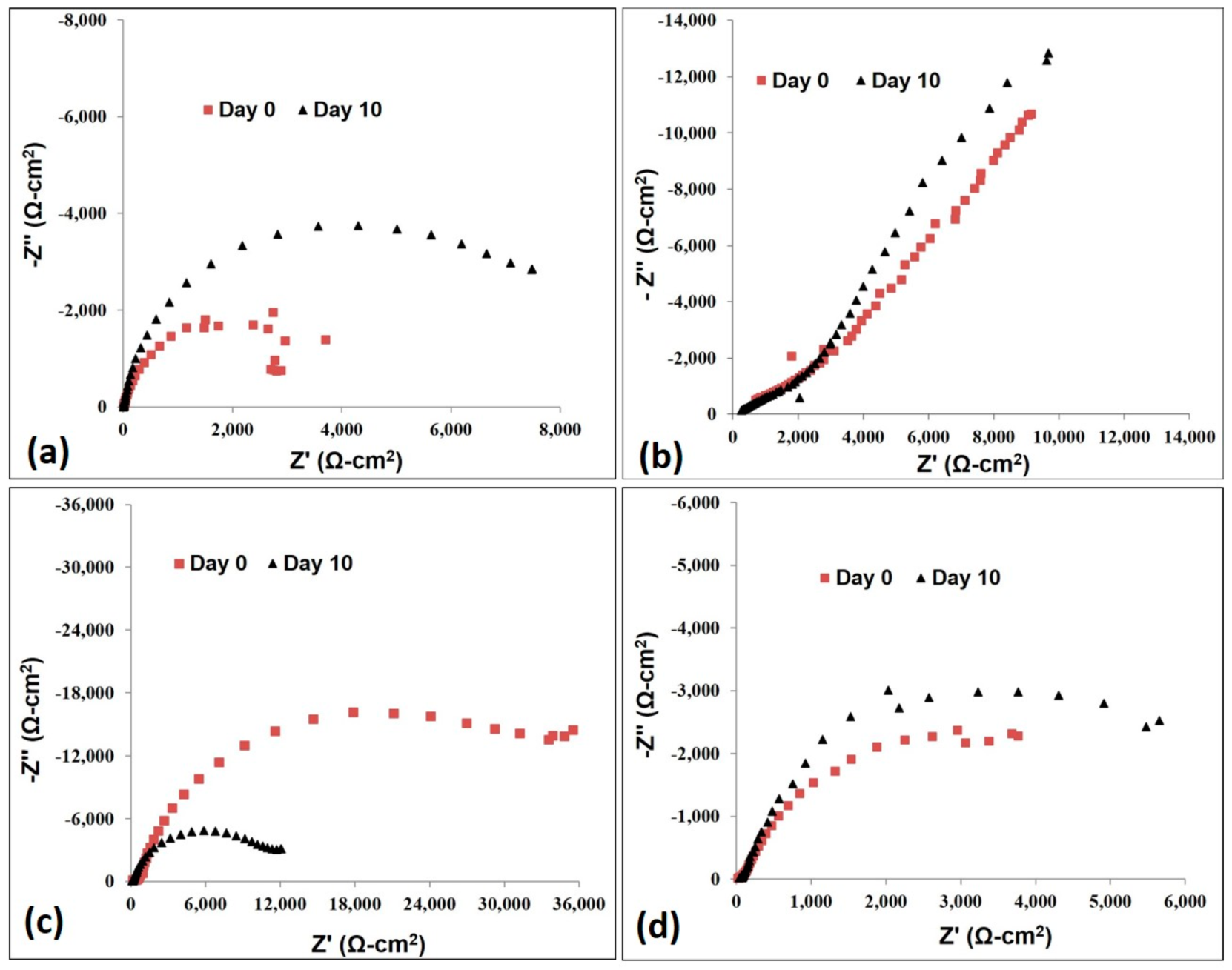
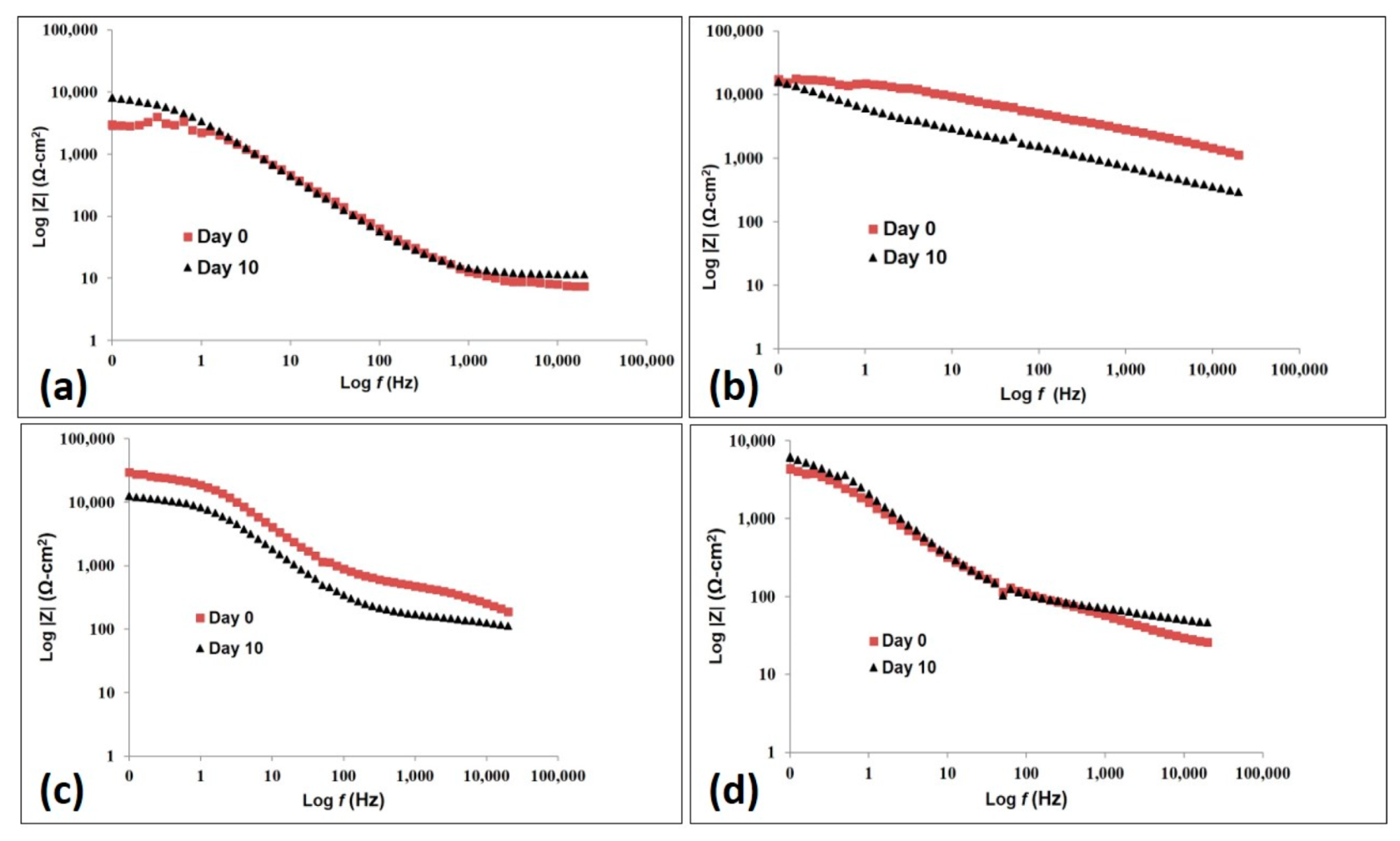
3.2. Electrochemistry
3.2.1. Inductive Coupled Plasma (ICP) Test
3.2.2. Mass Loss (Al3+) from Polarization Tests
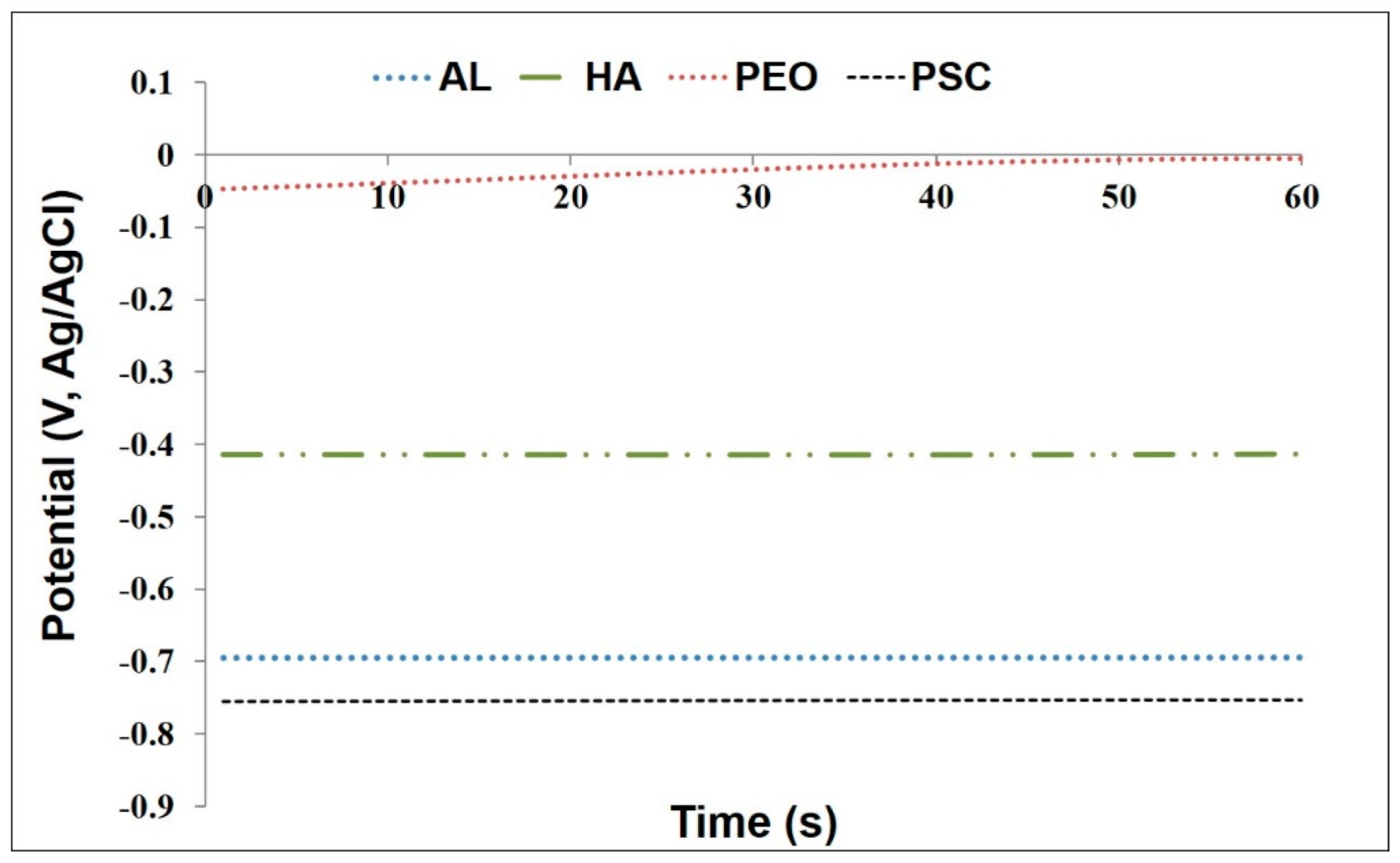
3.2.3. Open Circuit Potential (OCP) Measurements
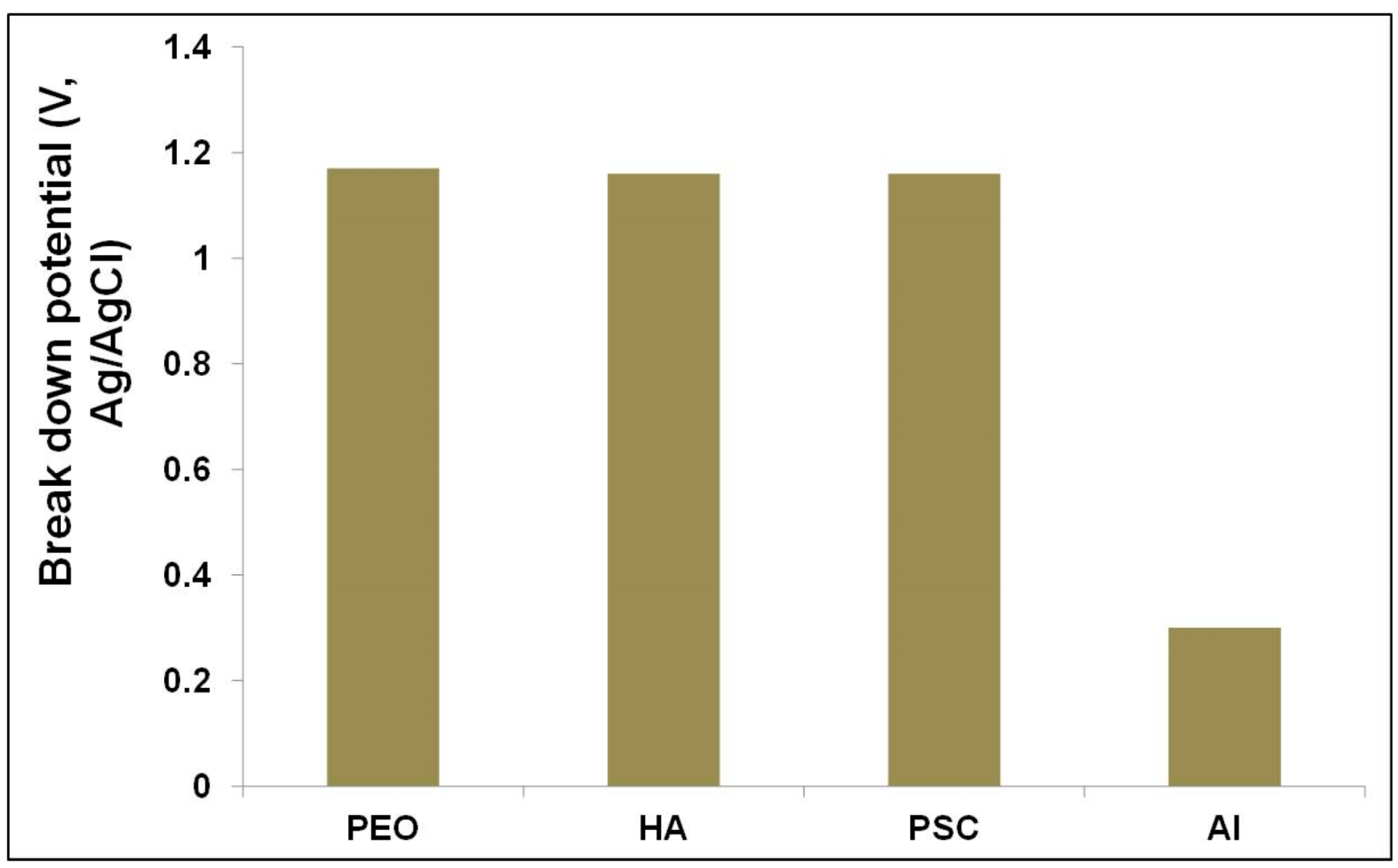
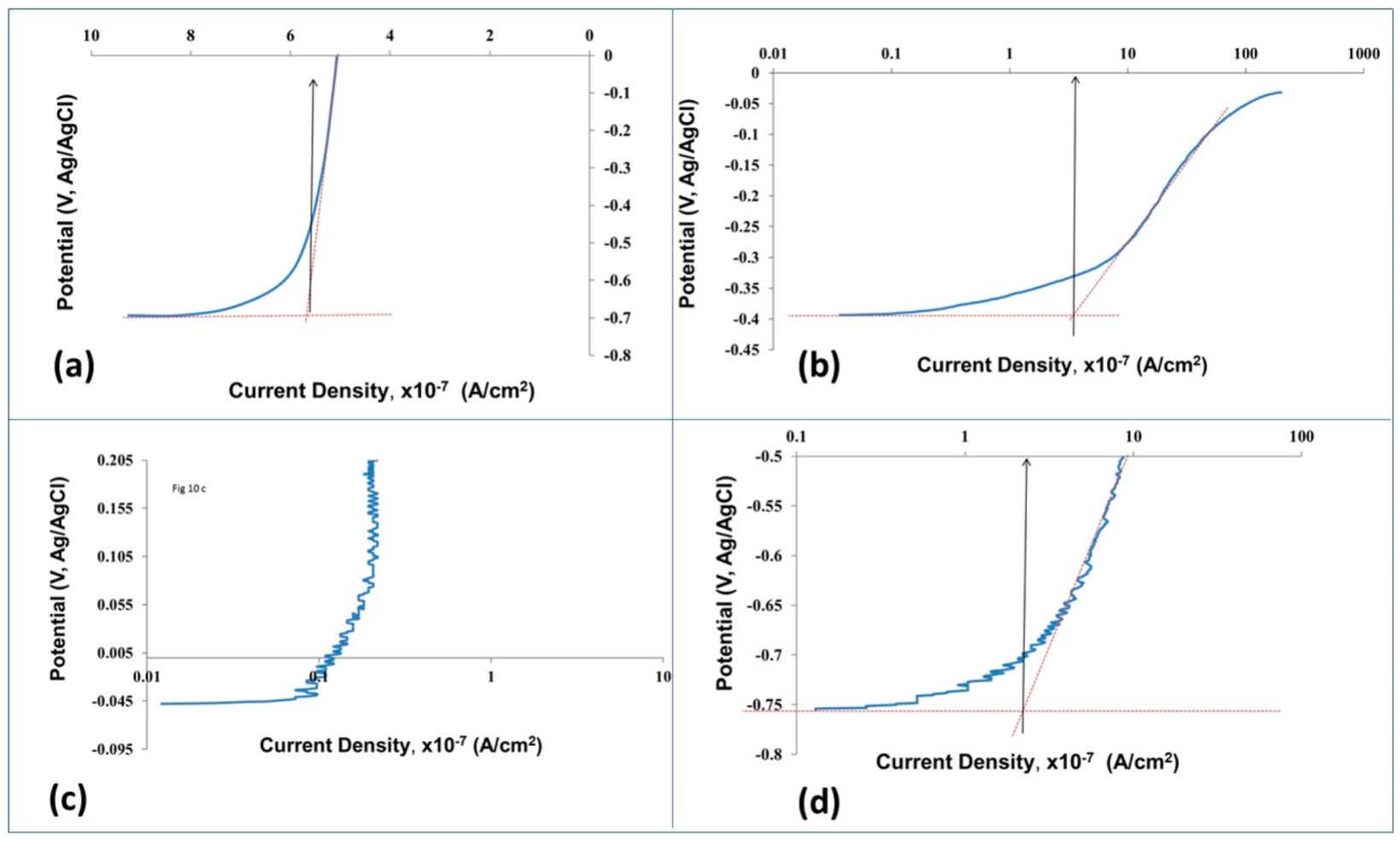
3.2.4. Anodic Polarization (AP) Resistance
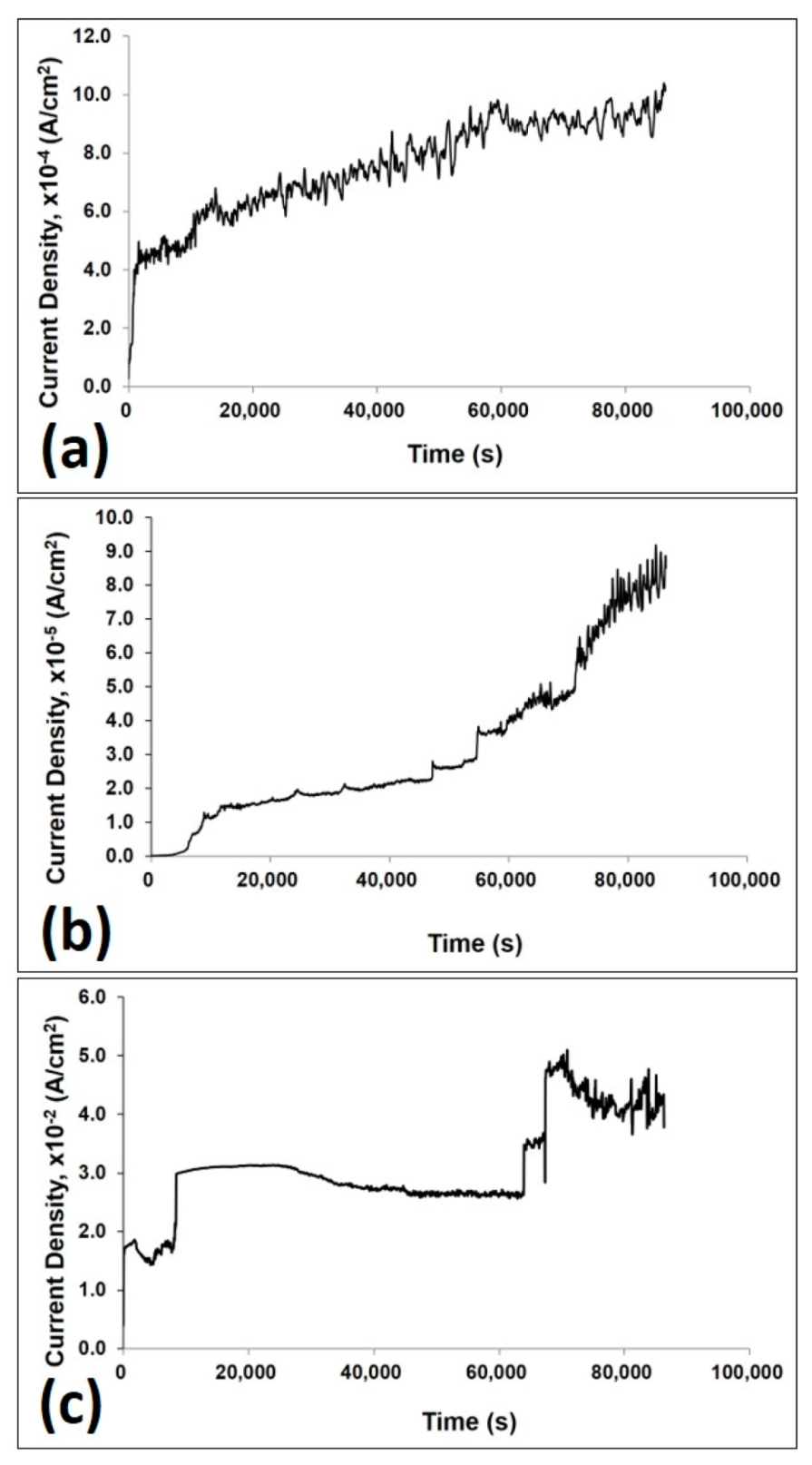
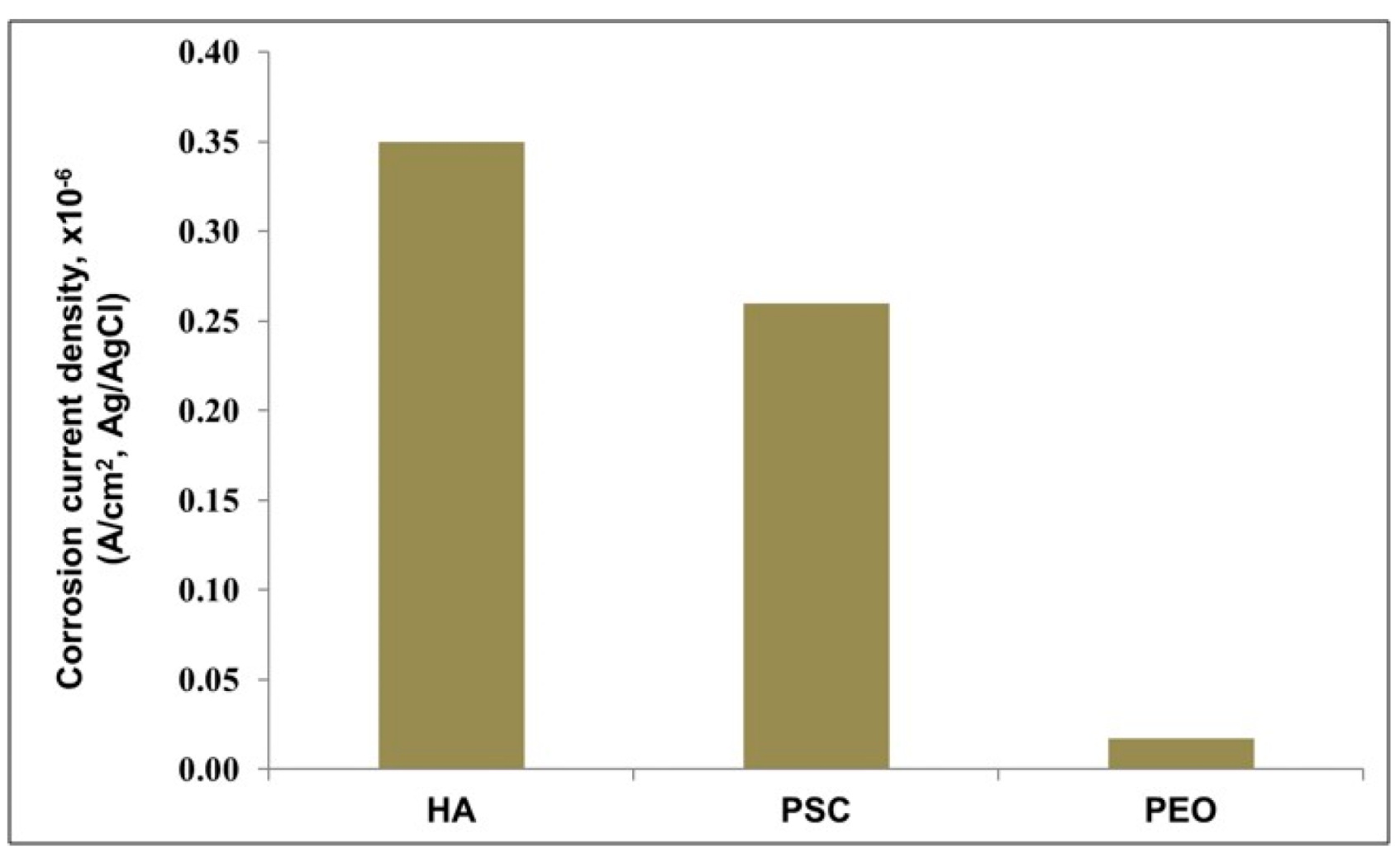
3.2.5. Corrosion Current Density
3.2.6. Optical Images of the Surfaces after Polarization tests
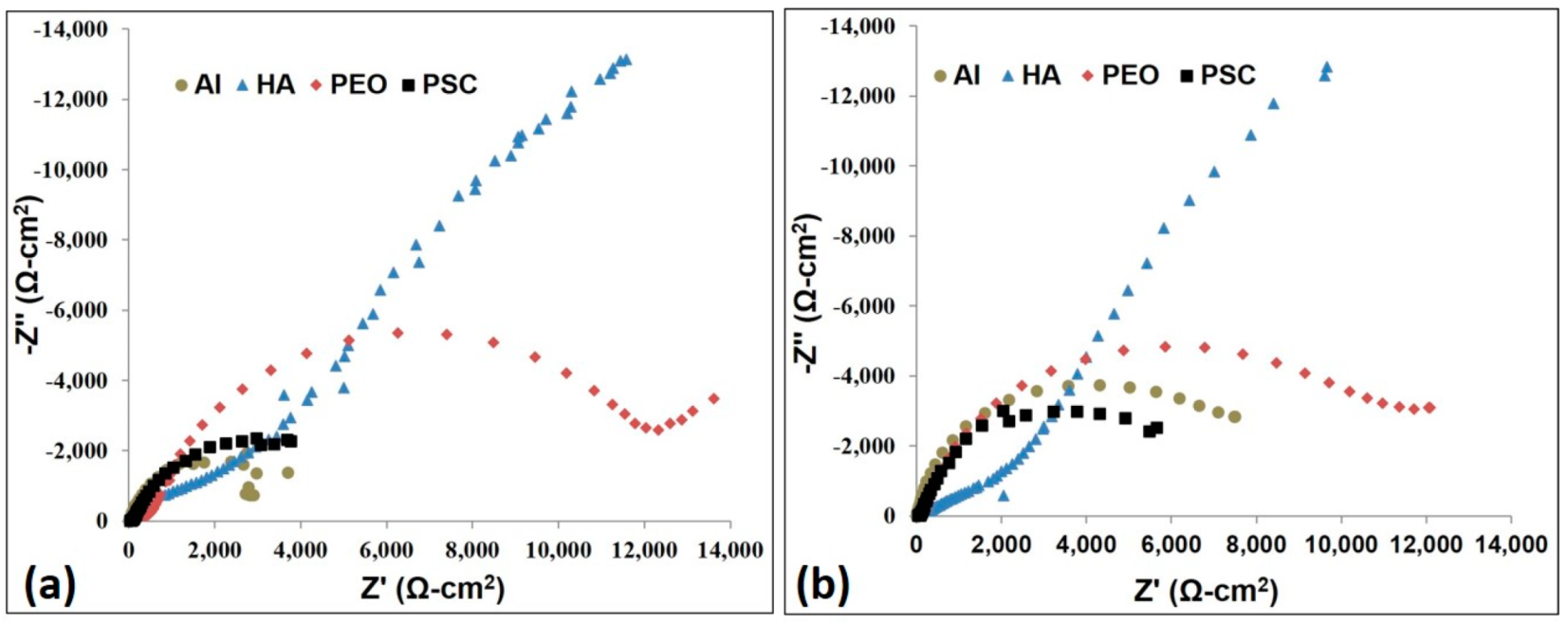
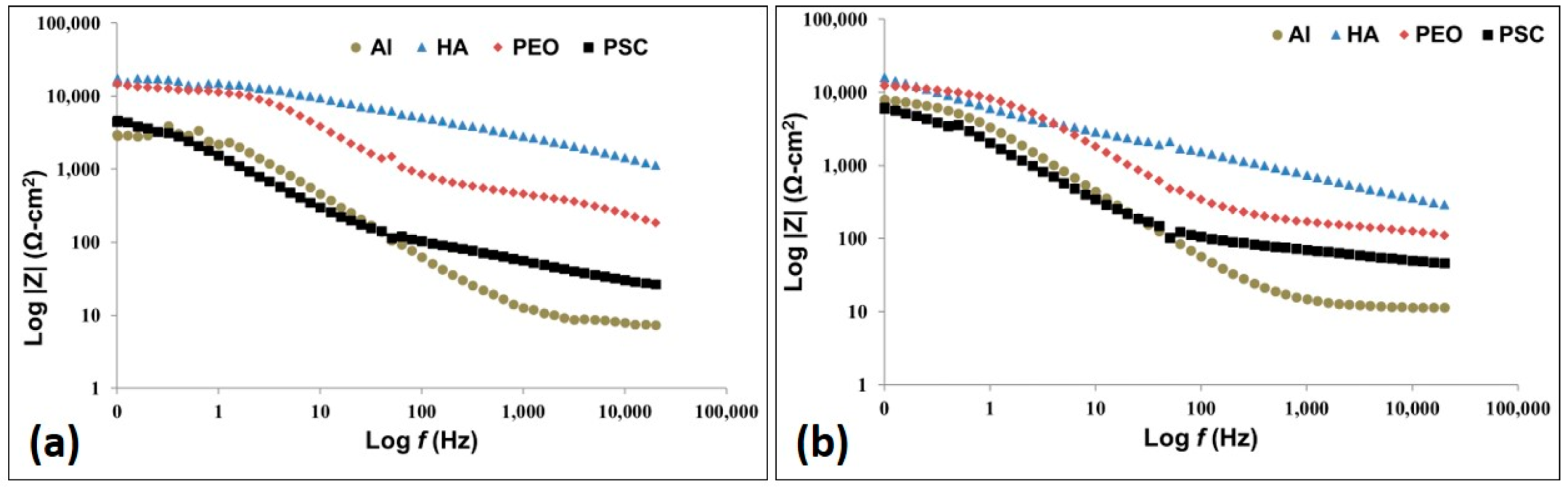
3.2.7. AC Impedance Test
4. Conclusions
- PEO coatings are denser, more uniform and well adhered to the substrate compared to the HA and the PSC coatings and show crystalline alumina phases (α-Al2O3, γ-Al2O3) structure.
- The PEO coating has the lowest amount of aluminum ions (Al3+) released from the coating samples after immersion in 3.5% NaCl solution for 24 h followed with HA then PSC samples.
- The impedance decreases as the immersion time increases. It is concluded that this is due to the degradation and deterioration of the protective coatings.
- All the three coatings (PEO, HA and PSC) have better corrosion resistance than the aluminum substrate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mraied, H.; Cai, W.; Sagüés, A.A. Corrosion resistance of Al and Al–Mn thin films. Thin Solid Film 2016, 615, 391–401. [Google Scholar] [CrossRef]
- Navaser, M.; Atapour, M. Effect of Friction Stir Processing on Pitting Corrosion and Intergranular Attack of 7075 Aluminum Alloy. J. Mater. Sci. Technol. 2017, 33, 155–165. [Google Scholar] [CrossRef]
- De Bonfils-Lahovary, M.; Laffont, L.; Blanc, C. Characterization of intergranular corrosion defects in a 2024 T351 aluminum alloy. Corros. Sci. 2017, 119, 60–67. [Google Scholar] [CrossRef]
- She, H.; Chu, W.; Shu, D.; Wang, J.; Sun, B. Effects of silicon content on microstructure and stress corrosion cracking resistance of 7050 aluminum alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2307–2313. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P. Corrosion and wear of 6082 aluminum alloy. Tribol. Int. 2009, 42, 886–889. [Google Scholar] [CrossRef]
- El-Mahdy, G.A.; Kim, K.B. AC impedance study on the atmospheric corrosion of aluminum under periodic wet-dry conditions. Electrochim. Acta 2004, 49, 1937–1948. [Google Scholar] [CrossRef]
- Nascimento, M.P.; Voorwald, H.J.C. Considerations on corrosion and weld repair effects on the fatigue strength of a steel structure critical to the flight-safety. Int. J. Fatigue 2010, 32, 1200–1209. [Google Scholar] [CrossRef]
- Olajire, A.A. Corrosion inhibition of offshore oil and gas production facilities using organic compound inhibitors—A review. J. Mol. Liq. 2017, 248, 775–808. [Google Scholar] [CrossRef]
- Wood, R.J.K. Tribo-corrosion of coatings: A review. J. Phys. D Appl. Phys. 2007, 40, 5502–5521. [Google Scholar] [CrossRef]
- Ibatan, T.; Uddin, M.S.; Chowdhury, M.A.K. Recent development on surface texturing in enhancing tribological performances of bearing sliders. Surf. Coat. Technol. 2015, 272, 102–120. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Cheng, F.T.; Lo, K.H. Developments in laser-based surface engineering processes: with particular reference to protection against cavitation erosion. Surf. Coat. Technol. 2016, 291, 189–204. [Google Scholar] [CrossRef]
- Bebelis, S.; Bouzek, K.; Cornell, A.; Ferreira, M.G.S.; Walsh, F.C. Highlights during the development of electrochemical engineering. Chem. Eng. Res. Des. 2013, 91, 1998–2020. [Google Scholar] [CrossRef]
- England, G. Surface Engineering in a Nutshell. Available online: http://www.surfaceengineer.co.uk/ (accessed on 10 November 2017).
- Wang, C.; Bai, S.; Xiong, Y. Recent advances in surface and interface engineering for electrocatalysis. Chin. J. Catal. 2015, 36, 1476–1493. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W. Review on corrosion-wear resistance performances of materials in molten aluminum and its alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 1715–1731. [Google Scholar] [CrossRef]
- Chi, Y.; Gu, G.; Yu, H.C.; Chen, C. Laser surface alloying on aluminum and its alloys: A review. Opt. Lasers Eng. 2018, 100, 23–37. [Google Scholar] [CrossRef]
- Morks, M.F.; Corrigan, I.C.P.; Kobayashi, A. Electrochemical Characterization of Plasma Sprayed Alumina Coatings. J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 107–111. [Google Scholar] [CrossRef]
- Kossenko, A.; Zinigrad, M. A universal electrolyte for the plasma electrolytic oxidation of aluminum and magnesium alloys. Mater. Des. 2015, 88, 302–309. [Google Scholar] [CrossRef]
- Martin, J.; Leone, P.; Nominé, A.; Veys-Renaux, D.; Belmonte, T. Influence of electrolyte ageing on the Plasma Electrolytic Oxidation of aluminum. Surf. Coat. Technol. 2015, 269, 36–46. [Google Scholar] [CrossRef]
- Martin, J.; Melhem, A.; Ihchedrina, I.; Duchanoy, T.; Belmonte, T. Effects of electrical parameters on plasma electrolytic oxidation of aluminum. Surf. Coat. Tech. 2013, 221, 70–76. [Google Scholar] [CrossRef]
- Kasalica, B.; Petkovic, M.; Belca, I.; Stojadinovic, S.; Zekovic, L. Electronic transitions during plasma electrolytic oxidation of aluminum. Surf. Coat. Tech. 2009, 203, 3000–3004. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Lampke, T. Electrolyte influence on ignition of plasma electrolytic oxidation processes on light metals. Surf. Coat. Tech. 2017, 315, 205–213. [Google Scholar] [CrossRef]
- Xie, H.; Cheng, Y.; Li, S.; Cao, J.; Cao, L. Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferrous Met. Soc. China 2017, 27, 336–351. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Bao, Y.; Yang, K. Sliding wear behavior of plasma electrolytic oxidation coating on pure aluminum. Wear 2011, 271, 1667–1670. [Google Scholar] [CrossRef]
- Massimiliano, B.; Manuela, C.; Roberto, G.; Andrea, B. Hard anodizing of AA2099-T8 aluminum-lithium-copper alloy: Influence of electric cycle, electrolytic bath composition and temperature. Surf. Coat. Technol. 2017, 325, 627–635. [Google Scholar]
- Andrea, B.; Roberto, G.; Tiziano, M.; Paolo, M. Pulsed current effect on hard anodizing process of 7075-T6 aluminum alloy. Surf. Coat. Technol. 2016, 270, 139–144. [Google Scholar]
- Massimiliano, B.; Roberto, G.; Andrea, B. Pulsed current hard anodizing of heat treated aluminum alloys: Frequency and current amplitude influence. Surf. Coat. Technol. 2016, 307, 861–870. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W. Anodizing treatments for magnesium alloys and their effect on corrosion resistance in various environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Huang, Y.; Shih, H. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.L.; Zheng, Y.G.; Ke, W.; Wang, J.Q. Effect of porosity sealing treatments on the corrosion resistance of high-velocity oxy-fuel (HVOF)-sprayed Fe-based amorphous metallic coatings. Surf. Coat. Tech. 2011, 206, 1307–1318. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, B.S. Use of plasma spray technology for deposition of high temperature oxidation/corrosion resistant coatings—A review. Mater. Corros. 2007, 58, 92–102. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, D.; Jain, J. Thermally sprayed alumina and ceria-doped-alumina coatings on AZ91 Mg alloy. Surf. Coat. Tech. 2017, 332, 533–541. [Google Scholar] [CrossRef]
- Thirumalaikumarasamy, D.; Shanmugam, K.; Balasubramanian, V. Corrosion performances of atmospheric plasma sprayed alumina coatings on AZ31B magnesium alloy under immersion environment. J. Asian Ceram. Soc. 2014, 2, 403–415. [Google Scholar] [CrossRef]
- Sivakumar, S.; Praveen, K.; Shanmugavelayutham, G.; Yugeswaran, S. Thermo-physical behavior of atmospheric plasma sprayed high porosity lanthanum zirconate coatings. Surf. Coat. Tech. 2017, 326, 173–182. [Google Scholar] [CrossRef]
- Li, C.; Wang, W. Quantitative characterization of lamellar microstructure of plasma-sprayed ceramic coatings through visualization of void distribution. Mater. Sci. Eng. A 2004, 386, 10–19. [Google Scholar] [CrossRef]
- Li, C.J. Thermal spraying of light alloys. In Surface Engineering of Light Alloys, 1st ed.; Hanshan, D., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 184–241. ISBN 9781845699451. [Google Scholar]
- Wang, R.Q.; Wu, Y.K.; Wu, G.R.; Chen, D.; He, D.L.; Li, D.; Guo, C.; Zhou, Y.; Shen, D.; Nash, P. An investigation about the evolution of microstructure and composition difference between two interfaces of plasma electrolytic oxidation coatings on Al. J. Alloys Compd. 2018, 753, 272–281. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, J. Effects of negative voltage on microstructure and corrosion resistance of red mud plasma electrolytic oxidation coatings. Surf. Coat. Tech. 2018, 352, 15–25. [Google Scholar] [CrossRef]
- Cerchier, P.; Luca, P.; Emanuela, M.; Leonardo, C.; Marie, G.M.O.; Isabella, M.; Maurizio, M. Antifouling properties of different Plasma Electrolytic Oxidation coatings on 7075 aluminum alloy. Int. Biodeterior. Biodegrad. 2018, 133, 70–78. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Walid, M.O.; Amany, M.F. Characterization and Corrosion behavior of anodized Aluminum alloys for military industries applications in artificial seawater. Surf. Interfaces 2018, in press. [Google Scholar] [CrossRef]
- Massimiliano, B.; Roberto, G. Hard anodizing of AA2011-T3 Al-Cu-Pb-Bi free-cutting alloy: improvement of the process parameters. Corros. Sci. 2018, 141, 63–71. [Google Scholar]
- Vatan, H.N.; EbrHAimi-kHArizsangi, R.; Kasiri-asgarani, M. Structural, tribological and electrochemical behavior of SiC nanocomposite oxide coatings fabricated by plasma electrolytic oxidation (PEO) on AZ31 magnesium alloy. J. Alloys Compd. 2016, 683, 241–255. [Google Scholar] [CrossRef]
- Tjiang, F.; Ye, L.; Huang, Y.; Chou, C.; Tsai, D. Effect of processing parameters on soft regime behavior of plasma electrolytic oxidation of magnesium. Ceram. Int. 2017, 43, s567–s572. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. The effect of processing parameters and substrate composition on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloys. Surf. Coat. Tech. 2013, 237, 357–368. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, R.; Wu, X.; Zhang, Y. Influences of Current Density on the Structure and Corrosion Resistance of Ceramic Coatings on ZK60 Mg Alloy by Plasma Electrolytic Oxidation. Int. J. Electrochem. Sci. 2013, 8, 1957–1965. [Google Scholar]
- Majid, V.; Hyoung, S.K. A combination of severe plastic deformation and ageing phenomena in Al–Mg–Si Alloys. Mater. Des. 2012, 36, 735–740. [Google Scholar] [CrossRef]
- Grażyna, M.N.; Sieniawski, J. Influence of heat treatment on the microstructure and mechanical properties of 6005 and 6082 aluminum alloys. J. Mater. Process. Technol. 2005, 162, 367–372. [Google Scholar] [CrossRef]
- Malayoglu, U.; Tekin, K.C.; Malayoglu, U.; Shrestha, S. An investigation into the mechanical and tribological properties of plasma electrolytic oxidation and hard-anodized coatings on 6082 aluminum alloys. Mater. Sci. Eng. A 2011, 528, 7451–7460. [Google Scholar] [CrossRef]
- Shrestha, S.; Shashkov, F. Microstructural and thermo-optical properties of black Keronite PEO coating on aluminum alloy AA7075 for spacecraft materials applications. In Proceedings of the 10th International Symposium, Materials in a Space Environment, Collioure, France, 19–23 June 2006. [Google Scholar]
- Metco, S. Metco® 101NS Grey Alumina Powder. Available online: https://www.oerlikon.com/ecomaXL/files/metco/oerlikon_DSMTS-0083.2_Al2O3_40TiO2.pdf&download=1 (accessed on 22 March 2017).
- Krishna, L.R.; Purnima, A.S.; Sundararajan, G. A comparative study of tribological behavior of microarc oxidation and hard-anodized coatings. Wear 2006, 261, 1095–1101. [Google Scholar] [CrossRef]
- Emregül, K.C.; Aksüt, A.A. The behavior of aluminum in alkaline media. Corrosion Science 2000, 42, 2051–2067. [Google Scholar] [CrossRef]
- Wang, C.; Neville, A. Erosion-corrosion of engineering steels—Can it be managed by use of chemicals? Wear 2018, 267, 2018–2026. [Google Scholar] [CrossRef]
- Cai, F.; Yang, Q.; Huang, X. The Roles of Diffusion Factors in Electrochemical Corrosion of TiN and CrN (CrSiCN) Coated Mild Steel and Stainless Steel. In Materials Processing and Interfaces; Wiley Online Library: Hoboken, NJ, USA, 2012; Volume 1. [Google Scholar] [CrossRef]
- Ren, J.; Zuo, Y. The growth mechanism of pits in NaCl solution under anodic films on aluminum. Surf. Coat. Technol. 2005, 191, 311–316. [Google Scholar] [CrossRef]
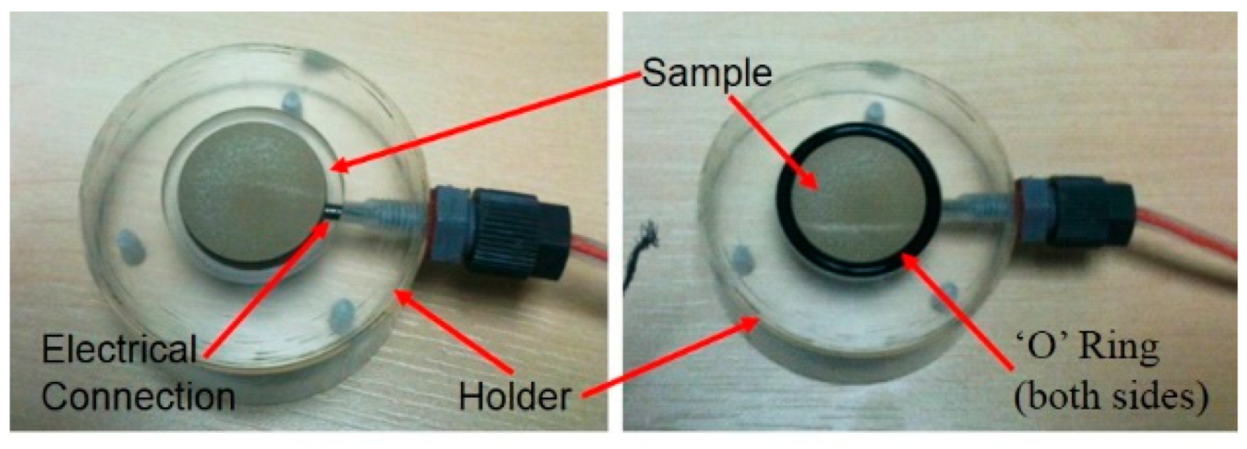




| Si | Mg | Mn | Fe | Cr | Cu | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 0.957 | 0.836 | 0.634 | 0.55 | 0.25 | 0.12 | 0.24 | 0.13 | Balance |
| Test Condition | HA | PEO | PSC |
|---|---|---|---|
| Free Corrosion at 20 °C | 0.000 | 0.000 | 0.000 |
| Free Corrosion at 80 °C | 0.000 | 0.000 | 0.590 |
| Free Corrosion at 20 °C after 80 °C | 0.008 | 0.000 | 0.077 |
| Corrosion Parameter | PEO | HA | PSC | Al |
|---|---|---|---|---|
| Open circuit potential (OCP) (V) | −0.05 ± 0.00246 | −0.40 ± 0.01375 | −0.76 ± 0.05542 | −0.7 ± 0.005 |
| Breaking down potential (Eb) (V) | 1.07 ± 0.1 | 1.11 ± 0.05 | 1.12 ± 0.04 | 0.2 ± 0.1 |
| Corrosion current density (icorr) (A/cm2) from AP Curve | 1.7 × 10−8 | 3.5 × 10−7 | 2.6 × 10−7 | 1.2 × 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algahtani, A.; Mahmoud, E.R.I.; Khan, S.Z.; Tirth, V. Experimental Studies on Corrosion Behavior of Ceramic Surface Coating using Different Deposition Techniques on 6082-T6 Aluminum Alloy. Processes 2018, 6, 240. https://doi.org/10.3390/pr6120240
Algahtani A, Mahmoud ERI, Khan SZ, Tirth V. Experimental Studies on Corrosion Behavior of Ceramic Surface Coating using Different Deposition Techniques on 6082-T6 Aluminum Alloy. Processes. 2018; 6(12):240. https://doi.org/10.3390/pr6120240
Chicago/Turabian StyleAlgahtani, Ali, Essam R.I. Mahmoud, Sohaib Z. Khan, and Vineet Tirth. 2018. "Experimental Studies on Corrosion Behavior of Ceramic Surface Coating using Different Deposition Techniques on 6082-T6 Aluminum Alloy" Processes 6, no. 12: 240. https://doi.org/10.3390/pr6120240
APA StyleAlgahtani, A., Mahmoud, E. R. I., Khan, S. Z., & Tirth, V. (2018). Experimental Studies on Corrosion Behavior of Ceramic Surface Coating using Different Deposition Techniques on 6082-T6 Aluminum Alloy. Processes, 6(12), 240. https://doi.org/10.3390/pr6120240






