1. Introduction
This introduction examines the complex factors affecting drug stability and quality, with emphasis on radiation exposure and physical modifications like tablet crushing. We start with a broad overview of drug stability and then focus on photodegradation and tablet crushing practices. We explore drug-radiation interactions, including reflectance and emissivity properties, and the significance of dyes in pharmaceutical preparations. The introduction concludes by presenting our study’s objectives, which investigate how tablet form modifications influence radiation interaction and overall pharmaceutical quality.
Drug stability directly correlates with therapeutic efficacy and patient safety while also impacting economic and logistical considerations. Among numerous factors affecting drug stability, radiation represents one of the primary concerns. Active pharmaceutical ingredients and excipients that exhibit UV sensitivity may undergo photodegradation, resulting in physicochemical alterations. Light exposure can trigger various processes including dimerization, transformation, and oxidation [
1]. When drugs absorb light, photodegradation may occur, leading to molecular decomposition. This process can result in the formation of free radicals or initiate photosensitization, where energy transfer occurs between molecules. These mechanisms encompass both primary (photochemical) and secondary (chemical) reactions, ultimately yielding final degradation products [
2].
The determination of active pharmaceutical ingredient (API) stability, defined as the state without API degradation, involves various analytical tests. Stability testing protocols recommend examining multiple factors, including humidity, temperature, pH, oxidizing agents, and light exposure [
3,
4]. The introduction of photostability testing as an integral component of stability studies in 1993 enabled the assessment of drug responses to sunlight exposure. Light interaction can trigger not only API structural modifications but also radical processes, energy transfer, and luminescence, potentially leading to unexpected outcomes, particularly in solid-state formulations. Exposure to electromagnetic radiation may result in significant API degradation and the formation of decomposition products [
2,
3,
4].
An example of an active ingredient that changes its structure under the influence of UV radiation is tetracycline. The sensitivity of tetracyclines to UV radiation is, on the one hand, a disadvantage as it reduces the stability of the drug, but, on the other hand, this effect can be used to remove tetracyclines from the environment via UV irradiation [
5].
Tablet crushing is a common practice both in domestic settings and hospital environments, particularly in intensive care units (ICUs). This mechanical modification of dosage forms removes the protective outer layer, potentially exposing the active pharmaceutical ingredient to UV radiation. Such exposure may result in diminished therapeutic efficacy or the formation of undesirable degradation products. For patients with conditions such as dysphagia that impair their ability to swallow intact tablets, crushing represents a common alternative administration method. However, tablet crushing, whether using a mortar and pestle or a tablet crusher, should only be performed when explicitly permitted by the material safety data sheet (MSDS) [
6].
Crushing the tablets leads to an increase in the surface area of the active substance molecules, which can affect their optical properties such as light absorption and scattering. These changes may accelerate photochemical processes, leading to the degradation of the active substance and a reduction in the stability of the drug. Additionally, fragmentation can affect the dissolution rate of the drug, which in turn can alter its bioavailability and therapeutic efficacy [
6].
The degradation of the API can lead to the formation of adducts that may be therapeutically ineffective or pose a risk of toxicity. Additionally, an altered particle size distribution can affect the dissolution rate, affecting the bioavailability of the API and potentially leading to under- or over-dosing [
7].
Electromagnetic radiation encompasses multiple frequency and wavelength ranges, originating from both natural and artificial sources. While the Sun emits radiation across a broad spectral range, the Earth’s surface receives primarily UV, UV-VIS, and IR radiation. The spectral ranges are categorized as follows: UVA (320–400 nm), UVB (290–320 nm), UVC (200–290 nm), and visible radiation (400–700 nm) [
2,
4,
8]. When electromagnetic radiation interacts with an object in space, three distinct phenomena occur: transmission, absorption, and reflection. The relationship between these phenomena can be expressed by the equation:
α represents the absorption coefficient; ρ denotes the reflection coefficient (reflectance); τ indicates the transmissivity (transmittance).
A solar radiation incident on a pill surface undergoes reflection and absorption at varying intensities. The reflectance coefficient serves as a parameter quantifying the proportion of solar light that is absorbed and reflected from the pill’s surface [
9]. The directional reflectance (directional reflectance determines how effectively a surface reflects light in a given direction compared to the incident light) coefficient is specifically defined as the ratio of total energy reflected from the intermediate half-space to the energy incident on the surface from the directions θi and φi (
Figure 1) [
10].
Visible radiation can trigger various reactions, such as oxidation, which decreases the active ingredient content in tablets and alters their properties [
9,
11].
Hemispherical total emittance (ε) is the ratio of the total radiant power emitted by a surface to the radiant power emitted by a black body at the same temperature. It is a dimensionless quantity that takes into account the emission in all directions and for all wavelengths. The hemispherical total emittance value depends on the surface temperature and can vary from 0 to 1, with 1 corresponding to a black body. In the context of solid drug testing, hemispherical total emittance can be of significant importance. It can be used to assess the thermal and optical properties of drug substances and pharmaceutical forms. Measurement of this quantity can provide information on the drug’s ability to absorb and emit thermal radiation, which can affect its thermal stability and storage. In addition, knowledge of hemispherical total emittance can be useful in the design of manufacturing processes, such as drying or tablet coating, where temperature control is crucial for maintaining product quality [
12].
The study of emissivity and reflectance coefficients is crucial for analyzing API degradation under UV radiation exposure. These optical properties directly influence how tablets interact with electromagnetic radiation, determining the amount of energy absorbed and potentially triggering photodegradation processes. By examining these parameters, we can better understand the susceptibility of crushed tablets to UV-induced degradation, as changes in surface area and crystal structure following crushing may significantly alter a drug’s optical characteristics. Furthermore, this analysis provides valuable insights into the potential impact of tablet modifications on drug stability and efficacy, enabling the development of more robust formulations and storage recommendations to maintain pharmaceutical quality.
The impact of drug formulation modification, particularly tablet crushing, on radiation interaction and pharmaceutical quality represents a significant concern in pharmacy. Tablet crushing can substantially alter physicochemical properties of medications, consequently affecting their radiation interaction patterns. This mechanical modification leads to two significant structural and property changes: First, crushing increases the specific surface area of the drug, potentially altering dissolution rates and active ingredient absorption. Second, the crushing process may disrupt the crystal structure of the active pharmaceutical ingredient, affecting its optical properties and radiation interaction characteristics. These alterations in radiation interaction can significantly impact pharmaceutical quality through two primary mechanisms: stability changes, particularly critical for photosensitive drugs, and bioavailability modifications resulting from structural and physicochemical alterations, which are fundamental to pharmaceutical quality. This research into the effects of tablet crushing on radiation interaction, including HDR measurements, provides valuable insights into pharmaceutical quality modifications. Understanding these mechanisms proves crucial for optimizing drug formulations and ensuring both safety and efficacy in pharmaceutical applications.
Organic and synthetic dyes play an important role in pharmaceutical preparations, affecting their esthetics, identification, and stability. Here are some key reasons for their use:
Product identification and differentiation: The color of a tablet makes it easier to identify, which is important for patients and healthcare personnel to avoid confusion. Different colors can indicate different dosages or types of medicines.
Masking the natural characteristics of active substances: Some active substances may have an unattractive color or may become discolored over time. Dyes help to hide these changes, improving the esthetics of the product.
Improving patient acceptance: an attractive appearance of a drug can increase patient acceptance, which can affect the regularity of use and the efficacy of therapy.
Light protection: some dyes can play a protective role by blocking harmful UV radiation that could lead to the degradation of active ingredients.
The choice between organic and synthetic dyes depends on a number of factors, such as cost, stability, safety, and the desired esthetic effect. Synthetic dyes often offer greater stability and color intensity, while natural dyes may be preferred due to their origin and potentially lower risk of allergies [
13,
14].
Tablet crushing, a common practice in healthcare settings, potentially exposes the active pharmaceutical ingredient to increased UV radiation by removing protective coatings and altering the drug’s physical structure. This study aims to investigate how these modifications affect the tablet’s interaction with radiation, particularly through changes in reflectance and emissivity properties. By examining these relationships, we seek to better understand the impact of crushing on drug stability and efficacy when exposed to UV light, ultimately contributing to improved pharmaceutical formulations and administration practices.
This study was undertaken to demonstrate that the physical form modification of tablets (through crushing) significantly impacts their interaction with radiation, consequently affecting their pharmaceutical quality.
The primary objective was to investigate the influence of tablet form and color on reflectance and emissivity properties. This investigation is particularly relevant as drug stability testing is crucial for ensuring pharmaceutical safety, efficacy, and quality throughout the storage period.
2. Materials and Methods
2.1. Test Samples
The reflectance and emissivity measurements were conducted on tablets in both intact and crushed forms, as illustrated in
Figure 2, which shows exemplary tablets in their intact and crushed forms.
The study included two groups of tablets:
White tablets (n = 15): anapran, calcium dobesilate, circadin, cyclonamine, debretin, deflegmin, diured, flawamed, metformax, metronidazole, nilogrin, polfenon, scopolan, siofor, and trittico.
Yellow-orange tablets (n = 10): aspargin, eplenocard, furaginum, moilec, neo-pancreatinum, nifuroksazyd, nospa, ozzion, ranloc, and rolpryna.
Their shapes are round and oblong, with sizes ranging from 8 mm to 15 mm in diameter for round tablets and 10 mm to 18 mm in length for oblong ones. The weight of the tablets varies between 200 mg and 800 mg, depending on the formulation. This ensures better clarity and context for the study.
For each measurement, three tablets were placed adjacent to each other on a plate, with measurements taken before and after crushing in a mortar.
White and yellow-orange tablets were used in this study, as these are some of the most commonly used colors in pharmaceutical formulations. White tablets are chosen for their neutrality and lack of effect on light-sensitive active ingredients, while yellow and orange colors are popular for their high visibility and ability to mask discoloration caused by degradation. This choice allows for results applicable to a wide range of practical situations.
The study was conducted under controlled conditions of room temperature (approximately 20–25 °C) and normal ambient humidity levels (40–60%), which represent typical environmental conditions encountered during the handling and storage of pharmaceuticals.
The test portion for reflectance and emissivity measurements was prepared to completely cover the measurement areas of both the reflectometer and the emissometer, ensuring a uniform surface without gaps or irregularities.
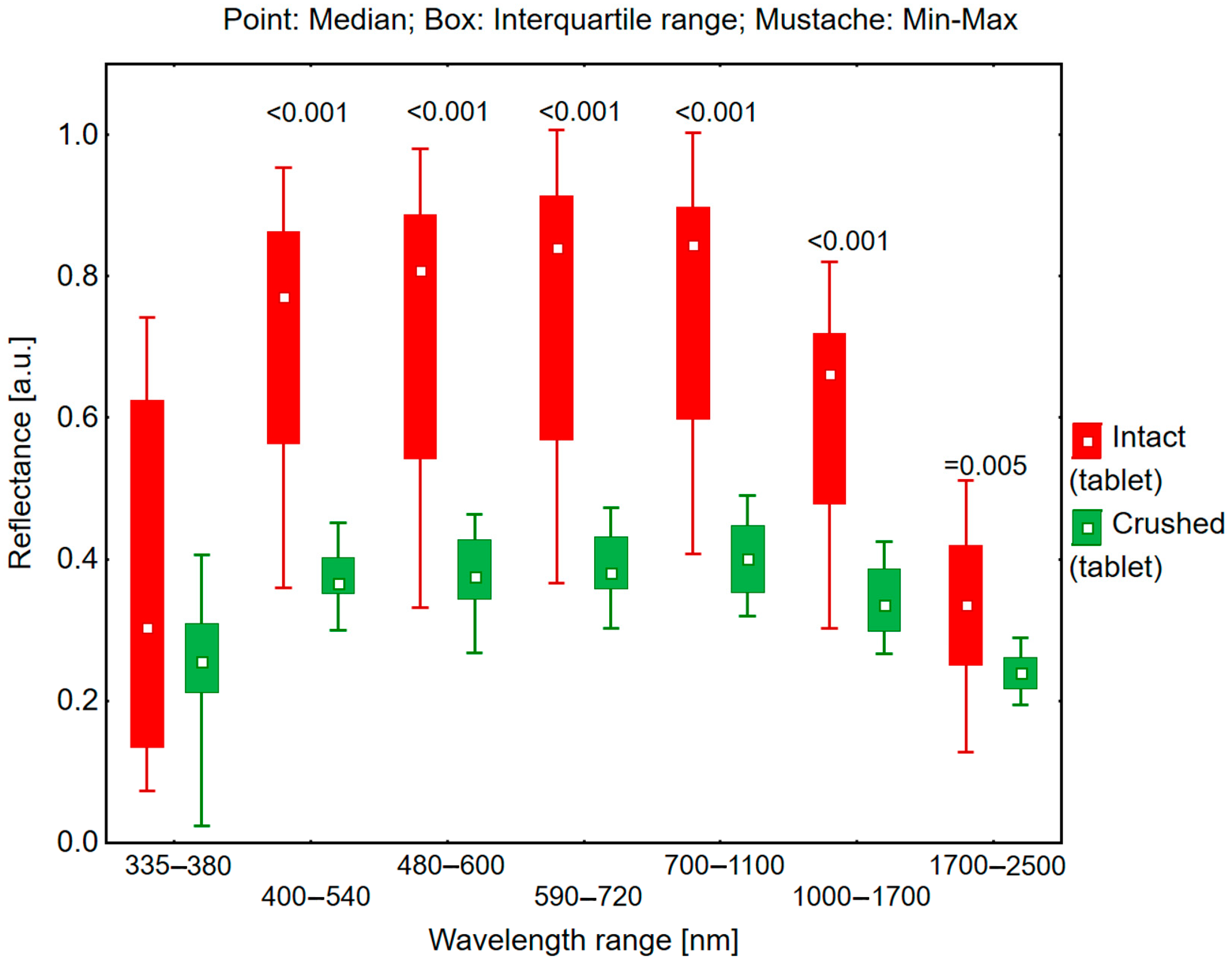
2.2. Reflectance Measurements in the 335–2500 nm Range
The reflectance measurements were performed using a Solar 410 directional reflectometer (Surface Optics Corporation, San Diego, CA, USA) to determine the total solar reflectance from both intact and crushed tablets of white and yellow-orange coloration.
Prior to the measurements, the Solar 410 reflectometer was calibrated using two standard calibration coupons. The calibration was conducted prior to the testing of intact tablets and then repeated before measuring the crushed tablet samples. The measurements were conducted at a 20° incident angle across seven wavelength bands: 335–380 nm, 400–540 nm, 480–600 nm, 590–720 nm, 700–1100 nm, 1000–1700 nm, and 1700–2500 nm. The reflectometer enables measurements in 7 wavelength bands.
The measurements were performed sequentially on intact tablets of both colors, followed by their crushed forms.
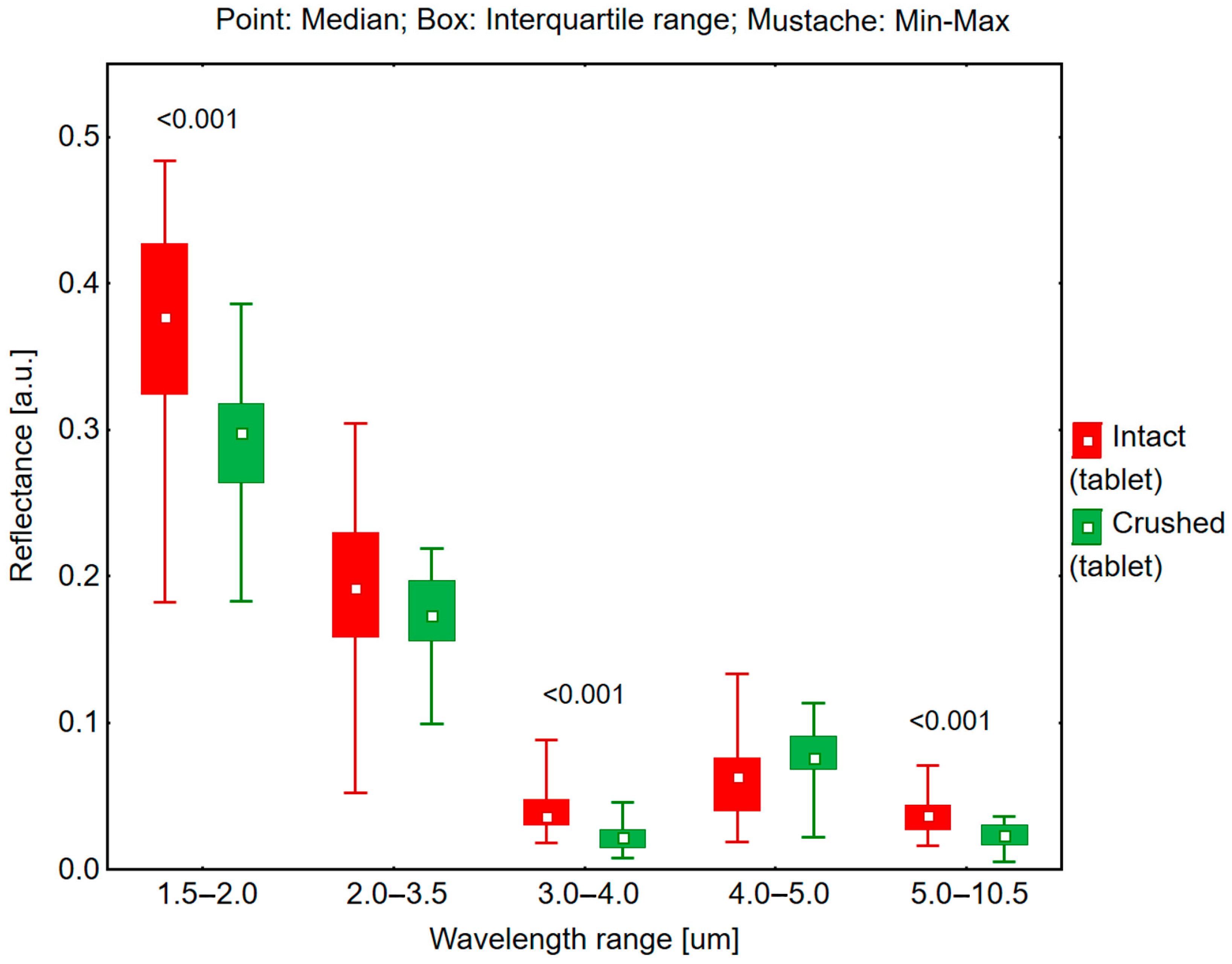
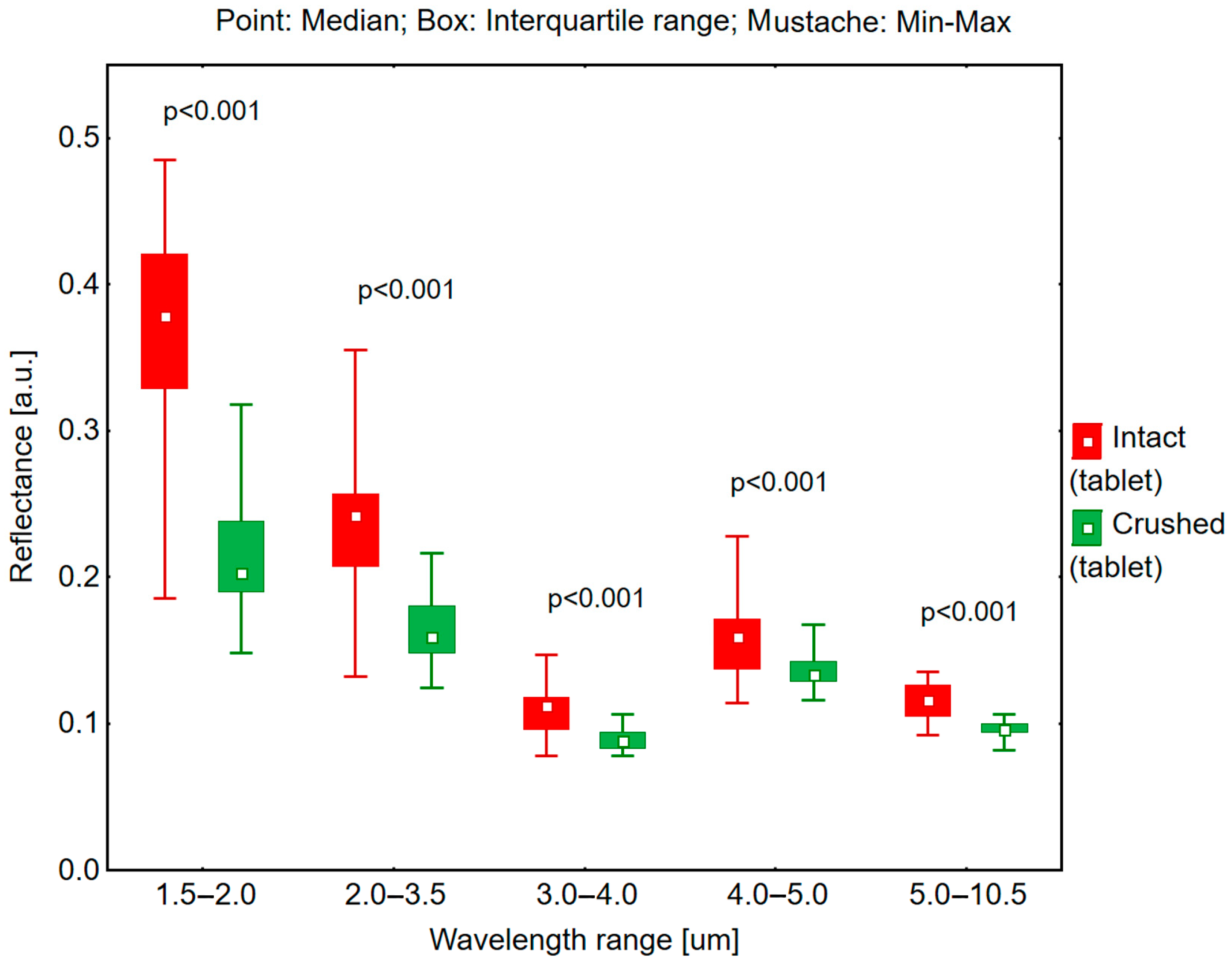
2.3. Reflectance Measurements in the 1.5–10.5 μm Range and Hemispherical Total Emittance
Reflectance and emissivity measurements were conducted using an ET100 emissiometer (Surface Optics Corporation, San Diego, CA, USA) to determine the reflectance and emittance of both intact and crushed tablets in white and yellow-orange colors.
The ET100 emissiometer was calibrated with a standard calibration coupon prior to measurements. The calibration process was carried out before testing intact white tablets and repeated prior to measuring crushed white tablets. Similarly, a separate calibration was performed before testing intact colored tablets and again before measuring the crushed colored tablets. The measurements were performed at incident angles of 20° and 60° across spectral ranges of 1.5–2.0 μm, 2.0–3.5 μm, 3.0–4.0 μm, 4.0–5.0 μm, and 5.0–10.5 μm. The measurements were first conducted on white tablets (intact and crushed), followed by yellow-orange tablets in the same sequence. All measurements were performed in triplicate under controlled conditions at an ambient temperature of 20 °C.
The spectral range chosen for this study, spanning from 335 nm to 10.5 μm, provides a comprehensive analysis of tablet interactions with electromagnetic radiation across ultraviolet, visible, and infrared regions. This broad range is crucial for several reasons:
The 335–2500 nm range covers UV, visible, and near-infrared regions, which are particularly relevant for photodegradation processes and interactions with ambient light. The 1.5–10.5 μm range extends into the mid-infrared region, allowing for the assessment of thermal properties and emissivity, which are important for understanding heat-related stability issues. By including both UV-VIS-NIR and infrared ranges, this study captures a full spectrum of potential radiation interactions that tablets may encounter during storage, handling, and administration. This comprehensive approach enables a thorough evaluation of how tablet crushing affects optical and thermal properties across a wide range of electromagnetic radiation, providing valuable insights into potential changes in drug stability and efficacy.
2.4. Statistical Analysis
Reflectance and emissivity measurements were compiled in Microsoft Excel and analyzed using the Statistica 13 software. The Shapiro–Wilk test was employed to assess data distribution normality. Due to non-normal distribution patterns, comparisons between intact and crushed tablets were conducted using the Wilcoxon signed-rank test. Statistical significance was established at p < 0.05.
4. Discussion
4.1. Reflectance Properties of Intact and Crushed Tablets
The European Pharmacopoeia provides guidelines for testing divided tablets but lacks specific protocols for crushed tablets. For divided tablets, mass loss must not exceed 3%, and the divided portions must maintain uniform composition and demonstrate disintegration times comparable to intact tablets [
15].
Research conducted at the Medical University of Silesia, examining the interaction between human serum albumin and ibuprofen stored at 25 °C and 45 °C, demonstrated that storage conditions significantly impact the binding properties of human serum albumin. The study concluded that adherence to manufacturer-recommended storage temperatures is crucial for ensuring therapeutic efficacy [
16].
Drug packaging systems are tailored to specific dosage forms, with liquid formulations requiring particular attention due to their increased potential for package interactions. Light protection is achieved through the use of opaque containers or amber glass bottles, which selectively filter light, permitting transmissions only above 470 nm [
2]. The extent of degradation correlates with both light intensity and the spectral distribution of the source. Research has demonstrated successful drug stabilization through spectral overlap using various dyes and excipients, as evidenced in studies of furosemide, haloperidol, and dihydroergotamine [
1]. Incorporating excipients with absorption spectra matching those of active pharmaceutical ingredients enables light absorption by the excipients, thereby protecting the active substance.
4.2. Emissivity Characteristics and Their Implications
Tablet coating with light-absorbing or light-reflecting agents represents one method of photostabilization, with protective efficacy dependent on coating thickness and absorbing agent concentration. When such coated tablets are crushed, the protective function is compromised, resulting in increased radiation absorption by the tablet [
2].
The current study, utilizing the ET100 emissiometer and the SOLAR 410 reflectometer, enabled the precise quantification of reflectance and emissivity in tablets of varying colors in both intact and crushed forms. The results clearly demonstrated that both tablet color and physical form significantly influence these parameters. The research objective was successfully achieved, confirming that tablet form and color affect reflectance and emissivity properties.
A key finding demonstrates that both white and yellow-orange tablets exhibit higher reflectance in their intact form compared to their crushed state. This phenomenon can be attributed to surface structure differences: intact tablets present a smooth surface conducive to radiation reflection, whereas crushed tablets, due to their irregular surface and increased porosity, promote greater light scattering, resulting in reduced reflectance. This distinction was particularly evident in the color-dependent analysis, where white tablets consistently demonstrated higher reflectance values than yellow-orange tablets in both intact and crushed forms.
4.3. Color-Dependent Effects on Optical Properties
The correlation between tablet color and its reflectance and emissivity properties reveals that lighter colored tablets, particularly white ones, demonstrate enhanced radiation reflection capacity beyond the visible spectrum. This phenomenon may be attributed to the presence of additives such as titanium dioxide, which effectively reflects radiation across a much broader spectral range than visible light alone.
Titanium dioxide is a semiconductor that can initiate photochemical reactions in the presence of UV light:
Photocatalysis: Under UV light, TiO2 absorbs energy, which leads to the excitation of electrons from the valence band to the conduction band. Electron-hole pairs are thus formed (e− i h+).
Generation of radicals: in the presence of water and oxygen, reactive oxygen species (ROS) are generated, such as hydroxyl radical (OH•) and superoxide (O2−), which can initiate the chemical degradation of other substances.
Potential degradation of active substances: If the active substances in a drug are susceptible to oxidation, the resulting ROS may accelerate their degradation. This may result in a reduction in the efficacy of the drug or the formation of undesirable by-products.
Temperature effects on tablets represent a critical factor in maintaining drug substance stability and pharmacological properties. Higher reflectance corresponds to reduced radiation absorption and increased reflection, highlighting the significance of tablet form. Similarly, higher emissivity correlates with more rapid radiation absorption. Intact tablets demonstrate superior reflectance and lower emissivity compared to their crushed counterparts, clearly indicating that the intact form offers optimal pharmacological benefits for patients. Consequently, surface smoothness can be considered a criterion for optimizing drug form stability: smoother surfaces correspond to increased reflectance and decreased emissivity, resulting in enhanced stability of the drug form.
4.4. Practical Implications for Pharmaceutical Applications
Beyond tablet form considerations, future research will investigate the role of packaging materials, specifically examining whether storage containers provide appropriate reflectance and emissivity properties to mitigate UV-induced degradation. Additionally, recommendations for storage guidelines, such as avoiding prolonged exposure to direct sunlight or storing crushed tablets in opaque, UV-resistant containers, have been proposed based on the findings.
Our findings have significant implications for practical pharmaceutical applications, particularly in the realm of drug stability and storage. As demonstrated by Ahmad et al. [
2], light exposure can trigger various processes in pharmaceuticals, including dimerization, transformation, and oxidation. Our results extend this understanding by quantifying the impact of tablet form and color on reflectance and emissivity properties. For instance, we found that intact white tablets consistently showed higher reflectance across all wavelength ranges compared to crushed tablets or yellow-orange tablets. This suggests that maintaining the integrity of tablet coatings is crucial for minimizing radiation absorption and potential photodegradation. Building on the work of Jamrógiewicz et al. [
1,
3,
4], who highlighted the importance of light protection in pharmaceutical preparations, our study provides quantitative data to support the development of more effective protective strategies. For example, our findings could inform the optimization of tablet coating processes to enhance surface smoothness and reflectance properties, particularly for medications likely to be exposed to sunlight. Furthermore, in line with the observations of Meisner et al. [
6] on the risks associated with tablet modification, our results underscore the need for clear guidelines and patient education regarding the potential consequences of crushing tablets, especially for photosensitive drugs. These insights could lead to improved storage recommendations, such as storing crushed tablets in opaque, UV-resistant containers, and emphasize the importance of protecting medications from direct light exposure.
In conclusion, this investigation demonstrated that both tablet color and physical form significantly influence optical properties. The findings have important implications regarding the safety considerations of administering crushed tablets. These insights emphasize the need for careful consideration of formulation design and patient practices, particularly for medications prone to photodegradation.