Abstract
Aiming at the high-value application of rare earth elements lanthanum (La), an Al-50% La alloy was selected and prepared in a vacuum medium-frequency induction furnace. The geometric characteristics of the Al-50% La alloy powders were compared and studied, with the powders prepared by two different methods: mechanical pulverization and gas atomization. The results showed that an Al-49.09% La master alloy was obtained, and the only intermediate phase containing La in the experimental alloy was Al11La3. From the perspectives of chemical and phase composition, La has a high yield. Additionally, an Al-La alloy with controllable rare earth intermediate phases can be obtained. The Al-La alloy powders prepared by the mechanical pulverization method are irregular in shape, but the particle size is relatively small, ranging from 0.25 to 66.9 μm. Submicron powders were obtained, with 4.38% of the powders having an equivalent particle size of less than 1 μm. Considering the characteristic of the selective laser melting (SLM) process forming micro-melt pools, a small amount of submicron Al-La alloy powders prepared by the mechanical pulverization method can be used as a trace additive for SLM preparation of CP-Ti. The powders prepared by gas atomization have good sphericity, with a particle size range of 1.65 to 76.0 μm. Among them, the powders with a size of 2–10 μm account for 75.52%, and this part of the powders can be used for the powder metallurgy preparation of composite materials.
1. Introduction
Titanium and its alloys are known for their light weight, superior strength, resistance to corrosion, and excellent biocompatibility. These properties have led to their extensive applications across various sectors, including aerospace, defense, biomedical, automotive, marine, and petrochemical industries [1,2]. However, the full potential of titanium and titanium alloys in industrial settings is somewhat hindered by the difficulties encountered during machining and hot working operations [3,4]. Additive manufacturing (AM) technology offers a promising solution to circumvent these processing obstacles [5,6], thereby potentially broadening the utilization of titanium and titanium alloys. Among various AM techniques, selective laser melting (SLM) stands out as one of the most prevalent [7]. When contrasted with conventional casting methods, commercial pure titanium (CP-Ti) produced via SLM exhibits heightened strength, albeit with a corresponding decrease in ductility [8,9]. Consequently, achieving a balance between enhanced ductility and maintained strength represents a pivotal challenge in optimizing the performance of CP-Ti [10].
In addition, SLM is characterized by a small melt pool, high temperature gradient, rapid cooling rate, and multiple thermal cycles of heating and cooling [11]. Such process features can result in various issues during the formation of components, including low density, the frequent occurrence of cracks and defects, the presence of both coarse columnar grains and non-uniformly distributed phase compositions, and challenges in achieving uniformity in both microstructure and performance [12,13]. This situation is particularly evident in titanium and its alloys. Hence, removing coarse columnar grains and attaining a uniformly distributed phase composition are crucial for obtaining consistently excellent mechanical properties in pure titanium components formed via SLM [14,15].
To achieve microstructural control and performance enhancement in metal powder bed fusion additive manufacturing, compositional modification, in conjunction with the adjustment of SLM process parameters, serves as a primary approach [16]. Research dating back to the 1950s has identified rare earth (RE) as the most promising alloying elements for titanium alloys. It was found that they can refine the as-cast microstructure of Ti and titanium alloys [17], reduce the proportion of columnar grains in the weld zone of Ti6Al4V [18], remove oxygen from the CP-Ti matrix [14], and enhance the corrosion resistance of Ti alloy [19] and thermal stability of CP-Ti [20]. RE has very active chemical properties. In traditional melting–casting processes, the addition of RE is difficult and the amount that can be added is limited, which leads to inconsistent research results on the effectiveness of RE among different researchers. SLM is similar to powder metallurgy in that both use metal powders as raw materials. Therefore, RE master alloys and RE compounds can be effectively incorporated by mixing them into the raw powder in a powdered form. In recent years, the application of RE in the additive manufacturing of titanium and titanium alloys has garnered attention [21,22,23]. Due to the relatively mature preparation methods for RE oxides, they are often added in the form of oxides. Wang et al. [14] found that the addition of nanoscale La2O3 in CP-Ti produced by SLM. Liu et al. [23] reported that adding nanoscale Y2O3 to Ti4Al4V alloy fabricated by laser powder bed fusion (LPBF). Moreover, the thermal accumulation resulting from multiple heating cycles during SLM may cause the decomposition of rare earth oxides, introducing oxygen. Oxygen acts as a double-edged sword [24,25]; it boosts strength but reduces the plasticity of titanium and titanium alloys. Therefore, to prevent the introduction of excessive oxygen, finding suitable RE master alloys and preparing them into powders suitable for LPBF processes is of urgent necessity. This study holds significant importance for the high-quality application of rare earth elements.
During the SLM process, the melt pool is micro-sized [4,5,21]. To ensure the even incorporation of trace rare earths throughout the shaping process, it is desirable for the rare earth alloy powder to more evenly adhere to the surface of CP-Ti powder particles post-mixing. Ideally, the rare earth master alloy and compound powders should be nano- or submicron-sized. While there is extensive research on nano-sized rare earth oxides, studies on the preparation and application of nano-sized rare earth master alloy powders are relatively scarce. Baotou in Inner Mongolia, China, boasts abundant RE resources, with La being particularly plentiful. The imbalance in rare earth applications remains unresolved. Developing high-value uses for the abundant La is a key objective for effectively leveraging the region’s unique resource advantages. In the SLM process, the choice of master alloys is pivotal for the effective integration of RE elements. The trace addition of Al will not alter the compositional system of CP-Ti. At the same time, it is crucial to balance the control of the RE alloy’s melting preparation technique to maximize the rare earth yield.
To expand the application of rare earth elements in materials, this paper designs and prepares a rare earth master alloy powder suitable for pure titanium, providing an important foundation for the further development of subsequent research.
2. Experimental Materials and Methods
2.1. Selecting and Melting of RE Master Alloy
The element of Al is a typical α-stabilizing element in CP-Ti. Trace additions of Al do not form the Ti-Al second phase particles, nor do they affect the composition system of industrial pure titanium. Additionally, considering the smelting preparation technology of RE alloys and the goal of maximizing the RE yield, combined with the research accumulation of the project team on the application of rare earths in metal materials [26,27], the Al-La master alloy was chosen as the rare earth additive. To ensure the introduction of as few additional elements as possible and to guarantee the efficient incorporation of rare earth elements, the content of rare earth elements should be maximized. Thus, the Al-50% La master alloy was chosen to be prepared as powders.
An Al-50% La (wt%) alloy was designed. The industrial high-purity (99.99 wt%) Al (Baotou Research Institute of Rare Earths, Baotou, China) and high-purity (99.99 wt%) La (Baotou Research Institute of Rare Earths, Baotou, China) were used as the raw materials to make the Al-La alloy, which supplied by Baotou Research Institute of Rare Earths (Baotou, China). By polishing and removing the surface oxide layer, the raw materials were cleaned with alcohol, dried and then sealed for storage. Then, 3 kg high-purity Al was placed into an alumina crucible with an inner diameter of 180 mm and a height of 320 mm and then melted in a DDVIF-25-60-5 vacuum medium-frequency induction furnace. The furnace was first pre-vacuumed to less than 2 Pa and then filled with argon to 104 Pa. This process of vacuuming and argon filling was repeated twice more, followed by another vacuuming. In accordance with the Al-La binary phase diagram [28], the liquidus of the Al-50% La alloy is approximately 1200 °C. Thus, when the vacuum reached 10−2 Pa, the high-purity Al was heated to 1230 °C with argon filling through the entire process until the high-purity Al was completely melted, followed by adding the 3 kg high-purity La into the Al liquid in five batches through the secondary charging port of the vacuum induction furnace with a 30 s interval between each batch at 900 °C. Once the La was completely melted, the alloy was cast in the vacuum chamber and then cooled naturally to room temperature. The mold was a cylindrical steel mold with a cross-sectional diameter of 60 mm, which had been preheated to 200 °C prior to casting.
2.2. Powder Preparation of RE Master Alloy
In the light of the microscale characteristics of the melting pool in SLM, in order to ensure the uniform addition of trace rare earths, the rare earth master alloy powders should be evenly attached to the surface of CP-Ti powder particles after mixing. Therefore, it was perfect for the rare earth master alloy powders to be added in the form of nano or submicron particles. Currently, the preparation technology for nanoscale rare earth oxides was relatively mature [23,29]; however, there were few reports on the application of nanoscale rare earth master alloy powders. In this study, the Al-La master alloy powders were prepared using both mechanical pulverization and atomization methods. The specific preparation principle of powder by mechanical pulverization is referred to in [30]; the specific preparation process of powder by gas atomization is referred to in [31]. Then, the adaptability of the powders produced by the two different methods to the SLM process was analyzed. For the method of mechanical pulverization, powder preparation was carried out using a DF-4 electromagnetic mill, and the produced powders were sieved through a 1000-mesh screen to obtain fine powders. The gas atomized powder was produced using the EIGA 50 electrode vacuum induction powder-making furnace, with a heating power of 20 kW and an atomization pressure ranging from 3.8 to 4.4 MPa. By characterizing the powder, a comparative analysis of its adaptability to the SLM process is conducted. The fabricated powders were sieved using an ultrasonic vibration screen to obtain fine powders. Samples were randomly taken from three different locations in the Al-La master alloy ingot, and the La content was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES).
2.3. Characterization
X-ray diffraction (XRD) tests and microstructure observations were conducted on samples at various heights along the ingot. The phase analysis of the Al-La master alloy ingots and powders were conducted using a SmartLab multifunctional X-ray diffractometer. For the XRD analysis, a Cu target was selected with a scanning range of 20 to 90 degrees and a step size of 0.02 degrees. The microstructure of the Al-La master alloy ingot and the morphology of the Al-La master alloy powders were observed using a Zeiss Supra55 field emission SEM. The particle size of the powders prepared by the two methods was statistically analyzed using the HELOS/RODOS dry laser particle size analyzer (PSA), which can test particle sizes ranging from 0.1 to 3500 μm, with a repeatability error of less than 0.04% (deviation of standard sample D50) and an accuracy error of less than 0.3% (deviation of standard sample D50). The experimental refractive index used in the test was 2.5, and the absorption rate was 3. In order to obtain the quantitative information such as powder morphology, equivalent diameter, and the ratio of the fine powders, the powders samples were analyzed using the Zeiss Versa610 three-dimensional high-resolution X-ray microscope (3D XRM) equipped with the Dragonfly 3.6 software (Comet Technologies Canada, Montreal, QC, Canada). The device has a pixel resolution of 0.68 μm, an exposure time of 2 s, and operates at a test voltage of 140 KV and a power of 21 W. Using the Dragonfly software, 3D XRM scan results are subjected to median filtering for noise reduction, grayscale threshold segmentation, and routine image processing. The binary images are then utilized to obtain quantitative information such as powder morphology and equivalent diameter.
3. Results and Discussion
3.1. Chemical Composition and Phase Analysis of Al-La Master Alloy Ingots
The ICP-OES tests showed that the La content of the Al-La master alloy from three random different positions of the ingot fluctuated within a range of ±0.05%. Thus, the average value of the La content, 49.09 wt%, was taken as the La content of the experimental alloy. The results indicated that the composition control during the alloy melting was precise, with the La content being very close to the designed value of 50 wt%; meanwhile, the alloy ingot composition was relatively uniform.
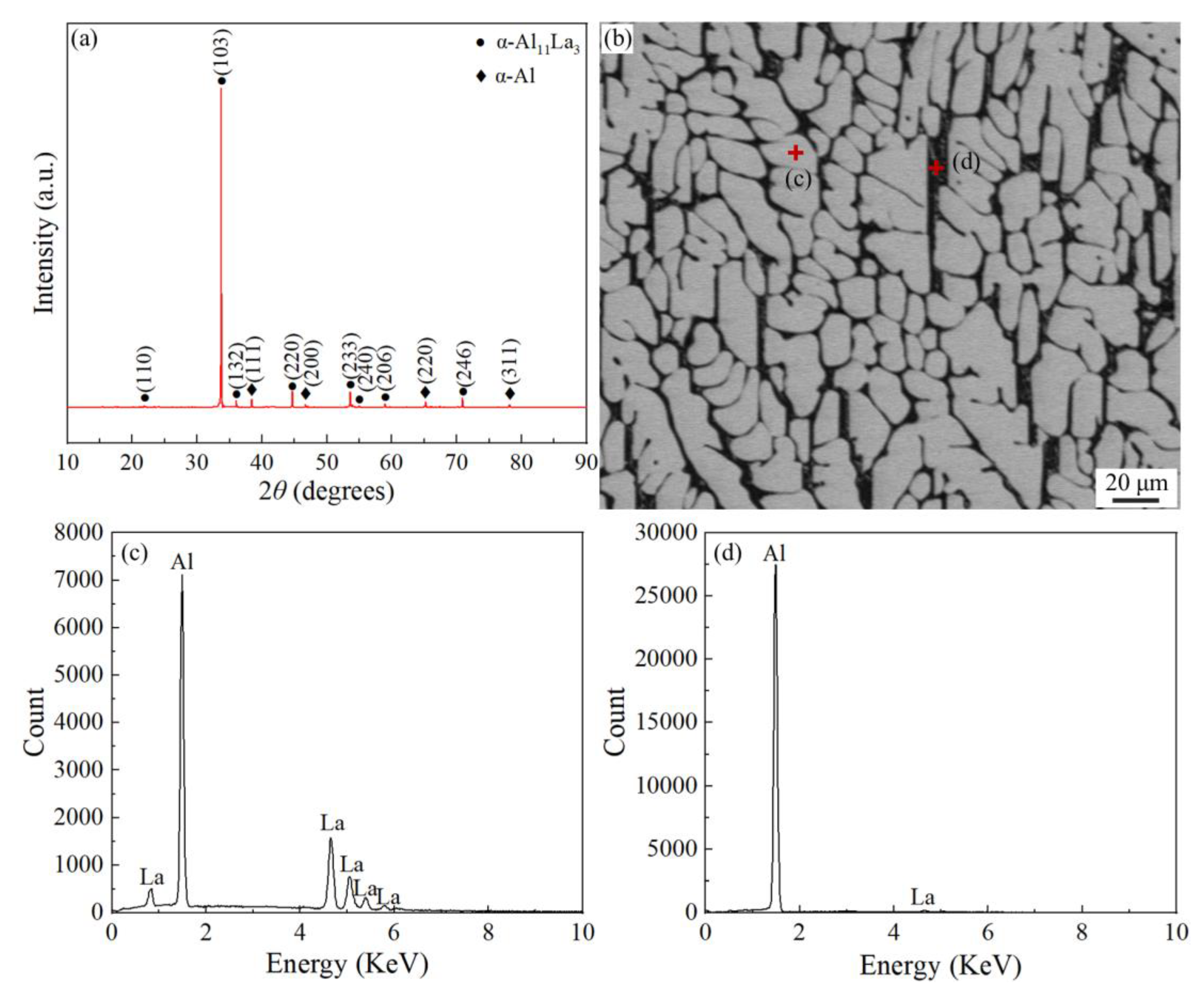
Figure 1a showed the XRD phase analysis results of the Al-La alloy ingot, which revealed that there were two phases of α-Al11La3 and α-Al in the Al-La alloy. According to the Al-La binary phase diagram [28], during the equilibrium solidification process of the experimental alloy, the Al11La3 phase, with a melting point of about 1240 °C, first crystallizes, followed by the formation of the eutectic products, (α-Al + Al11La3), at 640 °C. Additionally, during the cooling, the Al11La3 phase undergoes a crystallographic transformation from β-Al11La3 (bct) to α-Al11La3 (orth) at 915 °C, so the α-Al11La3 (orth) phase exists at room temperature. The results indicated that only the La-containing intermetallic compound phase formed in the prepared Al-La master alloy was the Al11La3 phase. Figure 1b displayed the typical SEM microstructure of the Al-La alloy ingot. The bright areas in the image were the Al11La3 phase, and the small amounts of the interspersed black and white parts were the eutectic structure of (α-Al + Al11La3).
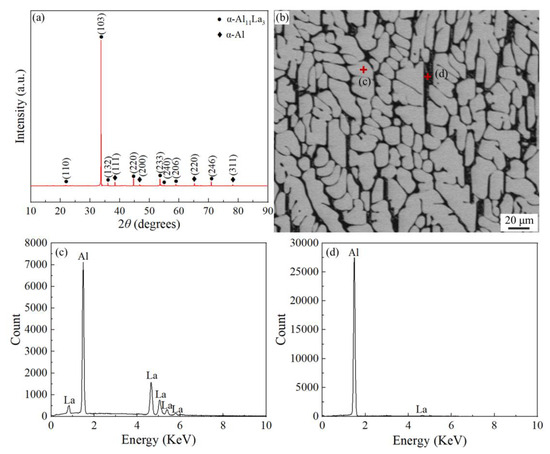
Figure 1.
Phase analysis and microstructure of as-cast Al-La alloy: (a) XRD pattern; (b) SEM morphology; and (c,d) EDS spectrum of area marked in (b).
The highest temperature of the melt pool in the SLM process can be roughly used to determine whether the SLM process parameters are reasonable. In theory, a melt pool can only form when the highest temperature of the melt pool reaches the melting point of the powder material. However, when the melt pool temperature exceeds the boiling point of the powder material, it will induce intense convection within the molten pool. The defects such as pores are prone to forming. Therefore, the appropriately highest temperature of the melt pool should be controlled between the melting point and the boiling point of the powder material. The melting point of CP-Ti is 1668 °C [32]. During the SLM process of CP-Ti with addition of the Al-La alloy, the Al11La3 phase in the Al-La alloy dissolves and reacts with the CP-Ti powders, releasing La and Al atoms. Al atoms can be solid-solved in the Ti matrix. Al is a stabilizer for α-Ti. Trace amounts of dissolved Al will not change the properties of pure titanium. La atoms will combine with oxygen in the matrix to form oxides. Additionally, the La2O3 particles could serve as nucleation sites for prior-β grains during solidification, thereby promoting the formation of equiaxed grains [14,16,22]. The results demonstrate that the Al-La master alloy, obtained from the experiment, is suitable for use as an additive, considering the aspects of chemical composition, phase constitution, and the effective incorporation of rare earth elements.
3.2. Comparison of Al-La Alloy Powders Prepared by Two Different Methods
3.2.1. Phase Composition
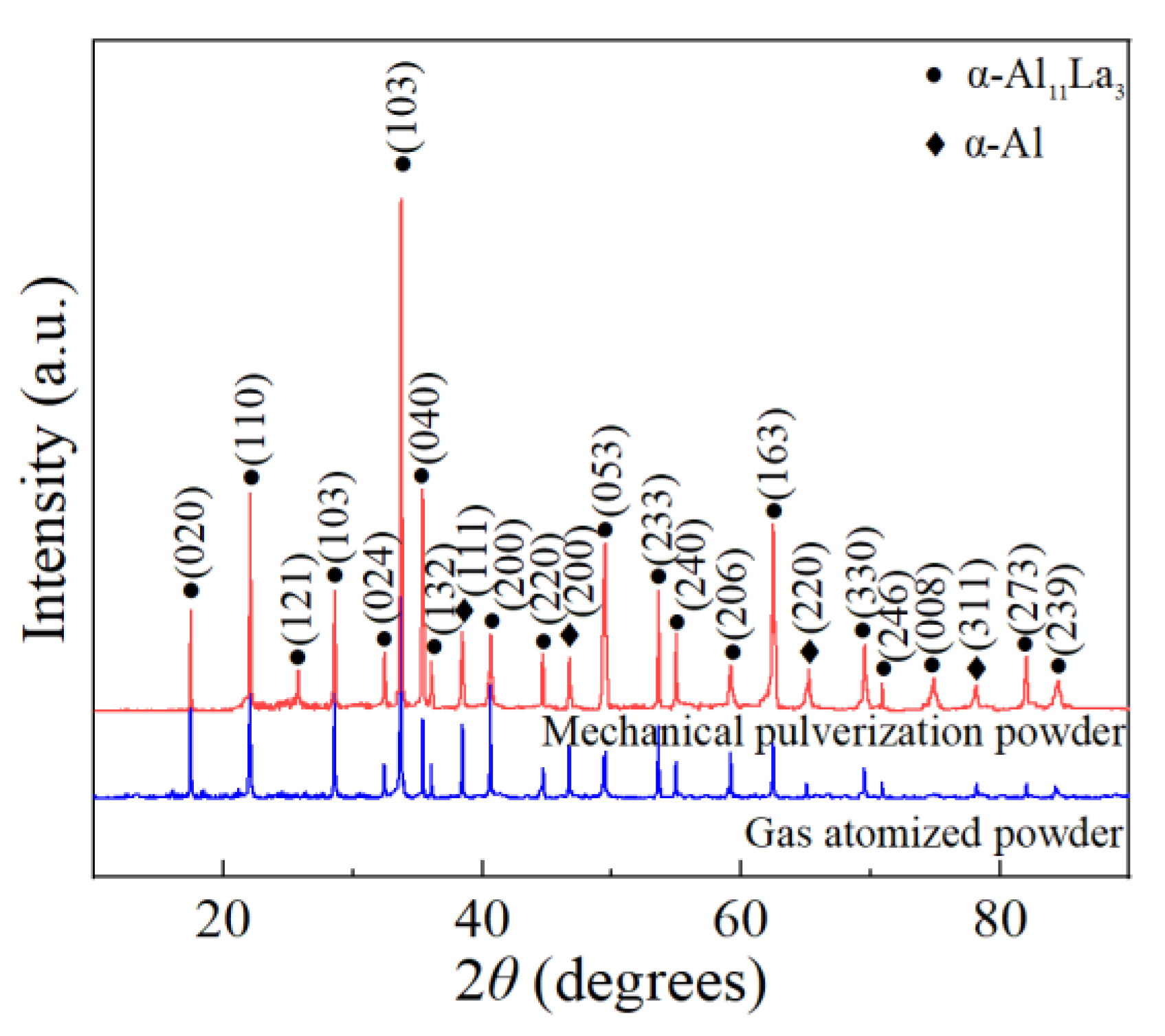
Figure 2 showed the XRD analysis results of the Al-La alloy powders prepared by mechanical pulverization and gas atomization methods. It was observed that the phase compositions of the powders prepared by the two different methods were both α-Al11La3 and α-Al phases, which was consistent with those of the Al-La master alloy ingot. It was indicated that neither of the two different powder-making methods had changed the phase composition of the alloy powders.
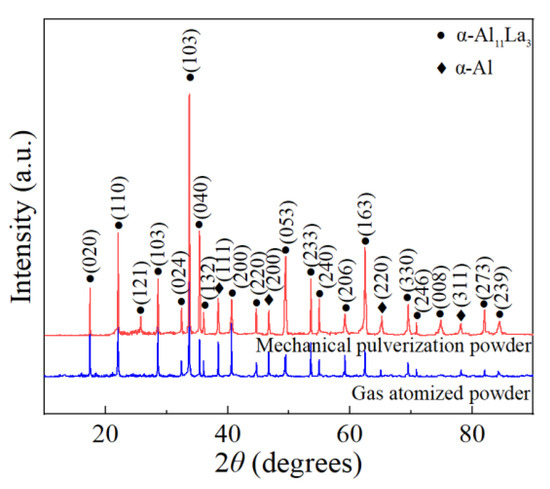
Figure 2.
XRD patterns of Al-La alloy powders produced by two different methods.
3.2.2. Morphology and Particle Size
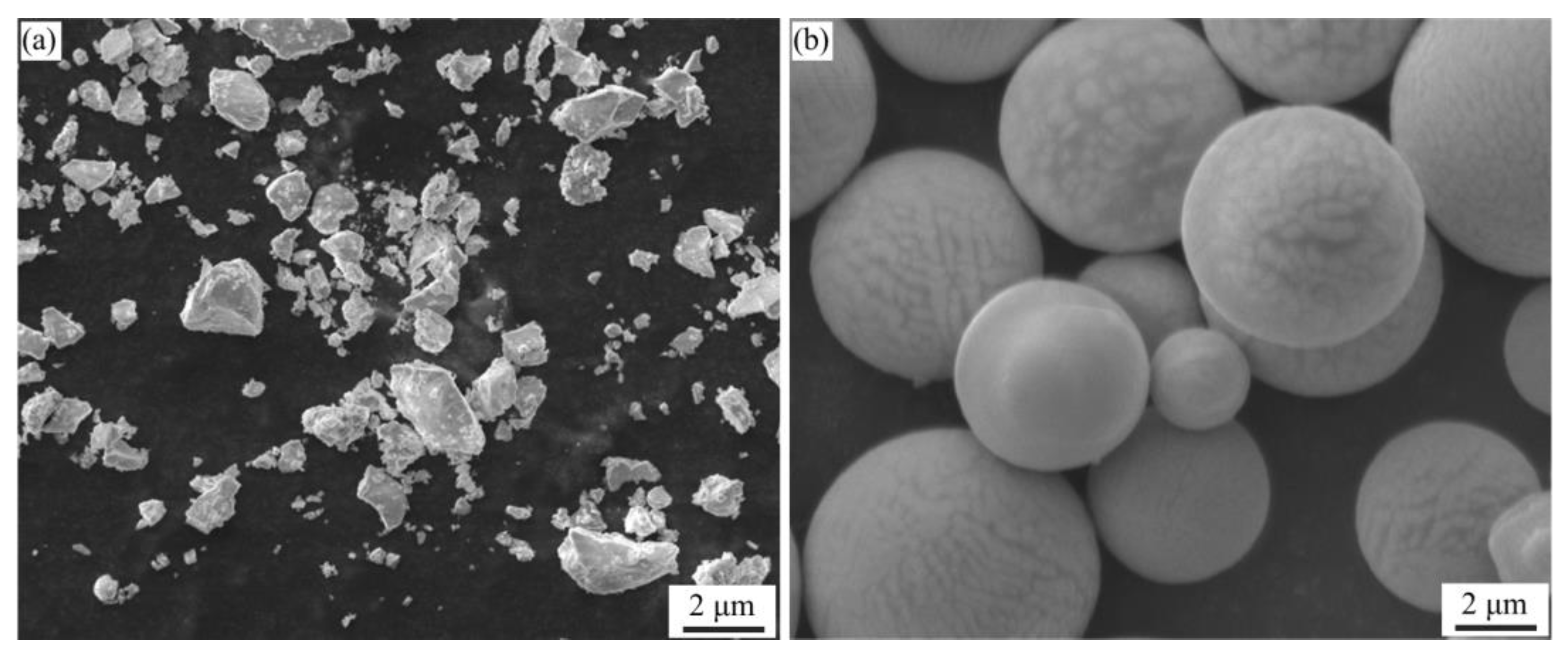
Figure 3 presented the SEM morphology of the Al-La alloy powders prepared by mechanical milling and gas atomization methods. It was observed that the powders prepared by mechanical pulverization had irregular shapes with significant variation in particle size. The powders prepared by gas atomization exhibited good sphericity, smooth surfaces, and relatively uniform size distribution. However, the overall particle size was relatively larger than that of the powders prepared by mechanical pulverization.
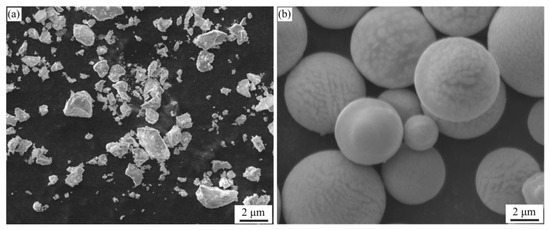
Figure 3.
SEM morphologies of Al-La alloy powders prepared by two different methods: (a) by mechanical pulverization and (b) by gas atomization.
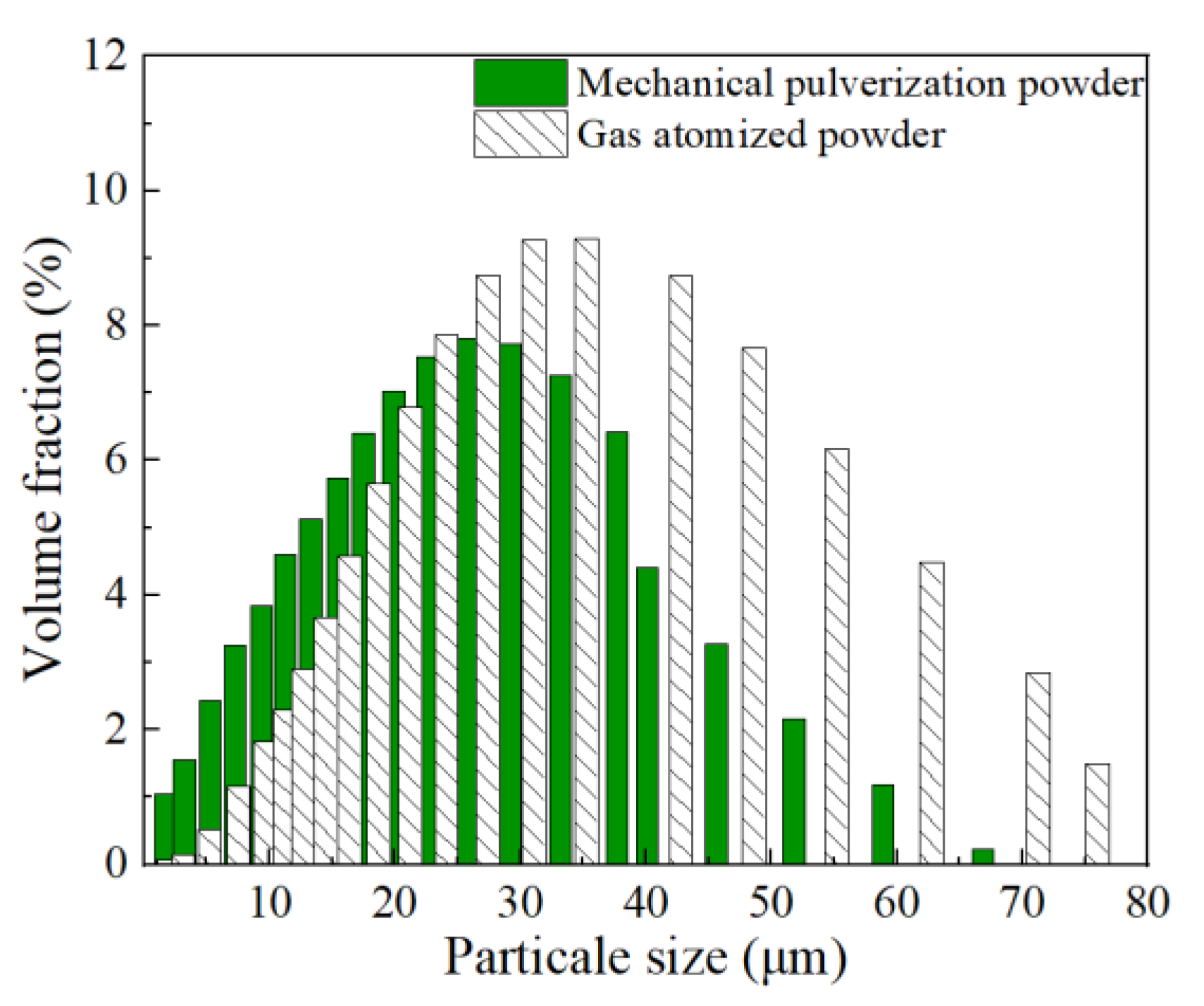
Figure 4 showed the particle size distribution results of the Al-La alloy powders prepared by mechanical pulverization and gas atomization methods. It indicated that the particle size range of the powders prepared by mechanical pulverization was 0.25~66.9 μm, with Dv (10, 50, 90) being 3.17 μm, 17.8 μm, and 41.6 μm, respectively, while those prepared by gas atomization was 1.65~76.0 μm, with Dv (10, 50, 90) being 12.9 μm, 31.1 μm, and 57.6 μm, respectively. By comparison, it can be found that the Al-La alloy powders prepared by the mechanical pulverization method, despite their more irregular shapes, had a smaller average particle size than those prepared by the gas atomization method.
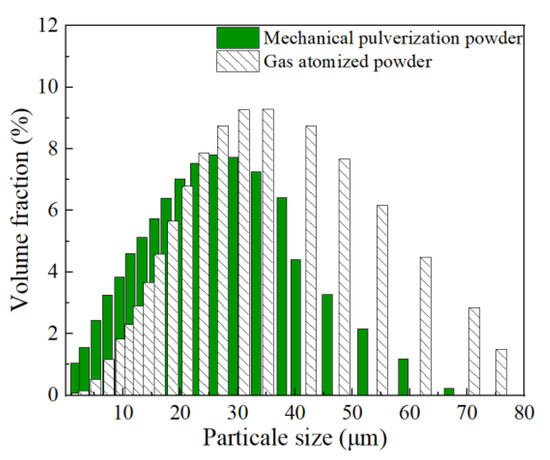
Figure 4.
Particle size distribution of Al-La alloy powders prepared by two different methods.
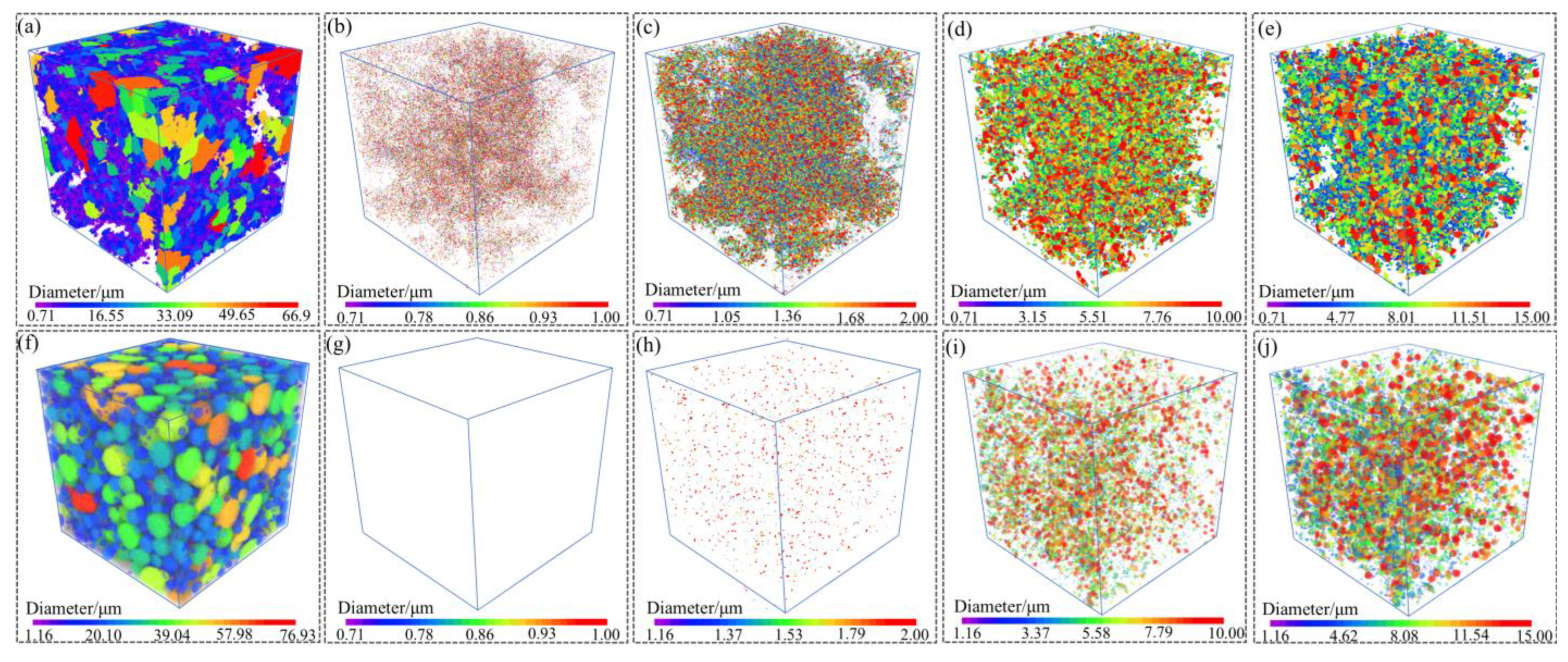
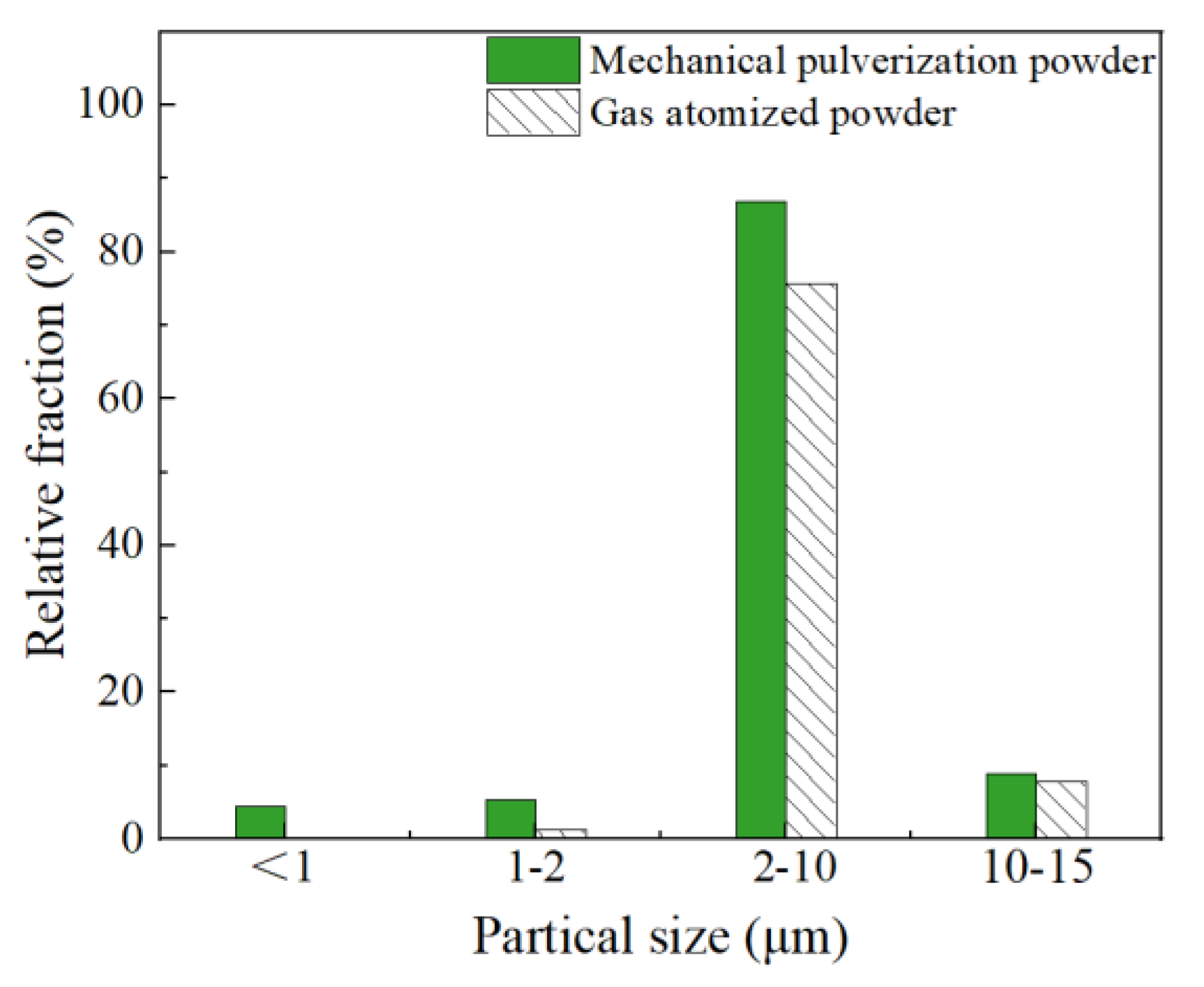
The 3D XRM characterization of powders can match the morphology with the size through the method of three-dimensional reconstruction. Figure 5 displayed the 3D XRM typical images of the Al-La alloy powders prepared by mechanical pulverization and gas atomization methods. The interactive display function of the Dragonfly software, equipped with 3D XRM, was used to investigate the proportion of powders with different particle sizes. Statistical analyses were conducted on powders with particle sizes of less than 1 µm, 1–2 µm, 2–10 µm, and 10–15 µm, respectively. The results are shown in Figure 6. The results indicate that the Al-La alloy powders prepared by gas atomization exhibit good sphericity, with no powders observed to be less than 1 μm in size. Powders with particle sizes between 1 and 2 μm account for 1.30%, those between 2 and 10 μm account for 75.62%, and those between 10 and 15 μm account for 1.3%. In contrast, the Al-La alloy powders prepared by mechanical pulverization have irregular morphologies. Statistical analysis reveals that the proportion of powders with equivalent diameters less than 1 μm is 4.38%, those with equivalent diameters between 1 and 2 μm is 9.70%, those between 2 and 10 μm is 86.93%, and those between 10 and 15 μm is 8.89%.
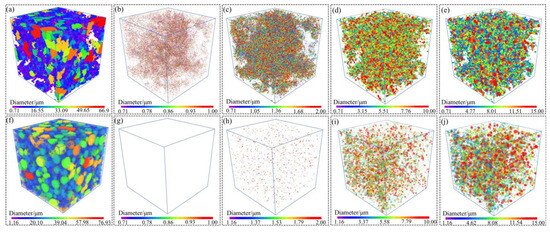
Figure 5.
Three-dimensional XRM reconstruction typical images of the Al-La powders prepared by two different methods. Powders prepared by mechanical pulverization: (a) 3D surface view and particle size of (b) less than 1 μm, (c) less than 2 μm, (d) less than 10 μm, and (e) less than 15 μm; powders prepared by gas atomization: (f) 3D surface view and particle size of (g) less than 1 μm, (h) less than 2 μm, (i) less than 10 μm, and (j) less than 15 μm.
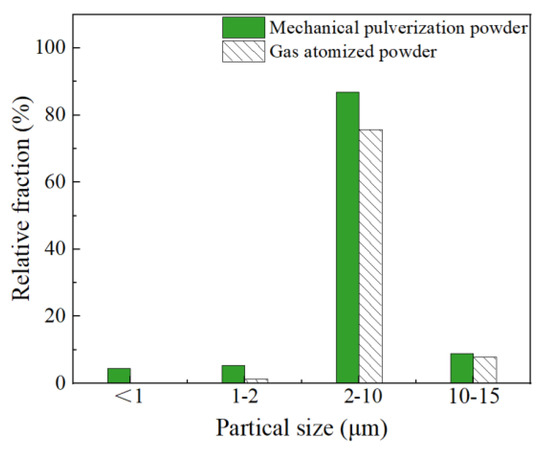
Figure 6.
Particle size distribution statistics of Al-La alloy powders prepared by different methods.
It is well known that particle sizes and shapes of additives powders can significantly affect the properties of the target material. The quality of components and their properties are very much dependent on the mixing of powders and uniformity of dispersion of additives when additives are fabricated through SLM or powder metallurgy [33]. Mechanically pulverization powders have irregular shapes, which can lead to reduced flowability. However, mechanically pulverization powders can achieve submicron particle sizes, which are crucial for use as additives. Powders that are too large increase the inhomogeneity of the mixed powders and tend to distribute unevenly in the melt pool, thereby affecting the stability of the melt pool and the quality of the final component. Compared with gas atomization methods, mechanical pulverization required simpler preparation conditions, and it is easier to obtain submicron powders. Therefore, for laboratory research, small quantities of submicron powders can be used for trace additions in LPBF fabricated of CP-Ti.
During the gas atomization process, metal droplets are fragmented into fine droplets by the impact of high-speed gas flow [34]. These droplets then cool and solidify rapidly under the influence of surface tension, which causes them to contract into the energetically favorable spherical shape. As a result, powders produced by gas atomization exhibit high sphericity and smooth surfaces, thereby providing excellent flowability. This characteristic is important for applications such as additive manufacturing and powder metallurgy. However, due to the limitations of gas atomization technology, the production of submicron powders remains challenging [30]. As additive powders, those prepared by gas atomization cannot achieve submicron- or even nano-level particle sizes, making them unsuitable for the SLM technology. Nevertheless, powders in the 2–10 µm range produced by gas atomization can be used as additives in powder metallurgy composites, thereby expanding the application scope of rare earth elements.
4. Conclusions
This study designed and prepared an Al-50% La master alloy, which was then prepared into powders using both mechanical pulverization and gas atomization methods. Additionally, the geometric characteristics of the two types of powders were investigated. This study demonstrates the potential value of the efficient utilization of RE and provides an experimental foundation for their industrial applications. The main conclusions are summarized as follows:
- The Al-La alloy was prepared in a vacuum medium-frequency induction furnace using the raw materials of high-purity Al and high-purity La. A master alloy with La content of 49.09% was obtained. The only La-containing intermetallic compound phase in the experimental alloy was the Al11La3 phase and the other phase was α-Al, which was consistent with thermodynamic predictions. It demonstrates that the composition of rare earth master alloys prepared by melting is controllable, with a yield of La reaching as high as 98.2%.
- This study compared Al-La alloy powders produced by two different methods. Compared to the gas atomization method, the mechanical pulverization method produces powders with irregular shapes and smaller equivalent particle sizes. The particle size range for these powders is 0.25 to 66.9 μm, with Dv (10, 50, 90) values of 3.17 μm, 17.8 μm, and 41.6 μm, respectively. Statistical research using 3D XRM revealed that gas atomization cannot produce powders with an equivalent particle diameter smaller than 1 μm, whereas mechanical pulverization can achieve 4.38% of powders with an equivalent particle diameter less than 1 μm. A small quantity of powders produced by mechanical pulverization powder can be used as a trace additive in CP-Ti fabricated by SLM.
- The powders produced by the gas atomization method have a high degree of sphericity, with a particle size range of 1.65 to 76.0 μm. The Dx (10, 50, 90) values are 12.9 μm, 31.1 μm, and 57.6 μm, respectively. Statistical studies conducted using 3D XRM show that the particle sizes of the powders produced by gas atomization are mainly concentrated in the range of 2–10 μm. These powders can be used for the powder metallurgy preparation of composite materials.
Author Contributions
Conceptualization, H.B. and Y.J.; methodology, Y.J. and Y.L.; investigation, H.B., H.W., X.K. and W.L.; resources, Y.J. and H.R.; data curation, H.B., Y.J. and X.K.; writing—original draft preparation, H.B.; writing—review and editing, H.B., Y.J., Y.L., H.W. and H.R.; visualization, H.B., Y.J., H.W. and W.L.; supervision, Y.J., X.K., H.R. and W.L.; project administration, Y.J., Y.L. and H.R.; funding acquisition, Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Program for Innovative Research Team in Universities of Inner Mongolia Autonomous Region (NMGIRT2401). This research was supported by Fundamental Research Funds for Inner Mongolia University of Science & Technology (2023RCTD001). This research was supported by Inner Mongolia Natural Science Foundation (2022MS05008). This research was supported by Fundamental Research Funds for Inner Mongolia University of Science & Technology (2022NS19).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Ding, W.; Tao, Q.; Chen, J.; Chen, G.; Qu, X.; Qin, M. Enhanced mechanical properties by laser powder bed fusion using cost-effective hydride-dehydride titanium powders. J. Mater. Process. Technol. 2023, 313, 117887. [Google Scholar] [CrossRef]
- Yeshanew, S.K.; Bai, C.; Jia, Q.; Xi, T.; Zhang, Z.; Li, D.; Xia, Z.; Yang, R.; Yang, K. Influence of Hot-Rolling Deformation on Microstructure, Crystalline Orientation, and Texture Evolution of the Ti6Al4V-5Cu Alloy. Acta Metall. Sin. 2023, 36, 1261–1280. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C. Design of titanium alloys by additive manufacturing: A critical review. Adv. Powder Mater. 2022, 1, 100014. [Google Scholar] [CrossRef]
- Baghi, A.D.; Nafisi, S.; Hashemi, R.; Ebendorff-Heidepriem, H.; Ghomashchi, R. Effective post processing of SLM fabricated Ti-6Al-4V alloy: Machining vs thermal treatment. J. Manuf. Process. 2021, 68, 1031–1046. [Google Scholar] [CrossRef]
- Tan, Q.; Fan, Z.; Tang, X.; Yin, Y.; Zhang, M.X. A novel strategy to additively manufacture 7075 aluminium alloy with selective laser melting. Mater. Sci. Eng. A 2021, 821, 141638. [Google Scholar] [CrossRef]
- Tao, Q.; Wang, Z.; Chen, G.; Cai, W.; Cao, P.; Zhang, C.; Ding, W.; Lu, X.; Luo, T.; Qu, X.; et al. Selective laser melting of CP-Ti to overcome the low cost and high performance trade-off. Addit. Manuf. 2020, 34, 101198. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rozniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Swieszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Li, G.X.; Chandra, S.; Rashid, R.A.R.; Palanisamy, S.; Ding, S.L. Machinability of additively manufactured titanium alloys: A comprehensive review. J. Manuf. Process. 2022, 75, 72–99. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Mohamad, B.; Tan, Q.; Yin, Y.; Fan, Z.; Liu, S.; Hattel, J.H.; Dargusch, M.; Zhang, M.X. Achieving high ductility in a selectively laser melted commercial pure-titanium via in-situ grain refinement. Scr. Mater. 2021, 191, 155–160. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, D.; Gibson, M.A.; Zheng, Y.; Fraser, H.L.; StJohn, D.H.; Eason, M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature 2019, 576, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Yin, Y.; Fan, Z.; Liu, Y.; Zhang, M.X. Uncovering the roles of LaB6-nanoparticle inoculant in the AlSi10Mg alloy fabri-cated via selective laser melting. Mater. Sci. Eng. A 2021, 800, 140365. [Google Scholar] [CrossRef]
- Zhang, J.; Bermingham, M.; Otte, J.; Liu, Y.; Hou, Z.; Yang, N.; Yin, Y.; Bayat, M.; Lin, W.; Huang, X.; et al. Ultrauniform, strong, and ductile 3D-printed titanium alloy through bifunctional alloy design. Science 2024, 383, 639–645. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, K.; Qiu, D.; Niu, W. Additive manufacturing of high-strength commercially pure titanium through lanthanum oxide addition. Mater. Charact. 2021, 176, 111074. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Narayana, P.L.; Hong, J.; Choi, S.; Kim, J.H.; Lee, S.W.; Park, C.H.; Yeom, J.; Mei, Q. Formation of equiaxed grains in selective laser melted pure titanium during annealing. J. Mater. Res. Technol. 2021, 11, 301–311. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Sha, G.; Jin, S.; Hou, Z.; Bayat, M.; Yang, N.; Tan, Q.; Yin, Y.; Liu, S.; et al. Designing against phase and property heterogeneities in additively manufactured titanium alloys. Nat. Commun. 2022, 13, 4660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wang, B.; Tang, H. Rare Earth Element: Is it a necessity for PM Ti alloys? Key Eng. Mater. 2012, 520, 41–48. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, C.; Zhang, F.; Qi, A.; Zhang, S.; Cheng, D. Effect of Yb2O3 on superplastic behavior of laser welded joint of TC4 titanium alloy. Int. J. Lightweight Mater. Manuf. 2023, 6, 278–284. [Google Scholar]
- Li, A.; Wang, Q.; Chen, R.; Ding, X.; Su, Y.; Fu, H. Application of alloying for enhancing the corrosion resistance of titanium alloys: A review. Mater. Today Commun. 2025, 42, 111111. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, H.; Lu, L.; Li, W.; Zhang, Z.; Lu, W.; Feng, Q.; Jia, B.; Song, K. Effect of rare earth yttrium and the deformation process on the thermal deformation behavior and microstructure of pure titanium for cathode rolls. J. Mater. Res. Technol. 2024, 33, 4192–4205. [Google Scholar] [CrossRef]
- Zhang, D.; Prasad, A.; Bermingham, D.; Todaro, C.J.; Benoit, M.J.; Patel, M.N.; Qiu, D.; StJohn, D.H.; Qian, M.; Easton, M.A. Refinement of Alloys in Fusion-Based Additive Manufacturing Processes. Metall. Mater. Trans. A 2020, 51, 4341–4359. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.; Guo, Y.; Zhu, G.; Fan, Y.; Wang, H.; Yan, W.; Zeng, X.; Wang, L. Simultaneously enhancing strength and ductility of LPBF Ti alloy via trace Y2O3 nanoparticle addition. J. Mater. Sci. Technol. 2024, 191, 146–156. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Y.; Bai, H.; Li, Y.; Kang, X.; Ren, H. Effect of CeO2 addition on the formability of CP-Ti manufactured by selective laser melting. J. Chin. Rare Earth Soc. 2024, 1–7. [Google Scholar]
- Shi, W.; Yang, Y.; Kang, N.; Wang, M.; Chen, B.; Li, Y.; Junko, U.; Kondoh, K. Microstructure and mechanical characterizations of additively manufactured high oxygen-doped titanium. Mater. Charact. 2022, 189, 112008. [Google Scholar] [CrossRef]
- Wang, X.; Han, W. Oxygen-gradient titanium with high strength, strain hardening and toughness. Acta Mater. 2023, 246, 118674. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.; Kang, X.; Ren, H. Research Progress of Effects of Rare Earth Elements on Metal Additive Manufacturing. Rare Met. Mat. Eng. 2022, 51, 3501–3523. [Google Scholar]
- Ji, Y.; Zhang, M.; Ren, H. Roles of Lanthanum and Cerium in Grain Refinement of Steels during Solidification. Metals 2018, 8, 884. [Google Scholar] [CrossRef]
- Gschneidner, K.; Calderwood, F. The Al-Re (Aluminum-Rare earth) systems. Bull. Alloy Phase Diagr. 1988, 9, 658–668. [Google Scholar] [CrossRef]
- Bao, Z.; Li, K.; Wang, S.; Gao, K.; Zhang, D.; Li, M. Preparation and characterization of submicron-cerium oxide byhypergravity coprecipitation method. Adv. Powder Technol. 2021, 32, 1611–1618. [Google Scholar] [CrossRef]
- Ren, P.; Yu, O.; Mu, J.; Luo, S.; Tang, Z.; Wu, Y.; Chu, L.; Oliveira, J.; Zou, Y.; Wang, H.; et al. Metal powder atomization preparation, modification, and reuse for additive manufacturing: A review. Prog. Mater. Sci. 2025, 152, 101449. [Google Scholar] [CrossRef]
- Lee, Y.; Nagarjuna, C.; Song, J.W.; Jeong, K.Y.; Song, G.; Lee, J.; Lee, J.H.; Hong, S.J. Powder characteristics of Al0.5CoCrFeMnNi high-entropy alloys fabricated by gas atomisation method. Powder Metall. 2021, 64, 219–227. [Google Scholar] [CrossRef]
- Guo, K.; Ji, Y.; Li, Y.; Kang, X.; Bai, H.; Ren, H. Numerical simulation of temperature field and melt pool characteristics of CP-Ti manufactured by laser powder bed fusion. Metals 2023, 13, 11. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, H.; Tao, L.; Gui, K.; Luo, L. Research progress and development of strengthening-toughening methods for molybdenum alloys prepared by powder metallurgy. J. Alloys Compd. 2025, 1010, 177099. [Google Scholar] [CrossRef]
- Sergachev, D.V.; Kuzmin, V.I.; Gulyaev, I.P.; Vaschenko, S.P. Study of the gas-driven atomization for a steel material. Thermophys. Aeromechanics 2024, 31, 557–562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).