Abstract
This study aimed to immobilise sucrase A (SacA) from Bacillus subtilis in E. coli using the AIDA-I system for the whole-cell biocatalysis to transform sucrose to lactate. The pAIDA-sacA plasmid, containing the sacA gene, was fused to the signal peptide of the toxin subunit B from Vibrio cholerae (ctxB) and the autotransporter of the aida gene, encoding a connector peptide and the β-barrel domain of the AIDA-I system. This plasmid was employed to transform E. coli strains W3110, WDHFAK, and WDHFAP, which are unable to naturally use sucrose. These strains were anaerobically cultured in batch fermentations using 10 g L−1 sucrose as the sole carbon source. All strains successfully hydrolysed and fermented sucrose, exhibiting a homolactic profile. Among them, WDHFAP/pAIDA-sacA achieved the highest lactic acid titre of 9.84 ± 0.15 g L−1 and a yield of 0.89 ± 0.02 g g−1. Deletion of the mgsA gene in WDHFAP/pAIDA-sacA confirmed that lactic acid production occurred via the methylglyoxal bypass pathway, as lactic acid titres were reduced by over 80%, while ethanol production increased to 4.27 ± 0.26 g L−1. Adaptive laboratory evolution of WDHFAK/pAIDA-sacA was conducted to improve its capacity and fermentation efficiency under elevated sucrose concentrations. The resultant strain, designated as WDHFAKEV/pAIDA-sacA, consumed up to 65 g L−1 sucrose, achieving 64.61 ± 1.65 g L−1 lactic acid with a yield of 0.99 ± 0.03 g g−1. These findings underscore AIDA-I-mediated SacA immobilisation as a robust strategy for whole-cell biocatalysis, enabling E. coli strains to efficiently ferment sucrose.
1. Introduction
Sucrose, a disaccharide composed of glucose and fructose units linked via a glycosidic bond, is the most abundant disaccharide on earth, predominantly extracted from sugarcane, molasses, and various fruit residues [1]. Due to its abundance in higher plant tissues, sucrose represents an economical and readily available resource for biobased chemical production, serving as one of the least expensive carbon sources for feedstocks in diverse biotechnological applications [2]. Despite its potential, many commonly used Escherichia coli strains, including K-12, B, and W3110, cannot utilise sucrose as their sole carbon source. This limitation arises from the absence of the sucrose operon, which encodes the saccharolytic enzymes and transport systems required for sucrose metabolism. These systems include the phosphotransferase system (PTS), which phosphorylates sucrose during transport into the cell, and alternative permease-based systems that allow for sucrose uptake without chemical modification [3,4]. Nevertheless, these E. coli strains are widely employed in research and industrial applications as workhorses for producing biobased chemicals such as organic acids, ethanol, and hydrogen [5,6,7]. Previous studies have attempted to engineer E. coli for sucrose utilisation by introducing heterologous sucrase genes, such as sacA from Bacillus subtilis, using plasmids with varying copy numbers. Although these approaches achieved gene expression, significant challenges arose: sucrase enzymes often remained cryptic in the cytoplasm due to the absence of a functional sucrose PTS in E. coli. Consequently, sucrose hydrolysis did not occur, and bacterial growth was not observed when sucrose was the sole carbon source [8,9]. These limitations underscore the need for integrated strategies that combine effective sucrose hydrolysis with efficient substrate uptake to optimise biotransformation processes. The autotransporter AIDA-I (Adhesin Involved in Diffuse Adherence I) system, derived from E. coli, provides a promising solution by enabling the immobilisation of heterologous proteins to the outer membrane of Gram-negative bacteria, facilitating extracellular enzymatic activity. This system comprises three key components: a signal peptide for translocation across the inner membrane, a β-barrel domain that embeds itself in the outer membrane, and a passenger domain that is immobilised on the surface to enable its functional activity [10,11,12]. Immobilisation of sucrase A (sucrose-6-phosphate hydrolase, SacA) on the surface membrane bypasses the reliance on the sucrose-PTS system, facilitates extracellular sucrose breakdown, and ensures that glucose and fructose are readily taken up by their respective transporters. In this study, the development of Escherichia coli strains capable of fermenting sucrose through the surface expression of SacA from Bacillus subtilis using the AIDA-I autotransporter system was investigated. This research explores whether this strategy can confer saccharolytic activity in non-sucrose-utilising strains and support the production of metabolites under anaerobic conditions. Additionally, the potential of adaptive laboratory evolution to enhance fermentation performance was performed, along with the implementation of metabolic pathway modifications to redirect carbon towards to the production of lactate or ethanol. This work provides a framework for optimising whole-cell biocatalysis and expanding the metabolic versatility of E. coli for the efficient conversion of sucrose.
2. Materials and Methods
2.1. Bacterial Strains and Molecular Cloning of E. coli
The protocols for generating the WDHFAK, WDHFAP, WDHFAKM, and WDHFAPM strains from E. coli W3110 were detailed previously [13,14]. Gene deletions were performed using P1 transduction [15] specifically targeting the genes ldhA (D-lactate dehydrogenase), frdDABC (fumarate reductase), ackA (acetate kinase), pta (phosphate acetyltransferase), hycA (repressor of the formate operon), and mgsA (methylglyoxal synthase). The success of the gene deletions and the loss of antibiotic resistance were confirmed via PCR, using the primers listed in Table 1.

Table 1.
Relevant characteristics of the strains, plasmids, and primers used in this work.
2.2. Construction of the pAIDA-sacA
The sacA gene encoding sucrase A was sourced from GenBank under accession number CP053102.1 and amplified via PCR from the Bacillus subtilis genome. The resulting amplicon was then cloned into the pGEM-T (Easy Vector Systems, Promega Corporation, Madison, WI, USA) and subsequently digested with the restriction enzymes AscI and XhoI (Thermo Scientific, Waltham, MA, USA). The digested fragment was then ligated into the pAIDA plasmid, previously generated by [5], which had been pre-digested with the same enzymes. The resulting plasmid, named pAIDA-sacA, includes the signal peptide from the β-subunit of Vibrio cholerae toxin (ctxB) for membrane translocation, the recombinant sucrase A from B. subtilis as the passenger protein, a linker region, and the β-barrel autotransporter AIDA-I in a single construct. The expression of this construct is driven by the gapAP1 promoter, a derivative of the constitutive gapA gene from E. coli, which ensures transcriptional regulation under both aerobic and anaerobic conditions [16]. DNA sequences were assembled using MacVector (MacVector Inc., Version 10.1, Apex, NC, USA) and SnapGene 3.3 software (GSL Biotech LLC, Version 3.3, San Diego, CA, USA). Following plasmid construction, E. coli strains DH5α, W3110, WDHFAK, WDHFAP, WDHFAKM, WDHFAPM, and WDHFAPMEV were heat-shock transformed.
2.3. Detection of Saccharolytic Activity on Plate
E. coli cells transformed with the pAIDA-sacA plasmid were cultivated on minimal medium and minimal medium agar plates, using sucrose as the sole carbon source. The minimal medium was prepared with the following composition (per litre): 10 g sucrose (Materiales y Abastos Especializados, Zapopan, México, ACS grade); 3.5 g KH2PO4 (CTR Scientific, Guadalajara, México, reagent grade); 3.5 g (NH4)2HPO4 (Karal, Guanajuato, México, reagent grade), 1.0 g MgSO4·7H2O (Fermont, Monterrey, México, ACS grade); 40 µg thiamine (Sigma-Aldrich, Burlington, MA, USA, ≥99% purity); and ampicillin (Pharmalife, Laboratorios Pisa, Guadalajara, México, ≥99% purity). To prepare the agar plates, 15 g agar (Santa Cruz Biotechnology, Dallas, TX, USA, reagent grade) was added to the liquid medium. The E. coli strains were incubated at 37 °C for 48 h on the agar plates to enable colony formation. After incubation, the presence of colonies was assessed to determine saccharolytic activity. Additionally, liquid cultures were evaluated by measuring optical density to quantify bacterial growth. Optical density at 600 nm (OD600) was measured using a Cary 50 Bio UV-Vis spectrophotometer (Varian, Palo Alto, CA, USA) with a 1 cm path length cuvette.
2.4. Culture Media and Anaerobic Fermentation Conditions
E. coli W3110, WDHFAK, WDHFAP, WDHFAKM, and WDHFAPM strains carrying the pAIDA-sacA plasmid were used to evaluate the production of anaerobic end products from sucrose. For preinocula preparation, cells were grown in 250 mL flasks with 100 mL of Luria Bertani medium (Sigma-Aldrich, Burlington, MA, USA) supplemented with 100 µg mL−1 ampicillin (Pharmalife, Laboratorios Pisa, Guadalajara, México, ≥99% purity) at 31 °C for 18 h with shaking at 200 rpm. After incubation, cells were harvested via centrifugation at 9500× g for 5 min using a Biofuge fresco centrifuge (Kendro laboratory products, Hannover, Germany) washed and resuspended in 120 mL fermentative serological bottles containing 110 mL of B medium [17], which, per litre, contained the following: 15 mg MnSO4·7H2O (Fermont, Nuevo León, México, reagent grade); 12.5 mg Na2MoO4·2H2O (Productos Químicos Nuevo León, México, reagent grade); 3 mg CoCl2·8H2O (Productos Químicos Nuevo León, México, reagent grade); 4500 mg NH4H2PO4 (Fermont, Nuevo León, México, reagent grade); 11,867 mg Na2HPO4 (CTR Scientific, Guadalajara, México, reagent grade); 75 mg ZnCl2 (Karal, Guanajuato, México, reagent grade); 100 mg MgCl2·6H2O (Fermont, Nuevo León, México, ACS grade); 0.5 g CaCl2 (Karal, Guadalajara, México, reagent grade); 125 mg K2HPO4 (CTR Scientific, Guadalajara, México, reagent grade); 25 mg FeSO4·6H2O (Tecnología industrial química, Tlaxcala, México, reagent grade); and 5 mg CuSO4·5H2O (Tecnología industrial química, Tlaxcala, México, reagent grade). Additionally, 1 g yeast extract (Difco Laboratories, San Diego, CA, USA, reagent grade), 1 mL trace element solution 1000 X [13], 1 g glucose (Materiales y abastos especializados, Zapopan, México, ACS grade), 100 mg ampicillin (Pharmalife, Laboratorios Pisa, Guadalajara, México, ≥99% purity), and 10 g of the respective carbon source—sucrose (Materiales y abastos especializados, Zapopan, México, ACS grade), glucose (Materiales y abastos especializados, Zapopan, México, ACS grade), or fructose (Materiales y abastos especializados, Zapopan, México, ACS grade)—were added. Cultures were inoculated to an initial OD600 of 0.1, pH was adjusted to 7.3, and the fermentation was carried out at 31 °C with shaking at 200 rpm under anaerobic conditions. All experiments were performed in triplicate.
2.5. Adaptive Evolution of E. coli Strains Under Glucose and Sucrose Conditions
The WDHFAPM and WDHFAK strains were cultured overnight in 250 mL flasks containing 100 mL of Luria–Bertani (Sigma-Aldrich, Burlington, MA, USA) broth at 31 °C with shaking at 200 rpm in an orbital incubator shaker (THZ-98A, Luzeren, Zhejiang, Hangzhou, China) with a 25 mm orbital diameter. After incubation, cells were harvested via centrifugation at 9500× g for 5 min, washed, and transferred to 120 mL fermentative serological bottles containing 110 mL of B medium, as detailed in Section 2.4. This medium was supplemented with 1 mL of 1000 X trace elements [13], 1 g L−1 yeast extract (Difco Laboratories, San Diego, CA, USA, reagent grade), and the appropriate carbon source. Cultures were initiated at an OD600 of 0.1, with the pH adjusted to 7.3, and incubated at 31 °C with shaking at 200 rpm. For the WDHFAPM strain, glucose (Materiales y abastos especializados, Zapopan, México, ACS grade) at 10 g L−1 was used as the carbon source, and the adaptation process involved serial transfers every 24 to 48 h into fresh B medium over four weeks. Mutants with faster growth rates in glucose were selected, and a single colony from the final culture was isolated. This colony, designated WDHFAPMEV, was tested for its glucose fermentation ability and subsequently transformed with the pAIDA-sacA plasmid to evaluate its capacity for sucrose fermentation. For the WDHFAK/pAIDA-sacA strain, the adaptation process began with sucrose (Materiales y abastos especializados, Zapopan, México, ACS grade) at 10 g L−1, and the concentration was gradually increased to 20, 40, 60, and finally 80 g L−1 through serial transfers. After the adaptation period, a single colony from the final culture was isolated and tested for sucrose fermentation at 65 g L−1. The resulting strain was designated WDHFAKEV/pAIDA-sacA.
2.6. Bioreactor Cultures
E. coli WDHFAKEV/pAIDA-sacA, stored in an Luria–Bertani medium with glycerol 50% (Jalmek, Nuevo León, México, ACS grade) at −80 °C, was transferred to Luria–Bertani agar and incubated overnight at 31 °C. A single colony was subsequently inoculated into 100 mL of Luria–Bertani (Sigma-Aldrich, Burlington, MA, USA) medium containing 100 µg mL−1 ampicillin (Pharmalife, Laboratorios Pisa, Guadalajara, México, ≥99% purity), and incubated overnight at 31 °C with shaking at 200 rpm. After incubation, the culture’s optical density at 600 nm (OD600) was measured to calculate the volume required to achieve an OD600 of 0.1 in the fermentation medium. This volume was centrifuged at 9500× g for 5 min at room temperature, and the resulting pellet was directly resuspended in the fermentation medium. Anaerobic experiments were conducted in B medium, as described in Section 2.4, supplemented with 1 g L−1 glucose (Materiales y abastos especializados, Zapopan, México, ACS grade), 1 g L−1 yeast extract (Difco Laboratories, San Diego, CA, USA, reagent grade), 1 mL L−1 of 1000 X trace element solution [13], 100 µg mL−1 ampicillin (Pharmalife, Laboratorios Pisa, Guadalajara, México, ≥99% purity), and 65 g L−1 sucrose (Materiales y abastos especializados, Zapopan, México, ACS grade) as the carbon source. Sucrose and ampicillin were sterilised via filtration, while yeast extract and glucose were sterilised separately. The pH was set to 6.5 and maintained using 5 M ammonium hydroxide (J.T. Baker, Phillipsburg, NJ, USA, ACS grade). Fermentation was performed in a 1.5 L bioreactor (Applikon® Biotechnology, Delft, The Netherlands) with a working volume of 1 L, equipped with two six-bladed Rushton turbines and an H/D ratio of 2.1. The pH was monitored with a sterilizable electrode (Applikon® Biotechnology, Delft, The Netherlands) connected to a Bioconsole ADI 1035 and regulated with a Biocontroller ADI 1030 (Applikon® Biotechnology, Delft, The Netherlands). Data were acquired using BioXpert 1.3 software (Applikon® Biotechnology, Delft, The Netherlands). Periodic 1 mL samples were collected to monitor cell growth, sucrose consumption, and metabolites.
2.7. Statistical Analysis
The statistical analysis of the experiments was performed using analysis of variance (ANOVA) and unpaired Student’s t-test. Results with p < 0.05 were considered statistically significant. All statistical analyses were conducted using Microsoft Excel version 16.0 (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Qualitative Confirmation of Saccharolytic Activity in E. coli Expressing AIDA-SacA
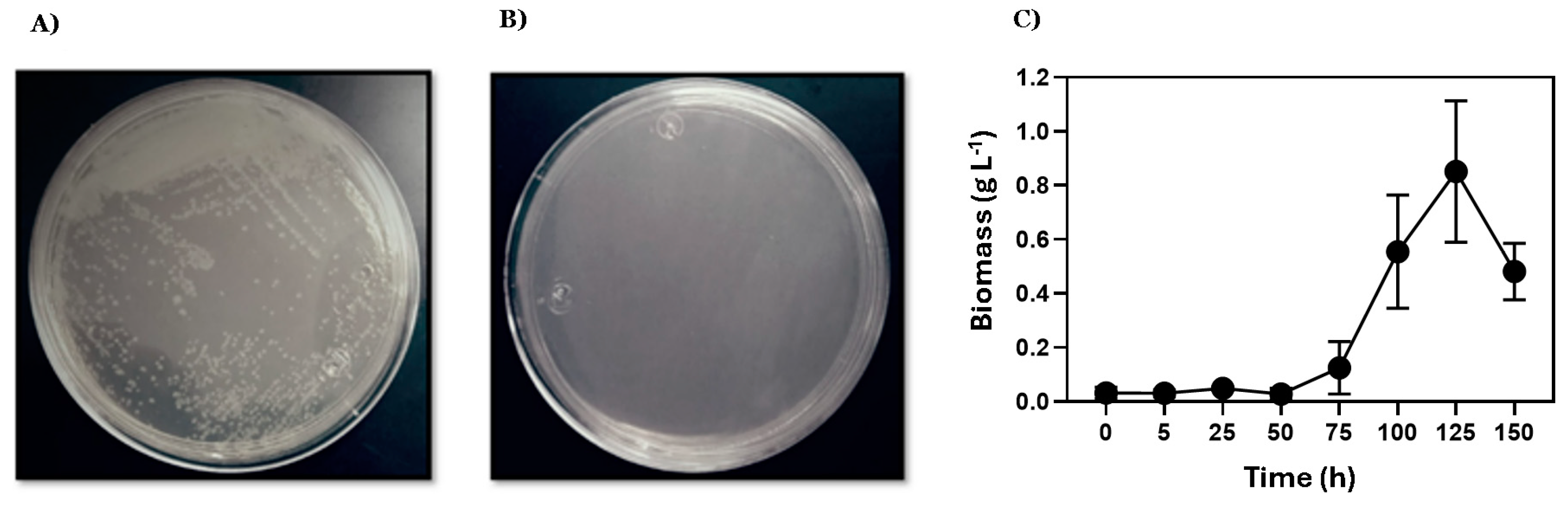
The saccharolytic activity of E. coli DH5α/pAIDA-sacA was assessed on minimal-medium agar plates with sucrose as the sole carbon source. Growth of E. coli DH5α/pAIDA-sacA at 37 °C confirmed the functionality of the AIDA-I-mediated SacA system for sucrose hydrolysis. In contrast, control plates with the pAIDA plasmid showed no growth, demonstrating that saccharolytic activity was conferred by the immobilisation of the AIDA-SacA system (Figure 1). Similarly, the strain W3110/pAIDA-sacA grew in minimal medium in 250 mL flasks, achieving a biomass concentration of up to 0.85 ± 0.26 g L−1.
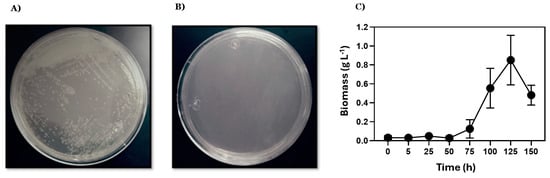
Figure 1.
Qualitative assessment of saccharolytic activity using sucrose as the sole carbon source. Agar minimal-medium dishes were incubated at 37 °C for 48 h: (A) E. coli DH5α/pAIDA-sacA; (B) E. coli DH5α transformed with pAIDA served as a negative control; (C) the E. coli W3110/pAIDA-sacA strain grown in minimal medium in 250 mL flasks up to 0.85 ± 0.26 g L−1 of biomass. The bars represent the standard deviation (n = 3).
3.2. Lactic Acid Production Prevails in Sucrose Fermentation
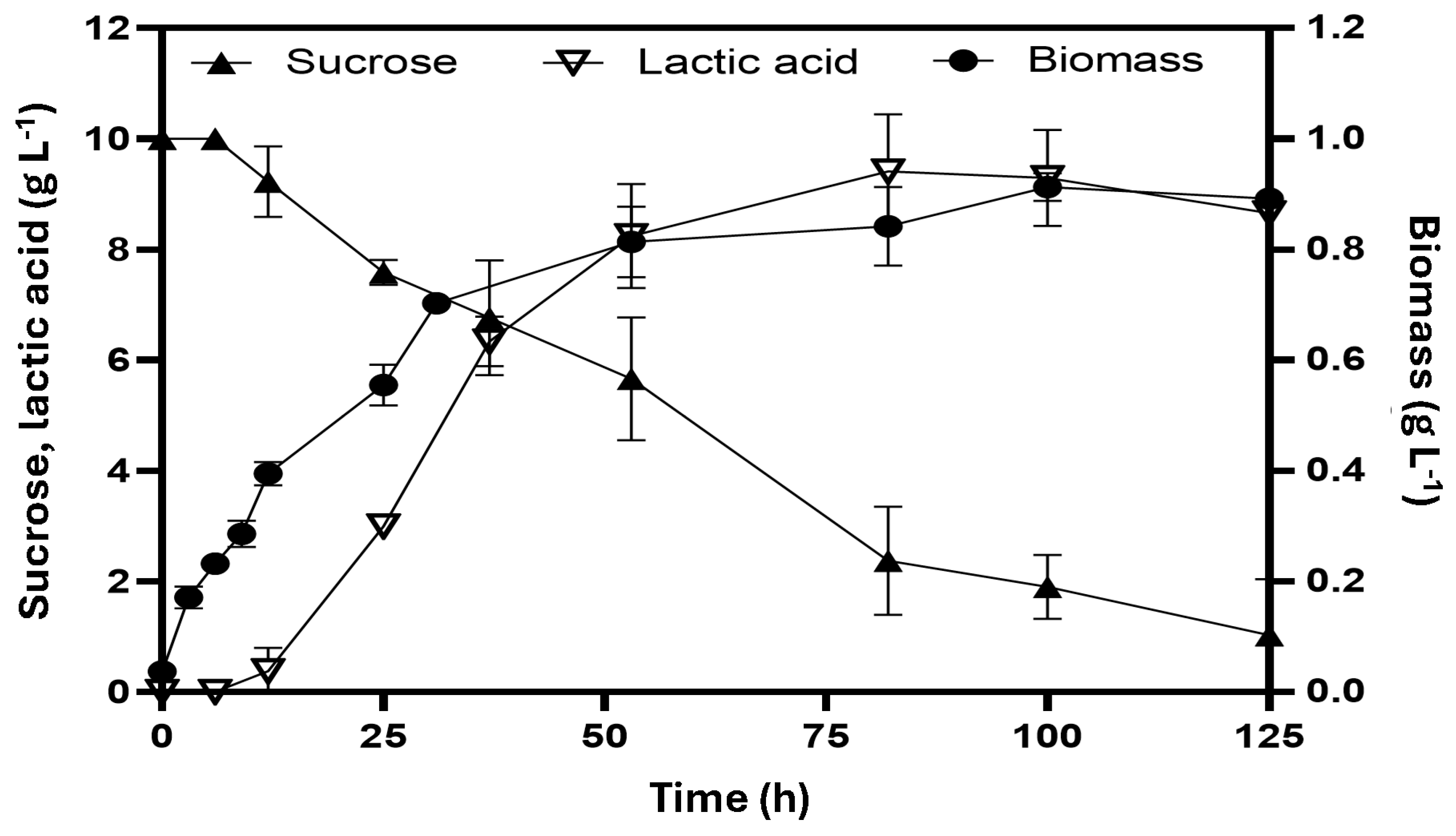
Figure 2 illustrates the anaerobic fermentation of E. coli W3110/pAIDA-sacA using sucrose as the carbon source. The strain achieved a maximum biomass concentration of 0.91 ± 0.02 g L−1 and peak cell growth within 53 h, with a specific growth rate of 0.09 ± 0.03 h−1. During fermentation, lactic acid was the predominant product, reaching 9.25 ± 0.88 g L−1 after 76 h, with trace amounts of acetic acid, succinic acid, and ethanol detected. Glucose and fructose, released from the hydrolysis of sucrose, were rapidly consumed, resulting in non-measurable concentrations in the medium. The lactic acid yield was 0.84 ± 0.09 g lactate g sucrose−1, with a production rate of 0.19 ± 0.05 g L−1 h−1. The small amount of glucose initially present (1 g L−1) likely supported cellular growth during the first 11 h.
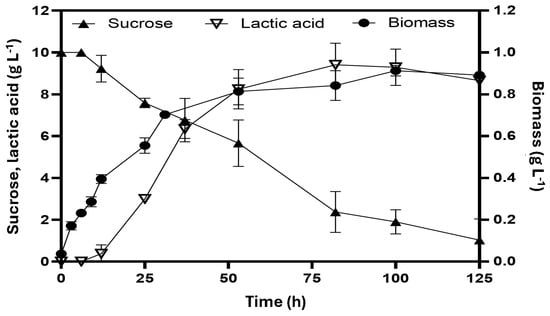
Figure 2.
Homolactic fermentation of E. coli W3110/pAIDA-sacA with sucrose as the carbon source at 31 °C. The bars represent the standard deviation (n = 3).
Typically, E. coli produces formate, CO2, H2, acetate, D-lactate, succinate, and ethanol, which is referred to as mixed-acid fermentation [18,19]. The introduction of the saccharolytic activity and the use of sucrose as a carbon source redirected the metabolic flux towards lactic acid production. In contrast, cultures with glucose or fructose resulted in lower lactic acid titres and higher by-products formation compared to sucrose cultivation (Table 2). Statistical analysis using one-way ANOVA revealed significant differences in lactic acid production among cultures with sucrose, glucose, and fructose (p < 0.05). A post hoc Tukey’s test confirmed that sucrose-supported cultures produced significantly higher lactic acid titres compared to glucose and fructose (p < 0.05), with no significant difference detected between glucose and fructose. These findings highlight the role of sucrose in enhancing lactic acid yields. Strategies to use alternative carbon sources often vary due to changes in the distribution of metabolic fluxes. Sucrose breakdown significantly influences the central metabolic flux by directing substrates into key pathways [8,20,21]. Comparisons highlight the role of sucrose as a selective substrate for lactic acid production under these conditions.

Table 2.
Fermentation products and yields of E. coli W3110/pAIDA-sacA with sucrose, glucose, and fructose as substrate at 10 g L−1.
3.3. Homolactic Fermentation on Sucrose in E. coli Strains Lacking ldhA Suggests Alternative Metabolic Pathways
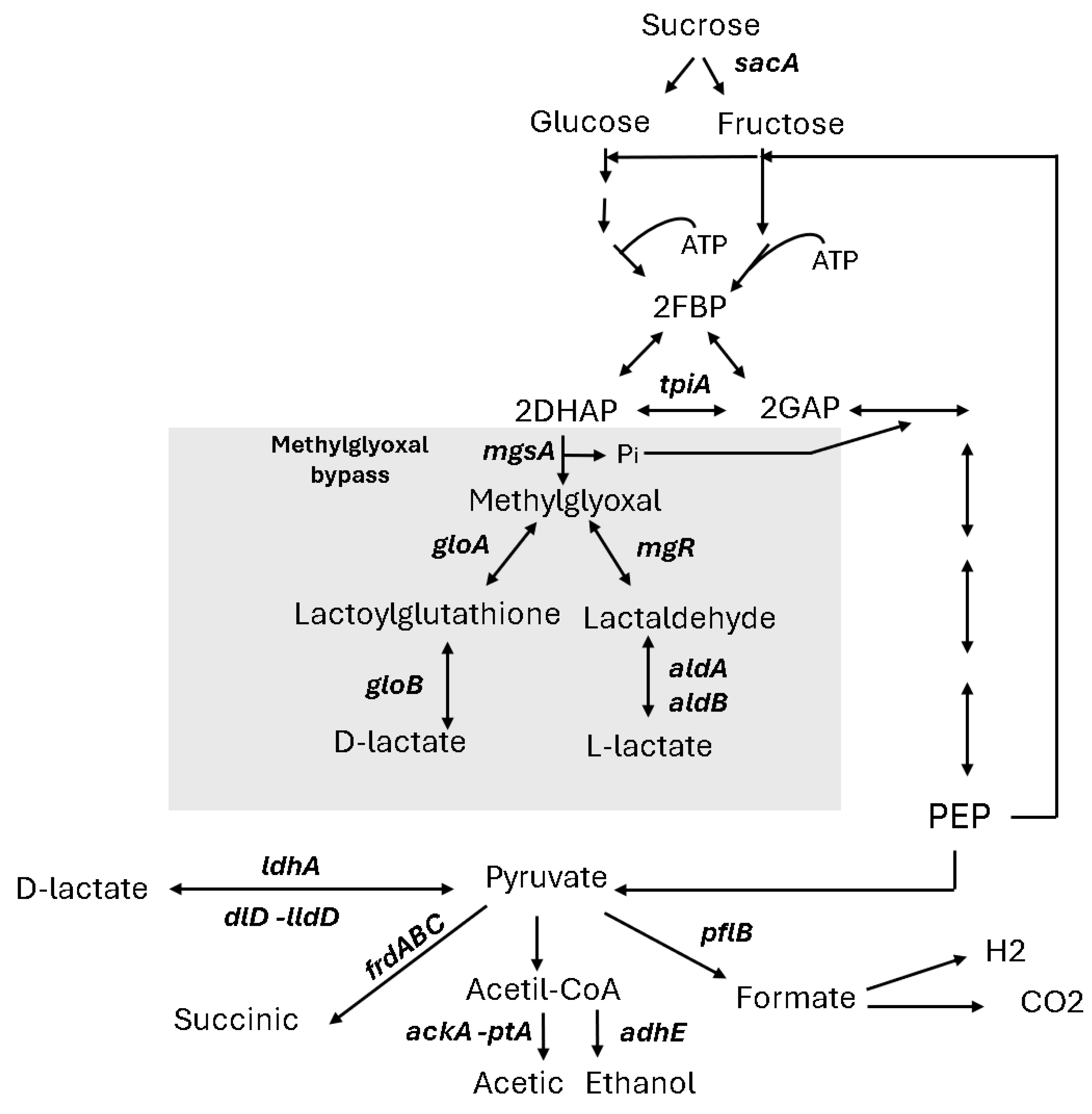
Figure 3 illustrates the conventional pathway for lactate production in Escherichia coli, which involves the NAD⁺-dependent lactate dehydrogenase (LDH, encoded by ldhA), catalysing the conversion of pyruvate to D-lactic acid under anaerobic conditions [22]. Lactate can also be synthesised by other lactate dehydrogenases, such as the FMN-linked LDH (lldD) and FAD-binding D-LDH (dld), although these enzymes are typically repressed under anaerobic conditions [23]. Beyond LDH-dependent pathways, E. coli can produce lactate via the methylglyoxal (MG) bypass. This alternative pathway involves methylglyoxal synthase encoded by mgsA, which converts dihydroxyacetone phosphate (DHAP) into methylglyoxal. Methylglyoxal can then be further oxidised to lactate through glyoxalase or aldehyde dehydrogenase activity [24]. The MG bypass not only contributes to lactic acid production but also functions as a detoxification system, converting potentially toxic intermediates such as methylglyoxal into lactate, thereby mitigating cellular stress under anaerobic conditions.
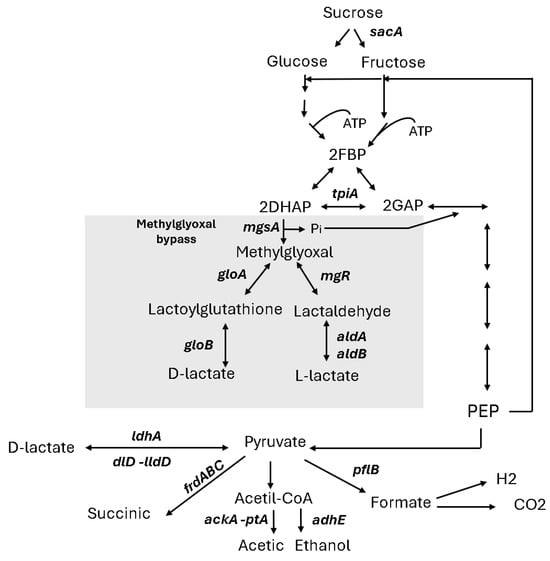
Figure 3.
Schematic diagram of the fermentation pathways used by E. coli. The metabolic intermediates are abbreviated as follows: FBP, fructose 1,6 biphosphate; GAP, glyceraldehyde-3-phosphate; DHAP, dihydroxyacetone phosphate; and PEP (phosphoenolpyruvate). Key genes encoding important enzymes are sacA (sucrose-6-phosphate hydrolase), tpiA (triosephosphate isomerase), gloA (lactoylglutathione lyase), gloB (hydroxyacylglutathione hydrolase), mgsA (methylglyoxal synthase), ldhA (anaerobic lactate dehydrogenase), dld (D-lactate dehydrogenase), lldD (L-lactate dehydrogenase), pta (phosphotransacetylase), frdDABC (fumarate reductase), and ackA (acetate kinase A).
To determine if the loss of ldhA affects lactic acid production, the performance of the wild-type W3110/pAIDA-sacA strain was compared with two genetically modified Escherichia coli strains, WDHFAP/pAIDA-sacA and WDHFAK/pAIDA-sacA, both carrying a deletion of the ldhA gene. This will show us whether alternative metabolic pathways E. coli could be used to produce different fermentation products. WDHFAP/pAIDA-sacA has additional deletions in pta (phosphotransacetylase), frdDABC (fumarate reductase), and hycA (repressor of the formate operon), while WDHFAK/pAIDA-sacA retains the pta gene but includes a deletion of ackA (acetate kinase A), as detailed in Table 1. These genetic modifications are expected to favour the co-production of ethanol and hydrogen. The deletions in pta and ackA hinder the production of acetate, while the deletion of frdDABC reduces the production of succinate. Additionally, the deletion of hycA promotes hydrogen production [13,14].
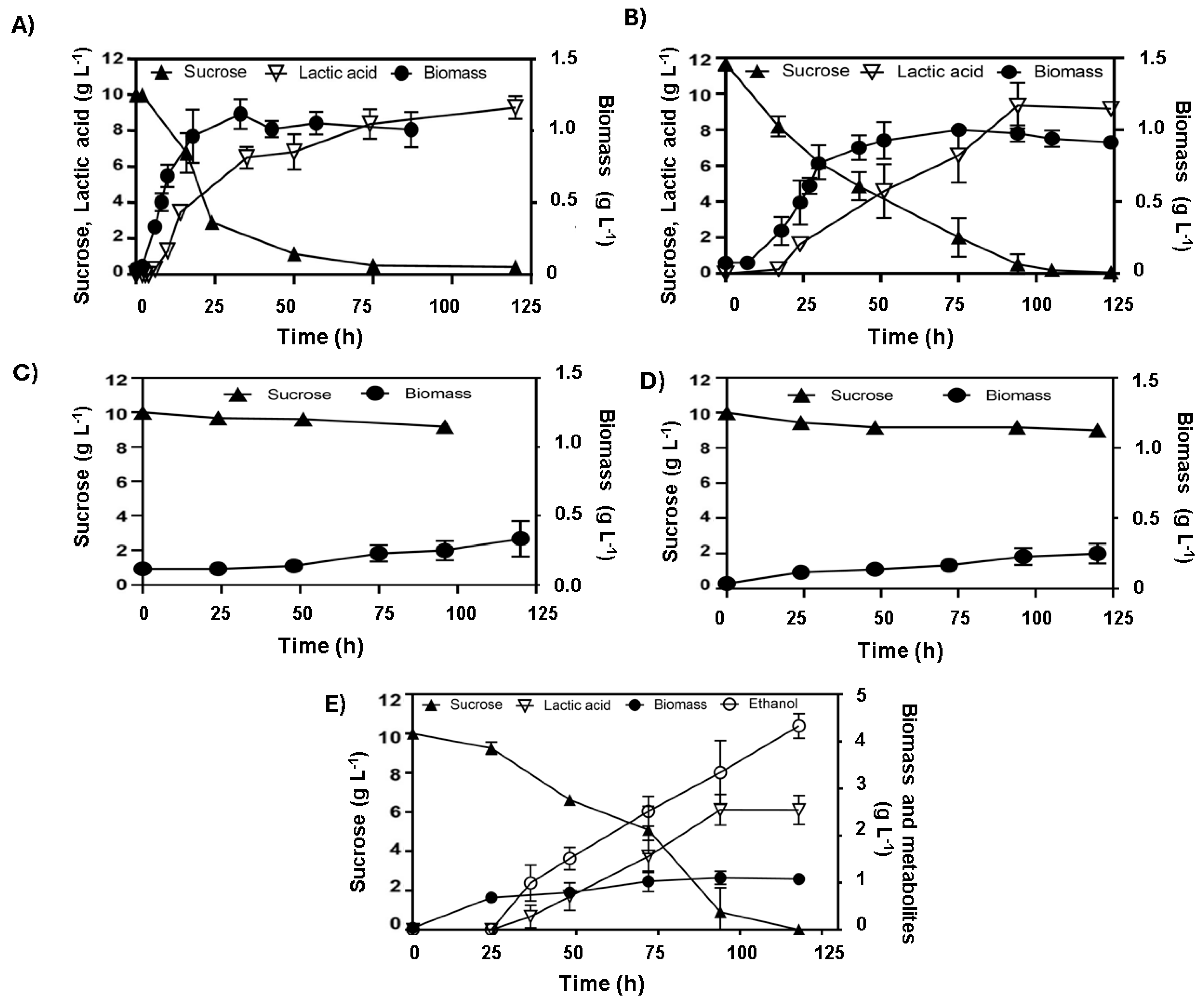
The genetically modified E. coli strains transformed with the pAIDA-sacA plasmid exhibited saccharolytic activity, enabling efficient sucrose utilisation. However, our results demonstrated that the absence of ldhA did not impair the ability of these strains to produce lactic acid from sucrose as both maintained a consistent homolactic fermentation profile. For WDHFAP/pAIDA-sacA, the maximum biomass concentration achieved was 1.01 ± 0.03 g L−1 at 18 h, with a specific growth rate of 0.32 ± 0.02 h−1. Lactic acid production started at 6 h and reached a peak of 9.84 ± 0.60 g L−1 by 74 h, resulting in a lactic acid yield of 0.89 ± 0.02 g lactic acid g sucrose−1 and an average production rate of 0.16 ± 0.07 g L−1 h−1 (Figure 4A). In comparison, WDHFAK/pAIDA-sacA showed an initial biomass of 0.07 g L−1, which increased to 1.15 ± 0.08 g L−1 by 33 h, with a specific growth rate of 0.13 ± 0.03 h−1. Lactic acid production commenced at 17 h and peaked at 9.32 ± 0.16 g L−1 by 76 h, achieving a lactic acid yield of 0.84 ± 0.08 g lactic acid g sucrose−1 and an average production rate of 0.13 ± 0.12 g L−1 h−1 (Figure 4B). This indicates that lactic acid production can occur via a metabolic pathway distinct from the conventional reduction of pyruvate to D-lactate. In exploring alternative pathways for lactic acid production, previous studies have indicated that sucrose metabolism can activate the MG bypass. This activation is attributed to the generation of elevated intracellular concentrations of fructose, glucose, or glucose-1-phosphate, which exceed levels typically observed during glucose metabolism [21]. Under anaerobic conditions, sugar phosphates such as DHAP allosterically activate methylglyoxal synthase, encoded by the mgsA gene [25]. Additionally, the metabolism of sugar mixtures, including sucrose, has been associated with high lactate concentrations, as co-fermentation modifies regulatory dynamics and promotes metabolic flux through the MG bypass [24]. This provides a plausible explanation for the exclusive homolactic fermentation profile observed in E. coli strains grown on sucrose, even in the absence of the ldhA gene, and for the absence of other typical fermentation end products, which are observed when the strains are cultivated on glucose.
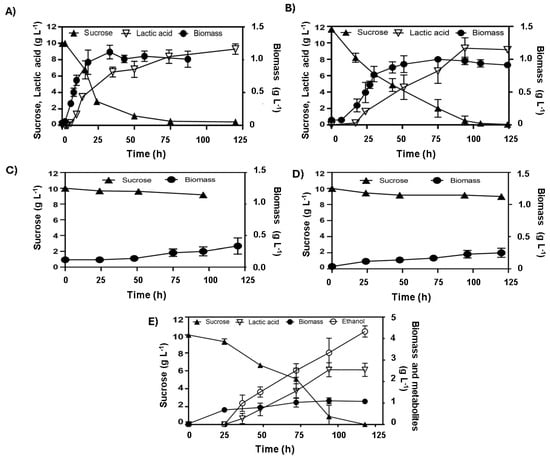
Figure 4.
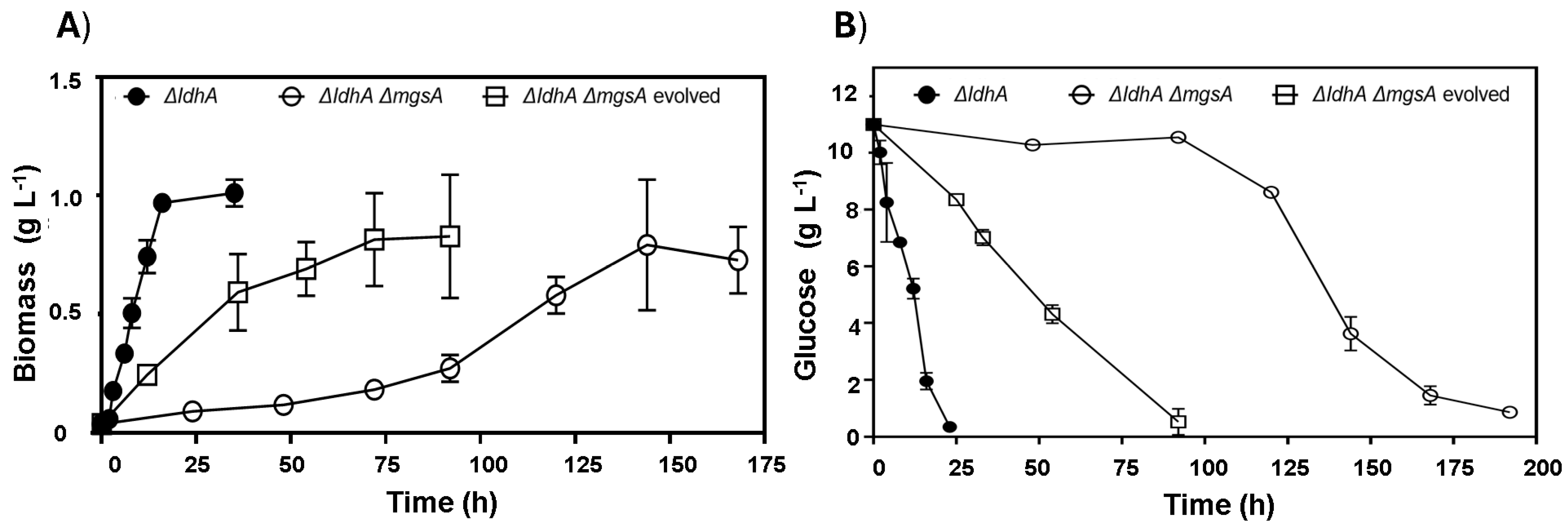
Anaerobic fermentation profiles of engineered E. coli strains cultivated on sucrose: (A) WDHFAP/pAIDA-sacA and (B) WDHFAK/pAIDA-sacA, with both (A,B), lacking the ldhA gene, exhibiting homolactic profiles; (C) WDHFAKM/pAIDA-sacA and (D) WDHFAPM/pAIDA-sacA, withing both (C,D) having deletions in both ldhA and mgsA, showing delayed growth; (E) WDHFAPMEV/pAIDA-sacA, an evolved strain with ldhA and mgsA deletions, demonstrating enhanced ethanol production.
3.4. The mgsA Gene Deletion Underscores the Role of the Methylglyoxal Bypass in Sucrose Metabolism and Lactate Production in E. coli
The WDHFAK and WDHFAP strains were selected for this study due to the deletion of the ldhA gene. In these strains, the mgsA gene was also deleted, effectively eliminating the two anaerobic pathways for lactate production (Figure 3). The deletion of mgsA redirects the metabolic flux towards glycolysis by removing the methylglyoxal bypass, significantly reducing the production of both lactate and its precursor, methylglyoxal [26]. Subsequently, both strains were transformed with the plasmid pAIDA-sacA to confer saccharolytic activity. The resultant strains were named WDHFAPM/pAIDA-sacA and WDHFAKM/pAIDA-sacA. Under anaerobic fermentation conditions, the ΔmgsA strains displayed slow growth. The WDHFAPM/pAIDA-sacA strain reached a maximum biomass of only 0.33 g L−1 after 125 h, consuming just 0.8 g of sucrose from the initial 10 g. Similarly, the WDHFAKM/pAIDA-sacA strain achieved 0.28 g L−1 biomass after 100 h, with minimal sucrose consumption (9.10 g L−1 remaining). In contrast, the parental and wild-type strains consumed all the sucrose within the same timeframe, demonstrating robust saccharolytic activity and efficient lactic acid production (Figure 4). These findings underscore the critical role of the MG bypass in sucrose metabolism and lactic acid production under anaerobic conditions. Furthermore, the lactate observed in the parental and wild-type strains suggests that the MG bypass is the primary source of lactic acid under these conditions.
The mgsA null strains WDHFAPM/pAIDA-sacA and WDHFAKM/pAIDA-sacA were also assessed and compared to their respective parental strains, WDHFAP/pAIDA-sacA and WDHFAK/pAIDA-sacA, under glucose fermentation conditions. In these evaluations, lactic acid and ethanol production were measured to evaluate the metabolic changes caused by the mgsA deletion. The mgsA null mutant strains showed a significant increase in ethanol production and a marked reduction in lactic acid levels (Table 3). Despite these advantages in ethanol production, the strains exhibited slower growth, reduced glucose consumption, and prolonged lag phases. In WDHFAPM/pAIDA-sacA, glucose depletion occurred by 216 h. The strain underwent a prolonged adaptation phase of approximately 50 h, after which exponential growth began, reaching the stationary phase by 144 h. Ethanol production increased significantly from 0.25 ± 0.05 to 4.29 ± 0.79 g L−1, representing an over-17-fold increase compared to the parental strain. However, its specific growth rate was markedly reduced to 0.02 h−1, reflecting a 17-fold decrease from the original 0.35 h−1. In contrast, the parental WDHFAP/pAIDA-sacA strain depleted glucose within 25 h and reached stationary phase by 16 h (Figure 5). Similar trends were observed in WDHFAKM/pAIDA-sacA, which achieved specific growth rate of 0.02 ± 0.01 h−1. This strain produced 4.00 ± 0.33 g L−1 of ethanol. In contrast, the parental strain WDHFAK/pAIDA-sacA exhibited a specific growth rate of 0.13 ± 0.02 h−1, yielding 2.47 ± 0.27 g L−1 of ethanol. Thus, the specific growth rate of WDHFAKM/pAIDA-sacA is approximately 7.22-times slower than that of its parental strain. However, it produced 1.61 times more ethanol than its parental counterpart. To address the prolonged lag phases and slow glucose consumption observed, the WDHFAPM strain was subjected to adaptive evolution through serial transfers in B medium with glucose as the carbon source. The evolved strain, WDHFAPMEV, exhibited a significantly shorter lag phase, reaching the stationary phase in 54 h and consuming all the glucose within 100 h, compared to 200 h required by the non-evolved strain (Figure 5). WDHFAPMEV was further transformed with pAIDA-sacA and evaluated under sucrose fermentation conditions. The evolved strain, WDHFAPMEV/pAIDA-sacA, achieved successful growth, reaching 1.26 g L−1 biomass within 24 h. Interestingly, this strain did not display a homolactic fermentation profile. Ethanol production increased up to 4.27 ± 0.26 g L−1, which represents 79.8% of the theoretical maximum (Figure 4E). Despite the deletion of both lactate-production pathways, residual lactate production of 2.54 ± 0.25 g L−1 was observed, potentially attributable to the lldD/dld pathway. Minor concentrations of acetic acid and succinic acid (0.5 g L−1 each) were consistent with the deletions of the frdDABC and pta genes.

Table 3.
Effect of ldhA and mgsA deletions on metabolite production in E. coli strains cultivated on glucose.
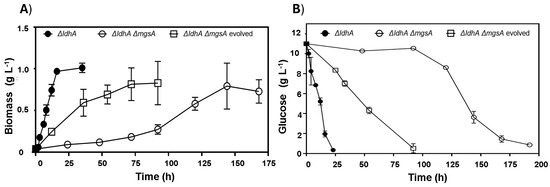
Figure 5.
Impact of ldhA and mgsA deletions on (A) biomass and (B) glucose consumption in E. coli strains: WDHFAP/pAIDA-sacA (ΔldhA), WDHFAPM/pAIDA-sacA (ΔldhA ΔmgsA), and WDHFAPMEV (ΔldhA ΔmgsA evolved).
3.5. Adaptive Evolution of Escherichia coli for Efficient Sucrose Fermentation Through the Methylglyoxal Bypass and the pAIDA-sacA System
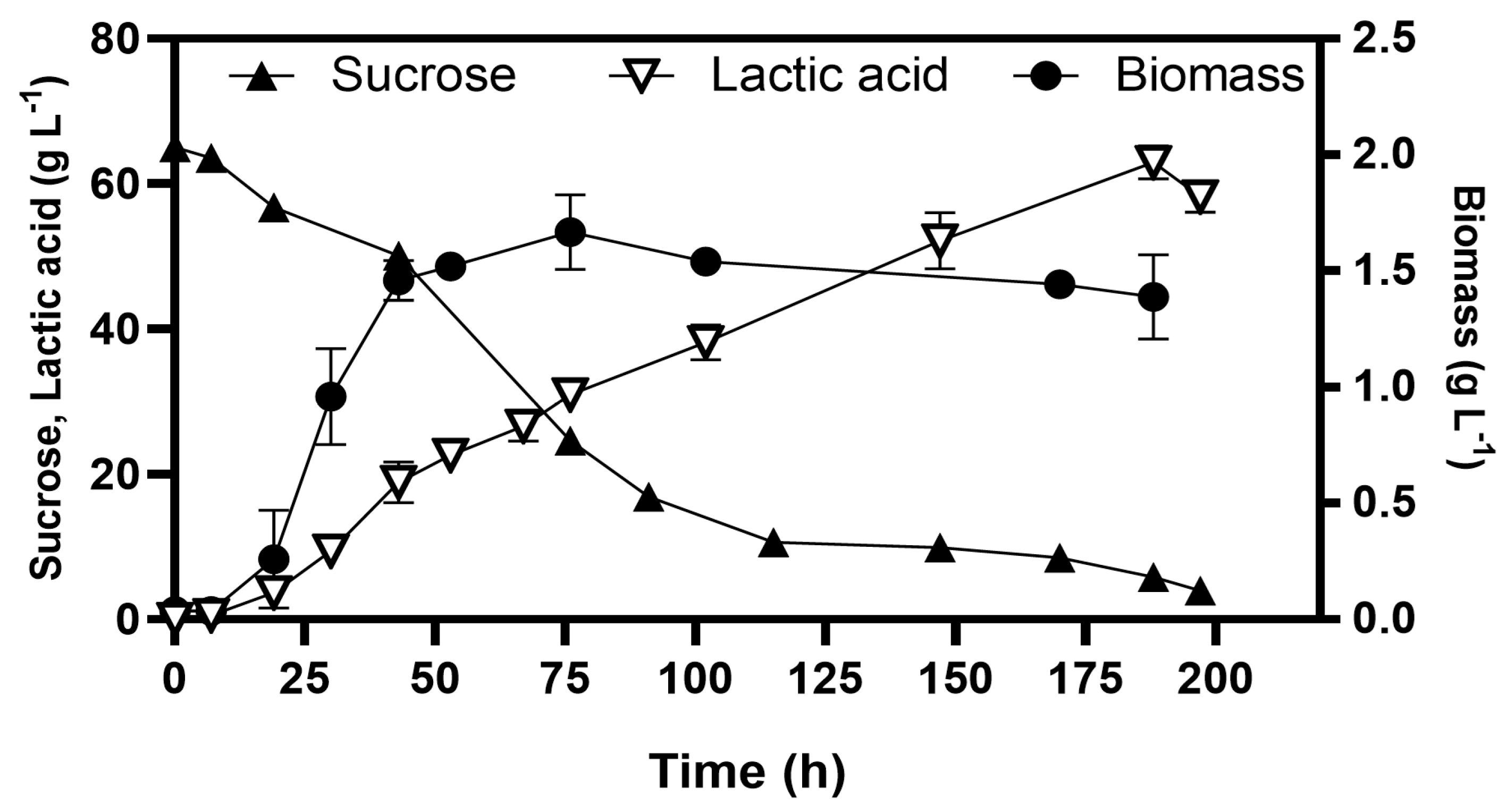
Methylglyoxal is a toxic metabolite that induces cell death at high concentrations; however, the glyoxalase pathway mitigates its effect; which converts MG into D-lactate (Figure 3), preventing accumulation and maintaining cellular viability [27,28]. We investigated whether the homolactic fermentation profile observed at 10 g L−1 sucrose could be sustained at higher sucrose concentrations. For this purpose, the strain WDHFAK/pAIDA-sacA was selected, which retains the mgsA gene and has deleted the ldhA. The strain evolved through serial transfers in B medium with sucrose concentrations up to 65 g L−1 and was subsequently characterised in batch fermentations. The evolved strain WDHFAKEV/pAIDA-sacA achieved biomass growth of 1.52 ± 0.05 g L−1 by 53 h (Figure 6). The cells reached the stationary phase by 43 h, at which point lactate production had reached 20.88 ± 1.65 g L−1. Interestingly, cell growth ceased after this point, lactate production continued, reaching a final concentration of 64.61 ± 1.65 g L−1 by 188 h, with a lactate yield of 0.99 ± 0.03 g g−1 sucrose consumed. Additionally, from the initial 65 g L−1 of sucrose, the release of glucose and fructose during hydrolysis resulted in peak concentrations of only 1.97 g L−1 and 1.61 g L−1, respectively, before these sugars were consumed. By 197 h, the residual sucrose concentration was 3.98 g L−1. Fermentations conducted with 10 g L−1 sucrose revealed no significant differences in maximum biomass (~1.5 g L−1), indicating that the MG pathway does not enhance cell growth. Instead, it efficiently redirects excess carbon into lactic acid, acting as a key detoxification mechanism. These results demonstrate the efficiency of the pAIDA-sacA system and the MG bypass for enabling lactic acid production in E. coli strains that naturally lack the ability to utilise sucrose. The strain maintained high lactic acid yields while efficiently utilising sucrose, a cost-effective and abundant carbon source.
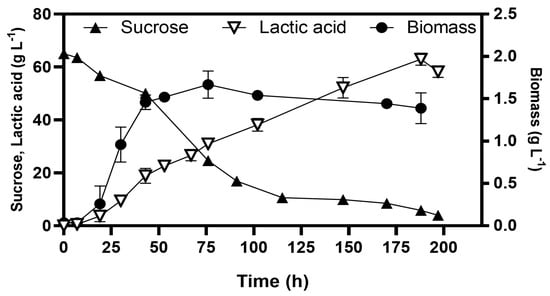
Figure 6.
Batch fermentation for lactic acid production from sucrose at 65 g L−1 via the methylglyoxal bypass in E. coli WDHFAKEV/pAIDA-sacA.
4. Discussion
Previously, expressing SacA in E. coli was challenging because the enzyme often remained inactive in the cytoplasm due to the lack of a functional sucrose-PTS system, resulting in the inability to support bacterial growth with sucrose as the sole carbon source. This limitation arises from the negligible uptake of sucrose by the cells [8,9]. The AIDA-SacA system overcomes this obstacle by immobilising sucrase A to the outer membrane, enabling the extracellular hydrolysis of sucrose. To the best of our knowledge, this is the first study to successfully express SacA in E. coli. This strategy is also adaptable to other industrially relevant processes, particularly for enabling sucrose metabolism in strains that naturally cannot utilise this sugar.
Strategies to utilise alternative carbon sources often vary due to changes in the distribution of metabolic fluxes [29]. Our findings underscore the critical role of the MG bypass in regulating carbon metabolism when sucrose is used as the primary carbon source. In our strains, the absence of the ldhA gene and the presence of mgsA indicate that lactate production is predominantly driven by the MG bypass rather than direct pyruvate reduction. Despite efforts to promote the co-production of ethanol and hydrogen, sucrose metabolism predominantly favoured lactate production. This contrasts with other carbon sources, such as glucose, xylose, or cheese whey, which enable the dual production of these biofuels [5,13,14]. Previous studies observed the emergence of the MG pathway in a ΔtpiA strain, which encodes triose phosphate isomerase—a key enzyme responsible for converting DHAP into glyceraldehyde 3-phosphate (GAP) during growth on glucose, splitting carbohydrate metabolism between EMP glycolysis from GAP and MG metabolism from DHAP. This demonstrates the metabolic flexibility of the MG pathway, which can serve as an alternative route for carbon flux distribution under specific conditions [25]. The MG bypass has also been studied in the context of glycerol metabolism, as glycerol can be directly converted into DHAP [30]. In strains lacking the tpiA gene, carbon assimilation relies almost exclusively on the MG bypass, described as a complete glycolytic bypass [31]. However, sucrose metabolism in E. coli is less understood due to the bacterium’s natural inability to utilise this sugar. Engineered strains have demonstrated activation of the MG pathway under oxygen-limiting conditions, a response linked to the higher levels of phosphorylated sugars observed with sucrose compared to glucose, as previously reported [21,32,33]. Our findings reveal that sucrose, like glycerol, can redirect the entire metabolic flux through the MG bypass, effectively replacing glycolysis without requiring mgsA overexpression or adaptive evolution, despite the high reactivity of its namesake intermediate. These observations highlight the flexible repurposing of metabolic pathways, enabling the creation of new metabolite links and the rewiring of central metabolism.
The emergence of the MG bypass appears to be a metabolic response to the accumulation of sugar phosphates, such as DHAP and GAP, during sucrose breakdown. The catabolism of sucrose through a hydrolase or phosphorylase leads to intracellular concentrations of fructose and glucose (or glucose-1-phosphate) that exceed those observed during glucose catabolism [8,15,21] doubling the formation of DHAP and GAP (Figure 3). This elevated production of sugar phosphates, particularly DHAP, serves as an allosteric activator of methylglyoxal synthase, encoded by the mgsA gene, driving the production of methylglyoxal, a highly toxic metabolite that necessitates effective detoxification mechanisms [28]. In E. coli, the MG bypass pathway mitigates MG toxicity via the glutathione-dependent glyoxalase system. Glyoxalase I converts MG and reduced glutathione into S-D-lactoylglutathione, which glyoxalase II subsequently hydrolyses to yield D-lactic acid while regenerating reduced glutathione. The resulting lactate can either be oxidised to pyruvate for respiration or excreted [24]. The MG bypass represents a low-energy alternative to the Embden–Meyerhof–Parnas (EMP) glycolytic pathway. Unlike EMP, the MG bypass consumes ATP during lactate production rather than generating it, emphasising its role as a survival mechanism rather than an energy-efficient pathway [24]. This mechanism explains why lactate production continues even after cell growth has stopped. A related process occurs during sudden shifts from low to high nutrient concentrations; under these conditions, the cells undergo an abrupt metabolic shift that leads to a rapid influx of carbon into central metabolic pathways, overwhelming glycolysis. The glycolytic pathway cannot efficiently manage the increased substrate availability, resulting in the accumulation of phosphorylated intermediates. Under normal conditions, methylglyoxal synthase is inhibited by free phosphate. However, the excessive accumulation of DHAP overrides this inhibition, triggering the activation of the bypass. As a result, excess DHAP is converted into methylglyoxal, which is detoxified through the glyoxalase system to produce D-lactate. While lactate continues to be produced under these circumstances, cellular proliferation does not occur, as the bypass primarily serves as a stress response mechanism to stabilise metabolic fluxes and prevent toxicity, rather than to support biomass synthesis [27,34,35].
A similar situation involving DHAP accumulation arises during the co-metabolism of sugars [36]. This has been observed when regulation of carbon source utilisation has been disrupted either by mutations or by manipulation of the cAMP concentration in the environment [37,38]. The release of catabolic repression increases carbon influx into the cell, leading to the accumulation of glycolytic intermediates such as DHAP and GAP. This accumulation activates methylglyoxal synthase, which converts DHAP into methylglyoxal. The MG bypass plays a critical role under these conditions, facilitating phosphate recycling and preventing excessive accumulation of sugar phosphates, as evidenced in mgsA null mutants [27]. The deletion of the mgsA gene has been shown to relieve catabolite repression, enabling the simultaneous metabolism of a complex mixture of five major sugars found in cellulosic biomass (glucose, xylose, arabinose, galactose, and mannose), while significantly reducing lactate and methylglyoxal levels [26]. In contrast, in our study on sucrose metabolism, no diauxic growth or fructose accumulation was observed. Instead, glucose and fructose were consumed simultaneously, with lactate being the sole detectable product. These findings suggest that the mgsA gene and the methylglyoxal pathway are critical for the controlled expression of sugar-specific transporters and other catabolic genes in native E. coli strains. Although methylglyoxal synthase and methylglyoxal are not typically linked to the carbon catabolite repression system, our results suggest they play a direct role in regulating mixed sugar metabolism in E. coli. This highlights the potential of targeting the methylglyoxal pathway to optimise sugar co-metabolism and enhance the efficiency of engineered microbial systems.
The bypass of methylglyoxal detoxification to lactic acid, driven by sucrose metabolism, offers a simpler and more streamlined route for lactic acid production compared to pyruvate reduction via lactate dehydrogenase. Additionally, the MG bypass holds potential for bioplastic production, providing a sustainable solution for value-added bioproducts [39,40]. To our knowledge, only [41] has explored this approach by introducing methylglyoxal synthase, a lactate/H⁺ symporter, and glyoxalase from E. coli into metabolically engineered cyanobacteria. This highlights its promise for enhancing biodegradable polymer production in industrial applications.
5. Conclusions
E. coli strains transformed with the pAIDA-sacA plasmid exhibit significant saccharolytic activity, enabling effective sucrose utilisation and playing a crucial role in regulating metabolic pathways in E. coli. This highlights the importance of selecting appropriate carbon sources for metabolic engineering. Activation of the methylglyoxal bypass enhances lactic acid production, while deletion of the mgsA gene redirects metabolic flux towards ethanol production. The pAIDA-sacA plasmid can also be introduced into other E. coli strains, providing the capability to confer saccharolytic activity. Overall, this research advances microbial biotechnology and opens promising avenues for developing specialised whole-cell biocatalysts that utilise sucrose for industrial applications. Although the engineered strains are efficient lactate producers, their ethanol production remains limited; therefore, further genetic modifications will be required to improve their performance for biofuel applications.
Author Contributions
Conceptualisation, J.S.-A. and A.D.L.-R.; Writing—Original Draft Preparation, J.S.-A.; Methodology, J.S.-A. and V.E.B.-H.; Writing—Review and Editing A.P.B.d.l.R., A.D.L.-R., and V.E.B.-H.; Resources, A.P.B.d.l.R. and A.D.L.-R.; Data Curation, J.S.-A. and V.E.B.-H.; Project Administration, A.D.L.-R.; Supervision, A.D.L.-R.; Funding Acquisition, A.P.B.d.l.R. and A.D.L.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Partial financial support from CONACyT-Basicas Grant 281700.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Acknowledgments
Jorge Sanchez thanks CONAHCyT for his fellowship 772331. We thank Luis M. Rosales-Colunga for his comments and Lucy R. McKenna for the English revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Li, K.; Huang, F.; Wang, J.; Zhao, J.; Zhao, X.; Garza, E.; Manow, R.; Grayburn, S.; Zhou, S. Engineering and adaptive evolution of Escherichia coli W for L-lactic acid fermentation from molasses and corn steep liquor without additional nutrients. Bioresour. Technol. 2013, 148, 394–400. [Google Scholar] [CrossRef]
- Koutinas, A.; Wang, R.; Webb, C. Evaluation of wheat as generic feedstock for chemical production. Ind. Crops Prod. 2004, 20, 75–88. [Google Scholar] [CrossRef]
- Reid, S.J.; Abratt, V.R. Sucrose utilisation in bacteria: Genetic organisation and regulation. Appl. Microbiol. Biotechnol. 2005, 67, 312–321. [Google Scholar] [CrossRef]
- Bruschi, M.; Boyes, S.J.; Sugiarto, H.; Nielsen, L.K.; Vickers, C.E. A transferable sucrose utilization approach for non-sucrose-utilizing Escherichia coli strains. Biotechnol. Adv. 2012, 30, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-García, A.K.; Alvarez-Guzmán, C.L.; De Leon-Rodriguez, A. Autodisplay of alpha amylase from Bacillus megaterium in E. coli for the bioconversion of starch into hydrogen, ethanol and succinic acid. Enzym. Microb. Technol. 2020, 134, 109477. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Hernandez, V.E.B.; De Leon-Rodriguez, A. Scale-up of hydrogen and ethanol co-production by an engineered Escherichia coli. Fuel 2021, 300, 121002. [Google Scholar] [CrossRef]
- Dien, B.S.; Nichols, N.N.; Bothast, R.J. Recombinant Escherichia coli engineered for production of L-lactic acid from hexose and pentose sugars. J. Ind. Microbiol. Biotechnol. 2001, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.; Park, J.H.; Vickers, C.E.; Nielsen, L.K.; Lee, S.Y. Development of sucrose-utilizing Escherichia coli K-12 strain by cloning β-fructofuranosidases and its application for L-threonine production. Appl. Microbiol. Biotechnol. 2010, 88, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Fouet, A.; Klier, A.; Rapoport, G. Cloning and expression in Escherichia coli of the sucrase gene from Bacillus subtilis. Mol. Gen. Genet. 1982, 186, 399–404. [Google Scholar] [CrossRef]
- Maurer, J.; Jose, J.; Meyer, T.F. Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions. J. Bacteriol. 1999, 181, 7014–7020. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.E.B.; Salas-Montantes, C.J.; la Rosa, A.P.B.-D.; De Leon-Rodriguez, A. Autodisplay of an endo-1,4-β-xylanase from Clostridium cellulovorans in Escherichia coli for xylans degradation. Enzym. Microb. Technol. 2021, 149, 109834. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Y. Constructing Escherichia coli co-display systems for biodegradation of polyethylene terephthalate. Bioresour. Bioprocess. 2023, 10, 91. [Google Scholar] [CrossRef]
- Rosales-Colunga, L.M.; Razo-Flores, E.; Ordoñez, L.G.; Alatriste-Mondragón, F.; De León-Rodríguez, A. Hydrogen production by Escherichia coli ΔhycA ΔlacI using cheese whey as substrate. Int. J. Hydrogen Energy 2010, 35, 491–499. [Google Scholar] [CrossRef]
- Balderas-Hernandez, V.E.; Maldonado, K.P.L.; Sánchez, A.; Smoliński, A.; Rodriguez, A.D.L. Improvement of hydrogen production by metabolic engineering of Escherichia coli: Modification on both the PTS system and central carbon metabolism. Int. J. Hydrogen Energy 2020, 45, 5687–5696. [Google Scholar] [CrossRef]
- Thomason, L.C.; Costantino, N.; Court, D.L. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 2007, 79, 1.17.1–1.17.8. [Google Scholar] [CrossRef] [PubMed]
- Thouvenot, B.; Charpentier, B.; Branlant, C. The strong efficiency of the Escherichia coli gapA P1 promoter depends on a complex combination of functional determinants. Biochem. J. 2004, 383 Pt 2, 371–382. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; de León-Rodríguez, A.; Alatriste-Mondragón, F.; Razo-Flores, E. The buffer composition impacts the hydrogen production and the microbial community composition in non-axenic cultures. Biomass Bioenergy 2011, 35, 3174–3181. [Google Scholar] [CrossRef]
- Förster, A.H.; Gescher, J. Metabolic Engineering of Escherichia coli for Production of Mixed-Acid Fermentation End Products. Front. Bioeng. Biotechnol. 2014, 2, 16. [Google Scholar] [CrossRef]
- El-Mansi, M. Control of central metabolism’s architecture in Escherichia coli: An overview. Microbiol. Res. 2023, 266, 127224. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gu, Y.; Quan, Y.; Gao, W.; Dang, Y.; Cao, M.; Lu, X.; Wang, Y.; Song, C.; Wang, S. Construction of energy-conserving sucrose utilization pathways for improving poly-γ-glutamic acid production in Bacillus amyloliquefaciens. Microb. Cell Fact. 2017, 16, 98. [Google Scholar] [CrossRef]
- Olavarria, K.; Fina, A.; Velasco, M.I.; van Loosdrecht, M.C.M.; Wahl, S.A. Metabolism of sucrose in a non-fermentative Escherichia coli under oxygen limitation. Appl. Microbiol. Biotechnol. 2019, 103, 6245–6256. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, H.; Zhang, Y.; Li, Y. Discovery of potential genes contributing to the biosynthesis of short-chain fatty acids and lactate in gut microbiota from systematic investigation in E. coli. Biofilms Microbiomes 2019, 5, 19. [Google Scholar] [CrossRef]
- Zhou, L.; Cui, W.; Liu, Z.; Zhou, Z. Metabolic engineering strategies for D-lactate over production in Escherichia coli. J. Chem. Technol. Biotechnol. 2016, 91, 576–584. [Google Scholar] [CrossRef]
- Cooper, R.A. Metabolism of methylglyoxal in microorganisms. Annu. Rev. Microbiol. 1984, 38, 49–68. [Google Scholar] [CrossRef]
- McCloskey, D.; Xu, S.; Sandberg, T.E.; Brunk, E.; Hefner, Y.; Szubin, R.; Feist, A.M.; Palsson, B.O. Adaptation to the coupling of glycolysis to toxic methylglyoxal production in tpiA deletion strains of Escherichia coli requires synchronized and counterintuitive genetic changes. Metab. Eng. 2018, 48, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yomano, L.P.; York, S.W.; Shanmugam, K.T.; Ingram, L.O. Deletion of methylglyoxal synthase gene (mgsA) increased sugar co-metabolism in ethanol-producing Escherichia coli. Biotechnol. Lett. 2009, 31, 1389–1398. [Google Scholar] [CrossRef]
- Tötemeyer, S.; Booth, N.A.; Nichols, W.W.; Dunbar, B.; Booth, I.R. From famine to feast: The role of methylglyoxal production in Escherichia coli. Mol. Microbiol. 1998, 27, 553–562. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Tötemeyer, S.; MacLean, M.J.; Booth, I.R. Methylglyoxal production in bacteria: Suicide or survival? Arch. Microbiol. 1998, 170, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Valdovinos, M.A.; Martínez-Antonio, A. Optimizing. Fermentation Strategies for Enhanced Tryptophan Production in Escherichia coli: Integrating Genetic and Environmental Controls for Industrial Applications. Processes 2024, 12, 2422. [Google Scholar] [CrossRef]
- Booth, I.R. Glycerol and Methylglyoxal Metabolism. EcoSal Plus 2005, 1, 10.1128/ecosalplus.3.4.3. [Google Scholar] [CrossRef] [PubMed]
- Iacometti, C.; Marx, K.; Hönick, M.; Biletskaia, V.; Schulz-Mirbach, H.; Dronsella, B.; Satanowski, A.; Delmas, V.A.; Berger, A.; Dubois, I.; et al. Activating Silent Glycolysis Bypasses in Escherichia coli. BioDes. Res. 2022, 2022, 9859643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, T.; Zhao, J.; Wang, J.; Yan, T.; Xu, L.; Liu, Z.; Garza, E.; Iverson, A.; Manow, R.; et al. Homofermentative production of D-lactic acid from sucrose by a metabolically engineered Escherichia coli. Biotechnol. Lett. 2012, 34, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.P.; de Araújo, E.F.; Silva, D.O.; Guimarães, W.V. Ethanolic fermentation of sucrose, sugarcane juice and molasses by Escherichia coli strain kO11 and klebsiella oxytoca strain p2. Braz. J. Microbiol. 2005, 36, 395–404. [Google Scholar] [CrossRef]
- Weber, J.; Kayser, A.; Rinas, U. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Microbiology 2005, 151 Pt 3, 707–716. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Chacko, A.D.; Lee, C.H.; Booth, I.R. The Activity of the High-Affinity K Uptake System Kdp Sensitizes Cells of Escherichia coli to Methylglyoxal. J. Bacteriol. 1996, 178, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, H.; Cocaign-Bousquet, M.; Lindley, N.D. Simultaneous consumption of glucose and fructose from sugar mixtures during batch growth of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 1997, 47, 600–603. [Google Scholar] [CrossRef]
- Zwaig, N.; Kistler, W.S.; Lin, E.C.C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J. Bacteriol. 1970, 102, 753–759. [Google Scholar] [CrossRef]
- Ackerman, R.S.; Cozzarelli, N.R.; Epstein, W. Accumulation of toxic concentrations of methylglyoxal by wild-type Escherichia coli K-12. J. Bacteriol. 1974, 119, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Zheng, Y.; Li, K.; Zhang, R.; Xu, J.; Wang, Z.; Zhang, Y.; Yin, H.; Li, J. Expression, Characterization, and Immobilization of a Novel D-Lactate Dehydrogenase from Salinispirillum sp. LH 10-3-1. Processes 2024, 12, 1349. [Google Scholar] [CrossRef]
- Ali, S.; Isha; Chang, Y.-C. Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review. Processes 2023, 11, 3445. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Goto, R.; Umetani, Y.; Hanai, T. Construction of a novel d-lactate producing pathway from dihydroxyacetone phosphate of the Calvin cycle in cyanobacterium, Synechococcus elongatus PCC 7942. J. Biosci. Bioeng. 2017, 124, 54–61. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).