Synthesis and Application of FeMg-Modified Hydrochar for Efficient Removal of Lead Ions from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation and Modification of Hydrochar
2.3. Adsorption Tests
2.4. Characterization of FeMg-PHC Before and After Pb2+ Removal
3. Results and Discussion
3.1. Characterization Before and After Pb2+ Adsorption Using FeMg-PHC
3.2. Influence of Initial pH
3.3. Influence of Contact Time and Kinetic Study
3.4. Influence of Initial Pb2+ Concentration and Isothermal Assay
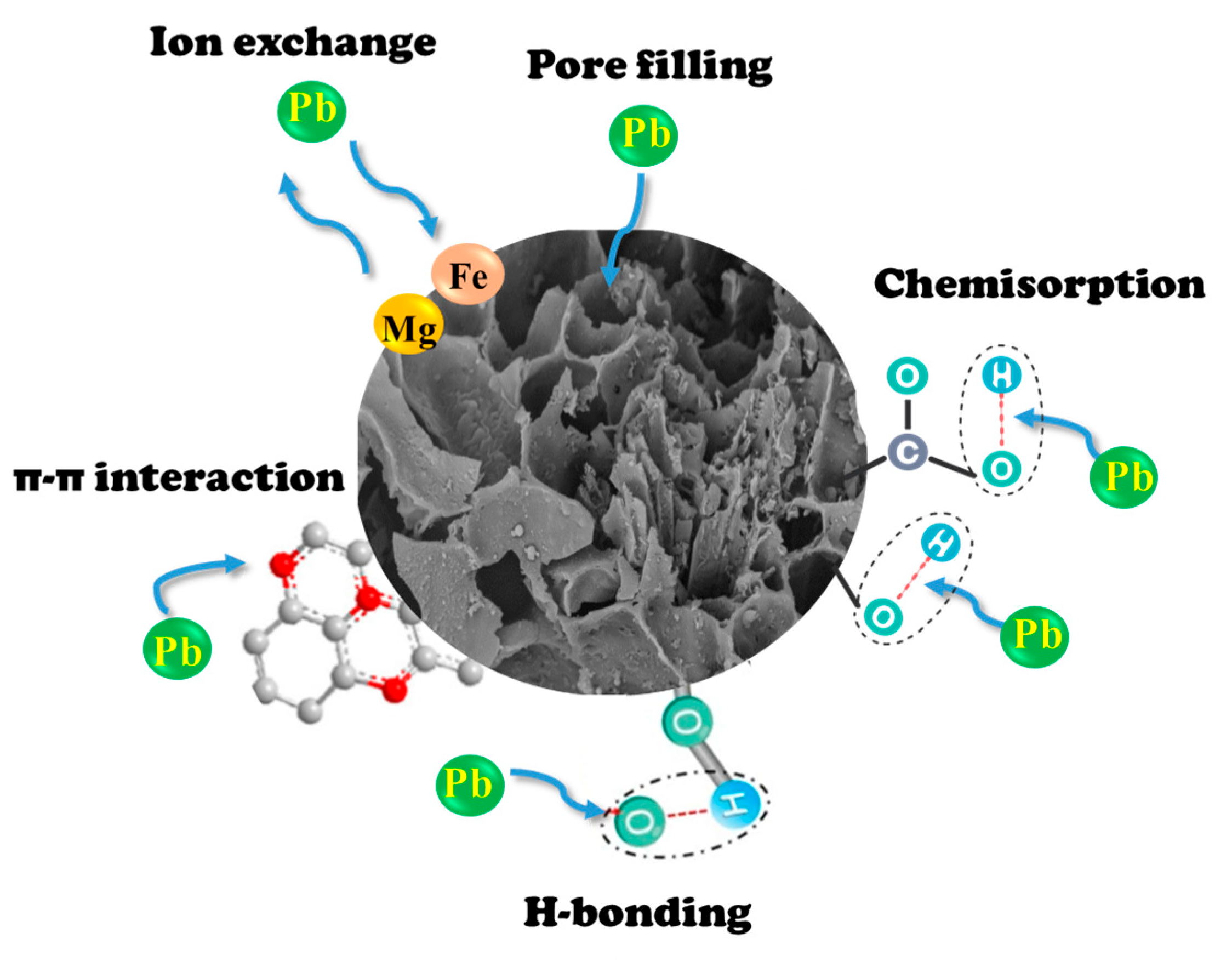
3.5. Potential Mechanism of Pb2+ Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simić, M.; Petrović, J.; Šoštarić, T.; Ercegović, M.; Milojković, J.; Lopičić, J.; Kojić, M. Mechanism assessment and differences of cadmium adsorption on raw and alkali-modified agricultural waste. Processes 2022, 10, 1957. [Google Scholar] [CrossRef]
- Fernández, L.A.G.; Castillo, N.A.M.; Polo, M.S.; Frómeta, A.E.N.; Cadre, J.E.V. Algal-Based Carbonaceous Materials for Environmental Remediation: Advances in Wastewater Treatment, Carbon Sequestration, and Biofuel Applications. Processes 2025, 13, 556. [Google Scholar] [CrossRef]
- Noor, A.; Ali Khan, S. Agricultural Wastes as Renewable Biomass to Remediate Water Pollution. Sustainability 2023, 15, 4246. [Google Scholar] [CrossRef]
- Pan, T.; Guo, Z.; Zhang, X.; Feng, L. Hydrothermal carbonization of biomass waste and application of produced hydrochar in organic pollutants removal. J. Clean. Prod. 2024, 457, 142386. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Pantić, J.J. Hydrothermal carbonization of waste biomass: A review of hydrochar preparation and environmental application. Processes 2024, 12, 207. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.Q.; Guo, J.Z.; Fu, S.Y.; Guo, M.; Yang, P. The polyaminocarboxylated modified hydrochar for efficient capturing methylene blue and Cu(II) from water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef]
- Koprivica, M.; Simić, M.; Petrović, J.; Ercegović, M.; Dimitrijević, J. Evaluation of Adsorption Efficiency on Pb(II) Ions Removal Using Alkali-Modified Hydrochar from Paulownia Leaves. Processes 2023, 11, 1327. [Google Scholar] [CrossRef]
- Hammud, H.H.; Karnati, R.K.; Al Shafee, M.; Fawaz, Y.; Holail, H. Activated hydrochar from palm leaves as efficient lead adsorbent. Chem. Eng. Commun. 2019, 208, 197–209. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Kalderis, D.; Koprivica, M.; Milojković, J.; Radulović, D. Novel Mg-doped pyro-hydrochars as methylene blue adsorbents: Adsorption behavior and mechanism. J. Mol. Liq. 2023, 376, 121424. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.Z.; Liu, J.L.; Fang, L.; Lv, J.Q.; Lv, K. Removal of aqueous-phase lead ions by dithiocarbamate-modified hydrochar. Sci. Total Environ. 2020, 714, 136897. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, A.K.; Al-Hazmi, H.; Kurniawan, T.A.; Majtacz, J.; Piechota, G.; Kumar, G.; Ezzati, P.; Saeb, M.S.; Rabiee, N.; Karimi-Maleh, H.; et al. Hydrochar as a bio-based adsorbent for heavy metals removal: A review of production processes, adsorption mechanisms, kinetic models, regeneration and reusability. Sci. Total Environ. 2024, 945, 173972. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.; Bandala, E.; Zhang, Z.; Mundree, S.; Goonetilleke, A. Removal of heavy metals from water using engineered hydrochar: Kinetics and mechanistic approach. J. Water Process Eng. 2021, 40, 101929. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Otero, M.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.; Rafatullah, M.; Hamedelniel, A.E. Heteroatom-doped magnetic hydrochar to remove post-transition and transition metals from water: Synthesis, characterization, and adsorption studies. Chemosphere 2019, 218, 1089–1099. [Google Scholar] [CrossRef]
- Kojić, M.; Mihajlović, M.; Marinović-Cincović, M.; Petrović, J.; Katanić, Đ.; Krstić, A.; Butulija, S.; Onjia, A. Calcium-pyro-hydrochar derived from the spent mushroom substrate as a functional sorbent of Pb²⁺ and Cd²⁺ from aqueous solutions. Waste Manag. Res. 2022, 40, 1629–1636. [Google Scholar] [CrossRef]
- Petrović, J.; Stojanović, M.; Milojković, J.; Petrović, M.; Šoštarić, T.; Laušević, M.; Mihajlović, M. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb²⁺ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Kaur, G.; George, N.; Walia, H.K.; Sillu, D.; Rath, S.K.; Saxena, S.; Rios-Solis, L.; Dwibedi, V. Waste to wealth: Microbial-based valorization of grape pomace for nutraceutical, cosmetic, and therapeutic applications to promote circular economy. Process Saf. Environ. Prot. 2024, 188, 1464–1478. [Google Scholar] [CrossRef]
- Eisenbies, M.H.; Volk, T.A.; Therasme, O.; Hallen, K. Three bulk density measurement methods provide different results for commercial scale harvests of willow biomass chips. Biomass Bioenergy 2019, 124, 64–73. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Pavkov, I.; Radojčin, M.; Stamenković, Z.; Bikić, S.; Tomić, M.; Bukurov, M.; Despotović, B. Hydrothermal Carbonization of Agricultural Biomass: Characterization of Hydrochar for Energy Production. Solid. Fuel Chem. 2022, 56, 225–235. [Google Scholar] [CrossRef]
- Hu, X.; Dai, L.; Ma, Q.; Xu, J.; Ma, J.; Liu, X. One-pot synthesis of iron oxides decorated bamboo hydrochar for lead and copper flash removal. Ind. Crop Prod. 2022, 187, 115396. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Obradović, B. Guidelines for general adsorption kinetics modeling. Hem. Ind. 2020, 74, 65–70. [Google Scholar] [CrossRef]
- Ercegović, M.; Petrović, J.; Koprivica, M.; Simić, M.; Grubišić, M.; Vuković, N.; Krstić, J. Efficient adsorption of pollutants from aqueous solutions by hydrochar-based hierarchical porous carbons. Water 2024, 16, 2177. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/biochar composites for methylene blue removal by adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Qu, J.; Du, Z.; Lei, Y.; Li, M.; Peng, W.; Wang, M.; Liu, J.; Hu, Q.; Wang, L.; Wang, Y.; et al. Microwave-assisted one-pot preparation of magnetic cactus-derived hydrochar for efficient removal of lead(II) and phenol from water: Performance and mechanism exploration. Bioresour. Technol. 2023, 388, 129789. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Chen, H.; Chen, J.; Mosa, A.; Gao, B. Hydrochar loaded with nitrogen-containing functional groups for versatile removal of cationic and anionic dyes and aqueous heavy metals. Water 2024, 16, 3387. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb²⁺ and Cd²⁺ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I.; Hassan, E.B. Novel oxone-treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liao, Q.; Xia, S.; Shen, X.; Zhu, J.; Liao, Y.; Wang, W.; Fang, Z.; Liu, D. Adsorption of heavy metal Pb(II) in dredged sediment using different biochar materials. Processes 2025, 13, 957. [Google Scholar] [CrossRef]
- Deng, W.; Kuang, X.; Xu, Z.; Li, D.; Li, Y.; Zhang, Y. Adsorption of Cadmium and Lead Capacity and Environmental Stability of Magnesium-Modified High-Sulfur Hydrochar: Greenly Utilizing Chicken Feather. Toxics 2024, 12, 356. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Liu, Y.; Duan, R.; Tang, Q.; Tian, L.; Xu, R.; Wang, H.; Xiang, T. Lead and antimony removal from wastewater via tea residue derived hydrochar: Immobilization capacity and industrial practicality. Ind. Crops Prod. 2025, 231, 121158. [Google Scholar] [CrossRef]
- Yang, W.; Lu, C.; Liang, B.; Yin, C.; Lei, G.; Wang, B.; Zhou, X.; Zhen, J.; Quan, S.; Jing, Y. Removal of Pb(II) from Aqueous Solution and Adsorption Kinetics of Corn Stalk Biochar. Separations 2023, 10, 438. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb(II) onto modified hydrochar: Modeling and mechanism analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef]
- Qin, X.; Meng, W.; Cheng, S.; Xing, B.; Shi, C.; Nie, Y.; Wang, Q.; Xia, H. Efficient removal of heavy metal and antibiotics from wastewater by phosphate-modified hydrochar. Chemosphere 2023, 345, 140484. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Z.; Lin, J.; Li, X.; Qui, J.; Liu, J.; Mao, X. Hydrothermal carbonization of sewage sludge and in-situ preparation of hydrochar/MgAl-layered double hydroxides composites for adsorption of Pb(II). J. Clean. Prod. 2020, 258, 120991. [Google Scholar] [CrossRef]






| Adsorbent FeMg-PHC | |
|---|---|
| qeq,exp (mg/g) | 139.6 ± 1.25 |
| Pseudo-First-Order Linear Model | |
| qeq,cal (mg/g) | 136.61 ± 1.08 |
| k1 (1/min) | 4.93 ± 0.37 |
| χ2 | 7.01 |
| R2 | 0.9235 |
| Pseudo-Second-Order Linear Model | |
| qeq,cal (mg/g) | 142.05 ± 2.17 |
| k2 (g/mg∙min) | 0.00087 ± 0.00003 |
| χ2 | 5.27 |
| R2 | 0.9998 |
| Pseudo-First-Order Non-Linear Model | |
| qeq,cal (mg/g) | 132.44 ± 0.75 |
| k1 (1/min) | 0.10 ± 0.03 |
| χ2 | 10.87 |
| R2 | 0.6742 |
| Pseudo-Second-Order Non-Linear Model | |
| qeq,cal (mg/g) | 138.73 ± 1.13 |
| k2 (g/mg∙min) | 0.0013 ± 0.0001 |
| χ2 | 2.26 |
| R2 | 0.9323 |
| Weber–Morris Diffusion Model | |
| Kid1 (mg/g∙min1/2) | 4.27 ± 0.15 |
| C1 (mg/g) | 85.02 ± 1.33 |
| R2 | 0.9568 |
| Kid2 (mg/g∙min1/2) | 0.16 ± 0.01 |
| C2 (mg/g) | 136.02 ± 2.21 |
| R2 | 0.7987 |
| Models | Parameters | Value |
|---|---|---|
| Langmuir | qm (mg g−1) | 145.55 ± 301 |
| KL (L/mg) | 0.16 ± 0.03 | |
| R2 | 0.9665 | |
| Freundlich | KF (mg/g)(L/mg)1/n | 77.77 ± 0.98 |
| 1/n | 10 | |
| R2 | 0.9393 | |
| Sips | qm (mg/g) | 157.24 ± 2.45 |
| KS (L/mg) | 0.37 ± 0.02 | |
| ns | 0.59 | |
| R2 | 0.9974 |
| Used Material | Preparation Method | pH | q (mg/g) | Ref. |
|---|---|---|---|---|
| Cactus hydrochar | Microwave-magnetic HTC | 5.0 | 139.34 | [29] |
| Corn straw biochar/rGO composite | HTC | 5.5 | 34.02 | [30] |
| Prosopis africana shell | Pyrolysis + HTC | 5.0 | 45.3 | [31] |
| Paulownia leaves | HTC | 5.0 | 49.62 | [7] |
| Pine wood | Oxone-modified hydrochar | 5.0 | 46.7 | [32] |
| Wheat straw | HTC | 5.0 | 64.97 | [33] |
| Spent mushroom substrate | HTC + Ca pyrolysis | 5.0 | 297 | [16] |
| Chicken feathers | Mg-modified hydrochar | 5.0 | 70.41 | [34] |
| Tea residues | Magnetic hydrochar | 5.6 | 328.2 | [35] |
| Corn stalk | Pyrolysis | 5.0 | 20.8 | [36] |
| Sawdust | H2O2 modified hydrochar | 5.0 | 92.8 | [37] |
| Poplar sawdust | Phosphate-modified hydrochar | 5.0 | 119.61 | [38] |
| Sewage sludge | MgAl-layered | 5.0 | 62.41 | [39] |
| Grape pomace | HTC | 5.0 | 27.8 | [17] |
| FeMg-PHC | HTC + Fe/Mg pyrolysis | 5.0 | 157.24 | This study |
| Sample | Pb (mg/L) | Cu (mg/L) | Zn (mg/L) | Cd (mg/L) | Ni (mg/L) |
|---|---|---|---|---|---|
| AFW | 6.05 | 9.23 | 3.98 | 0.98 | 4.99 |
| FeMg-PHC | 2.98 | 6.53 | 2.12 | 0.52 | 3.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, J.; Koprivica, M.; Ercegović, M.; Simić, M.; Dimitrijević, J.; Bugarčić, M.; Trifunović, S. Synthesis and Application of FeMg-Modified Hydrochar for Efficient Removal of Lead Ions from Aqueous Solution. Processes 2025, 13, 2060. https://doi.org/10.3390/pr13072060
Petrović J, Koprivica M, Ercegović M, Simić M, Dimitrijević J, Bugarčić M, Trifunović S. Synthesis and Application of FeMg-Modified Hydrochar for Efficient Removal of Lead Ions from Aqueous Solution. Processes. 2025; 13(7):2060. https://doi.org/10.3390/pr13072060
Chicago/Turabian StylePetrović, Jelena, Marija Koprivica, Marija Ercegović, Marija Simić, Jelena Dimitrijević, Mladen Bugarčić, and Snežana Trifunović. 2025. "Synthesis and Application of FeMg-Modified Hydrochar for Efficient Removal of Lead Ions from Aqueous Solution" Processes 13, no. 7: 2060. https://doi.org/10.3390/pr13072060
APA StylePetrović, J., Koprivica, M., Ercegović, M., Simić, M., Dimitrijević, J., Bugarčić, M., & Trifunović, S. (2025). Synthesis and Application of FeMg-Modified Hydrochar for Efficient Removal of Lead Ions from Aqueous Solution. Processes, 13(7), 2060. https://doi.org/10.3390/pr13072060









