Production of Elemental Tellurium During Processing of Tellurium-Containing Middling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.3. Characterization
3. Results and Discussion
3.1. Tellurium-Containing Condensate
3.2. Choice of Reducing Agent
3.3. Thermodynamic Justification of the Process
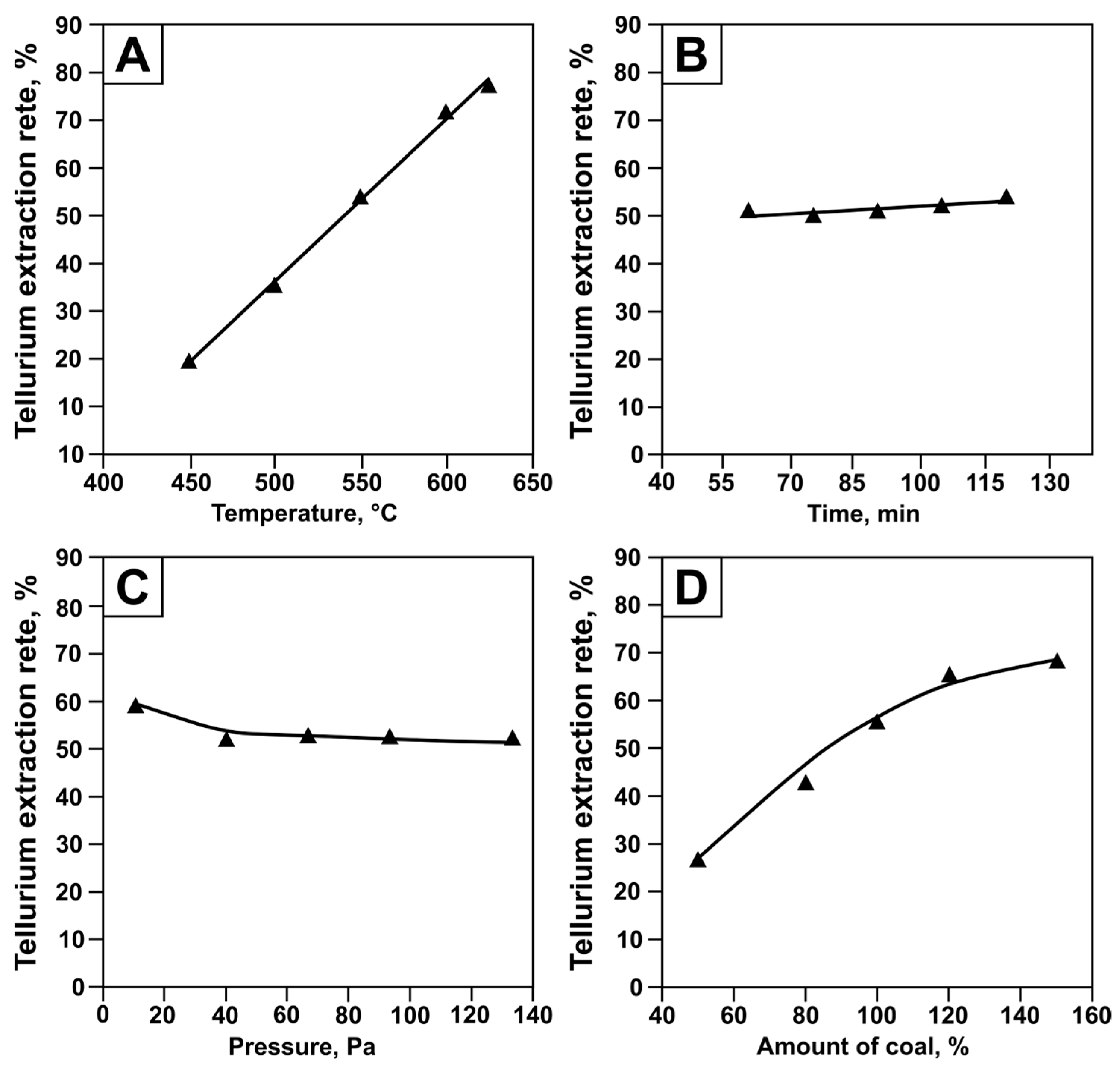
3.4. Experimental Data and Modeling
3.5. Production of Elemental Tellurium and Its Characterization
4. Conclusions
- The tellurium-containing condensate is deposited in four temperature zones during oxidative-distillation roasting. Phases of TeO2 and Te2O3(SO4) are present in the condensate deposited in low-temperature zones (100–350 °C). The condensate contains a single-phase TeO2 in higher-temperature zones (350–600 °C). In the carbothermic reduction process, the presence of Te2O3(SO4) does not cause technological difficulties since it decomposes to TeO2 and SO3 at the process temperatures.
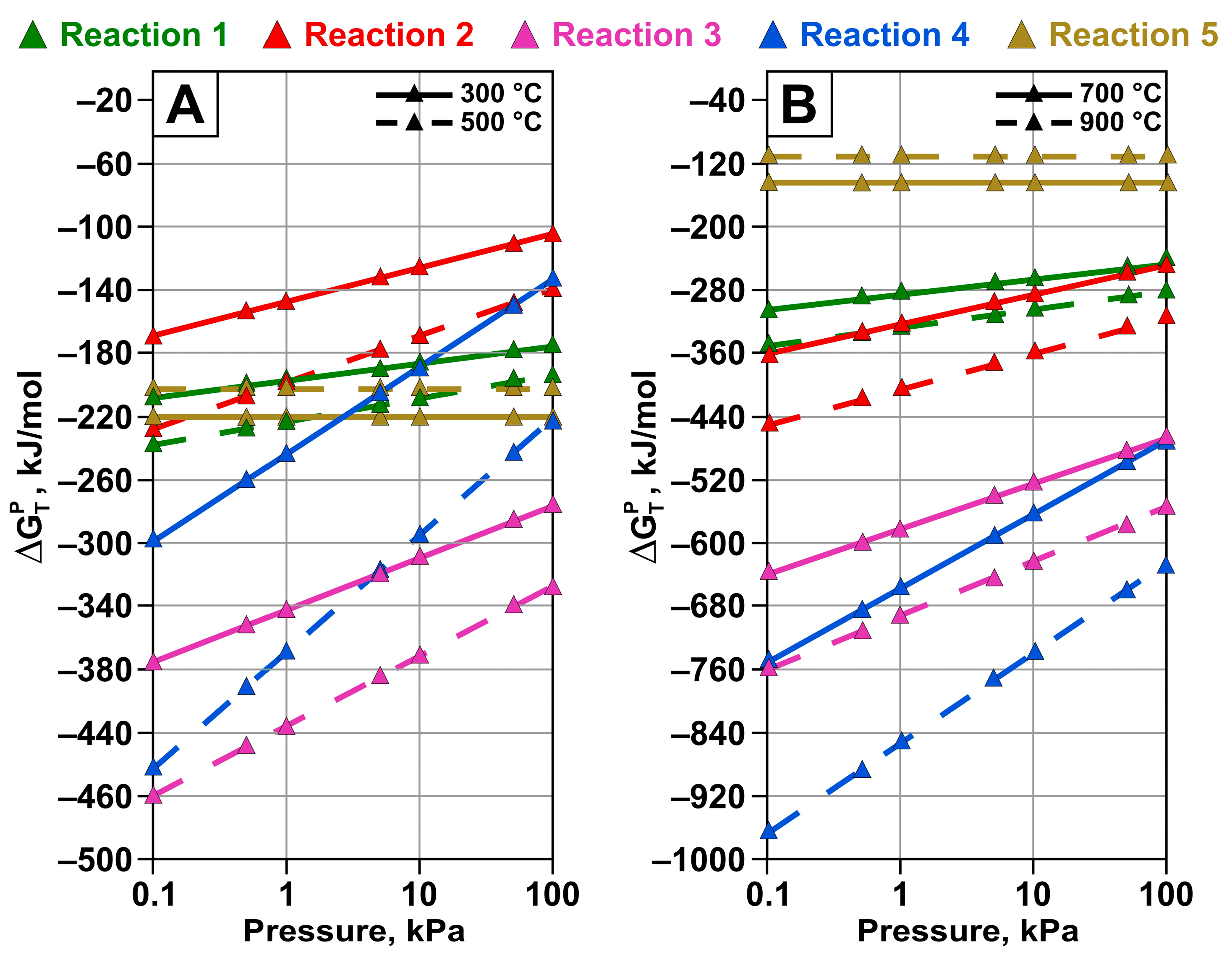
- Thermodynamic analysis demonstrated the feasibility of tellurium reduction reactions from TeO2 with the formation of elemental tellurium in both solid and gas phases at temperatures above 300 °C. Decreasing the pressure increases the possibility of all considered reactions leading to elemental tellurium formation. The reactions involving tellurium evaporation are thermodynamically the most favorable across the entire range of considered pressures and temperatures.
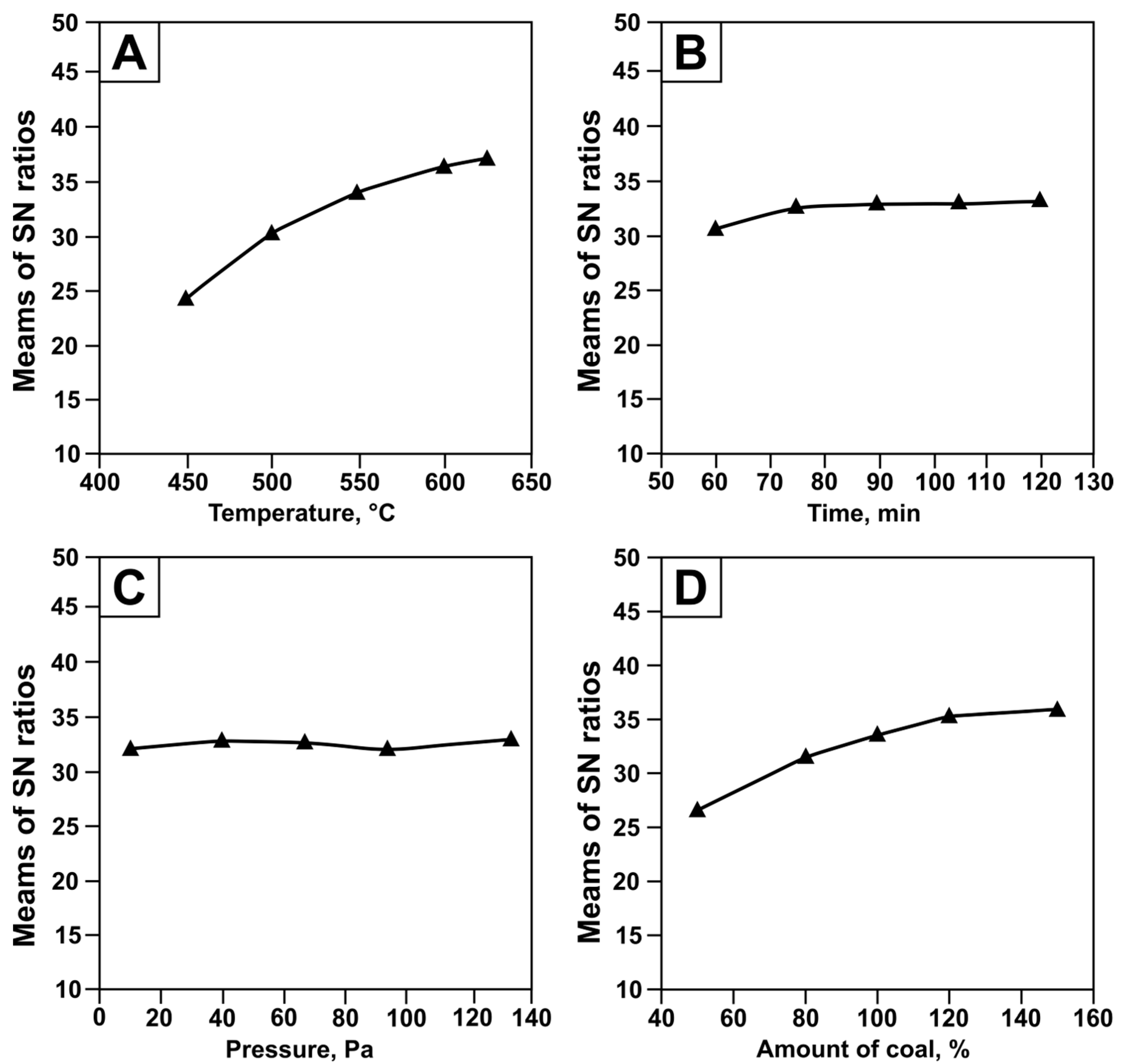
- A regression model and a generalized equation were constructed using mathematical planning methods based on experimental data. This model and equation describe the dependence of tellurium recovery from tellurium-containing condensate on the main process parameters. The calculated errors of the dependencies were 7–9%.
- Parameters for effective tellurium extraction were calculated based on the obtained dependencies. Experimental studies performed under the calculated optimal conditions confirmed the adequacy of the proposed model. The observed tellurium recovery values corresponded to theoretical predictions and exceeded 95%, indicating the reliability of the mathematical planning method and its applicability for process optimization.
- At a temperature of 625 °C, pressure of 66.5 Pa, processing time of 60 min, and addition of 150% charcoal, the tellurium recovery from tellurium-containing condensate reached 98.74%. The condensate contained only the elemental tellurium phase, with no impurity compounds, indicating high selectivity of the process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahmoudi, A.; Shakibania, S.; Mokmeli, M.; Rashchi, F. Tellurium, from copper anode slime to high purity product: A review paper. Metall. Mater. Trans. B 2020, 51, 2555–2575. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y.; Tang, A.; Pan, D.; Li, B. Recovery of scattered and precious metals from copper anode slime by hydrometallurgy: A review. Hydrometallurgy 2020, 197, 105460. [Google Scholar] [CrossRef]
- Mastyugin, S.A.; Naboichenko, S.S. Processing of copper-electrolyte slimes: Evolution of technology. Russ. J. Non-Ferrous Met. 2012, 53, 367–374. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. Leaching of gold and silver from anode slime with a mixture of hydrochloric acid and oxidizing agents. Geosyst. Eng. 2017, 20, 216–223. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.L.; Yu, Y.; Fu, G.Y.; Han, P.W.; Sun, Z.H.I.; Ye, S.F. An environmentally friendly process to selectively recover silver from copper anode slime. J. Clean. Prod. 2018, 187, 708–716. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Liu, B.; Li, B. Integrated process for recycling copper anode slime from electronic waste smelting. J. Clean. Prod. 2017, 165, 48–56. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Trebukhov, S.A.; Volodin, V.N.; Trebukhov, A.A.; Tuleutay, F.H. Selenium extraction out of metallurgical production middlings. Kompleks. Ispol. Miner. 2018, 307, 56–64. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Trebukhov, S.A.; Nitsenko, A.V.; Burabayeva, N.M.; Trebukhov, A.A. Determination of technological parameters of selenium recovery from metallurgical production middlings in a vacuum distillation unit. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 87–98. [Google Scholar]
- Chizhikov, D.M.; Shchastlivyi, V.P. Tellurium and Tellurides; Collet’s Publishers Ltd.: London, UK, 1970. [Google Scholar]
- Hoffman, J.E. Recovering selenium and tellurium from copper refinery slimes. JOM 1989, 41, 33–38. [Google Scholar] [CrossRef]
- Mastyugin, S.A.; Volkova, N.A.; Naboichenko, S.S.; Lastochkina, M.A. Slime from Electrolytic Refining of Copper and Nickel; Ural Federal University: Ekaterinburg, Russia, 2013. [Google Scholar]
- Wang, S. Tellurium, its resourcefulness and recovery. JOM 2011, 63, 90–93. [Google Scholar] [CrossRef]
- Shibasaki, T.; Abe, K.; Takeuchi, H. Recovery of tellurium from decopperizing leach solution of copper refinery slimes by a fixed bed reactor. Hydrometallurgy 1992, 29, 399–412. [Google Scholar] [CrossRef]
- Nitsenko, A.V.; Burabaeva, N.M.; Tuleytay, F.K.; Seisembaev, R.S.; Linnik, X.A.; Azlan, M.N. Study of physical and chemical properties of tellurium–containing middlings. Kompleks. Ispol. Miner. 2020, 315, 49–56. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, Y.; Song, Y.; Zhang, G.; Zhang, F.; Yang, Y.; Hua, Z.; Tian, Y.; You, J.; Zhao, Z. Recycling of copper telluride from copper anode slime processing: Toward efficient recovery of tellurium and copper. Hydrometallurgy 2020, 196, 105436. [Google Scholar] [CrossRef]
- Nitsenko, A.V.; Linnik, K.A.; Tuleutay, F.H.; Burabayeva, N.M.; Seisembayev, R.C. Physical and chemical characterization of tellurium–containing industrial product of Kazakhmys Smelting LLP. Theory Technol. Metall. Prod. 2021, 3, 10–16. [Google Scholar]
- Li, Z.; Qiu, F.; Tian, Q.; Yue, X.; Zhang, T. Production and recovery of tellurium from metallurgical intermediates and electronic waste—A comprehensive review. J. Clean. Prod. 2022, 366, 132796. [Google Scholar] [CrossRef]
- Ha, Y.-C.; Sohn, H.-J.; Jeong, G.-J.; Lee, C.K.; Rhee, K.-I. Electrowinning of tellurium from alkaline leach liquor of cemented Te. J. Appl. Electrochem. 2000, 30, 315–322. [Google Scholar] [CrossRef]
- Rhee, K.I.; Lee, C.K.; Ha, Y.C.; Jeong, G.J.; Kim, H.S.; Sohn, H.J. Tellurium recovery from cemented tellurium with minimum waste disposal. Hydrometallurgy 1999, 53, 189–201. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, S.; Feng, W.; Yu, T.; Zhang, H.; Cao, H.; Zheng, G. An efficient hydrometallurgical process for extracting tellurium from copper telluride slag. Metall. Mater. Trans. B 2022, 53, 2838–2851. [Google Scholar] [CrossRef]
- Xu, L.; Yao, D.; He, J.; Zhang, X.; Xiong, Y.; Hua, Z.; Tian, Y.; Zhao, Z. An efficient process for recycling of copper telluride residue out of copper anode slime. J. Environ. Chem. Eng. 2022, 10, 107987. [Google Scholar] [CrossRef]
- Nitsenko, A.; Linnik, X.; Volodin, V.; Tuleutay, F.; Burabaeva, N.; Trebukhov, S.; Ruzakhunova, G. Phase transformations and tellurium recovery from technical copper telluride by oxidative-distillate roasting at 0.67 kPa. Metals 2022, 12, 1774. [Google Scholar] [CrossRef]
- Nitsenko, A.V.; Volodin, V.N.; Linnik, X.A.; Tuleutay, F.K.; Burabaeva, N.M. Distillation recovery of tellurium from copper telluride in oxide form. Russ. J. Non-Ferrous Met. 2022, 63, 284–291. [Google Scholar] [CrossRef]
- Koga, N.; Mako, A.; Kimizu, T.; Tanaka, Y. Thermal decomposition of synthetic antlerite prepared by microwave-assisted hydrothermal method. Thermochim. Acta 2008, 467, 11–19. [Google Scholar] [CrossRef]
- Güner, E.K.; Kancan, D.; Naktiyok, J.; Özer, A. Synthesis, characterization, and thermal decomposition kinetics of copper hydroxide sulfate (Cu4(SO4)(OH)6) synthesized by chemical precipitation method. Asia-Pac. J. Chem. Eng. 2020, 16, e2583. [Google Scholar] [CrossRef]
- Ahmed, M.A.K.; Fjellvåg, H.; Kjekshus, A. Synthesis, structure and thermal stability of tellurium oxides and oxide sulfate formed from reactions in refluxing sulfuric acid. J. Chem. Soc. Dalton Trans. 2000, 24, 4542–4549. [Google Scholar] [CrossRef]
- Khasraw, D.; Spooner, S.; Hage, H.; Meijer, K.; Li, Z. Devolatilisation characteristics of coal and biomass with respect to temperature and heating rate for HIsarna alternative ironmaking process. Fuel 2021, 284, 119101. [Google Scholar] [CrossRef]
- Wang, T.; Mirgaux, O.; Patisson, F. Cleaner steelmaking using biomass: A novel ironmaking process. Chem. Eng. Sci. 2026, 319, 122319. [Google Scholar] [CrossRef]
- Galvão, M.L.; Batista, A.S.; Nobre, J.R.C.; Balboni, B.M.; Santos, I.S.; Fernandes, M.E.B. Chemical, physical, and mechanical wood properties of Rhizophora mangle L., Avicennia germinans (L.) L., and Laguncularia racemosa (L.) C.F. Gaertn. on the Brazilian Amazon coast. Ann. For. Sci. 2025, 82, 13. [Google Scholar] [CrossRef]
- Han, Y.; Bernasowski, M.; Ślęzak, M.; Migas, P.; Mohanty, S.; Cheisson, T.; Tangstad, M. A Sustainable Way to Produce Mn-Sinter with Charcoal. J. Sustain. Metall. 2025, 11, 1922–1936. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Du, C.; Han, Y.; Yang, J. High-temperature graphitization of coke and lithium storage properties of coke-based graphite. Int. J. Coal Prep. Util. 2023, 44, 19–36. [Google Scholar] [CrossRef]
- Ning, X.; Liang, W.; Zhang, J.; Wang, G.; Li, Y.; Jiang, C. Effect of ash on coal structure and combustibility. Int. J. Miner. Metall. Mater. 2019, 26, 973–982. [Google Scholar] [CrossRef]
- Graphite Properties and Characteristics. Available online: https://poco.entegris.com/content/dam/poco/resources/reference-materials/brochures/brochure-graphite-properties-and-characteristics-11043.pdf (accessed on 17 August 2025).
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Our Specialty Graphites for the Semiconductor Industry. Available online: https://www.sglcarbon.com/pdf/SGL-Brochure-Specialty-Graphites-for-the-Semiconductor-Industry-EN.pdf (accessed on 17 August 2025).
- Outotec Research Oy. HSC Chemistry for Windows, version 5.1; Outotec Research Oy: Pori, Finland, 2002. [Google Scholar]
- Nesmeyanov, A.N. Vapor Pressure of Chemical Elements; Akad. Nauk SSSR: Moscow, Russia, 1961. [Google Scholar]
- Chemistry and Technology of Rare and Scattered Elements, 2nd ed.; Bolshakov, K.A., Ed.; Higher School: Moscow, Russia, 1976; Volume 3. [Google Scholar]
- Malyshev, V.P. Mathematical Planning of Metallurgical and Chemical Experiments; Alma-Ata: Nauka, Russia, 1977. [Google Scholar]
- Protodyakonov, M.M.; Teder, R.I. Metdology of Rational Planning of Experiments; Nauka: Moscow, Russia, 1970. [Google Scholar]
- Chung, H.; Friedrich, S.; Qu, M.; Friedrich, B. Hydrogen reduction of tellurium oxide in a rotary kiln, initial approaches for a sustainable process. Crystals 2025, 15, 478. [Google Scholar] [CrossRef]



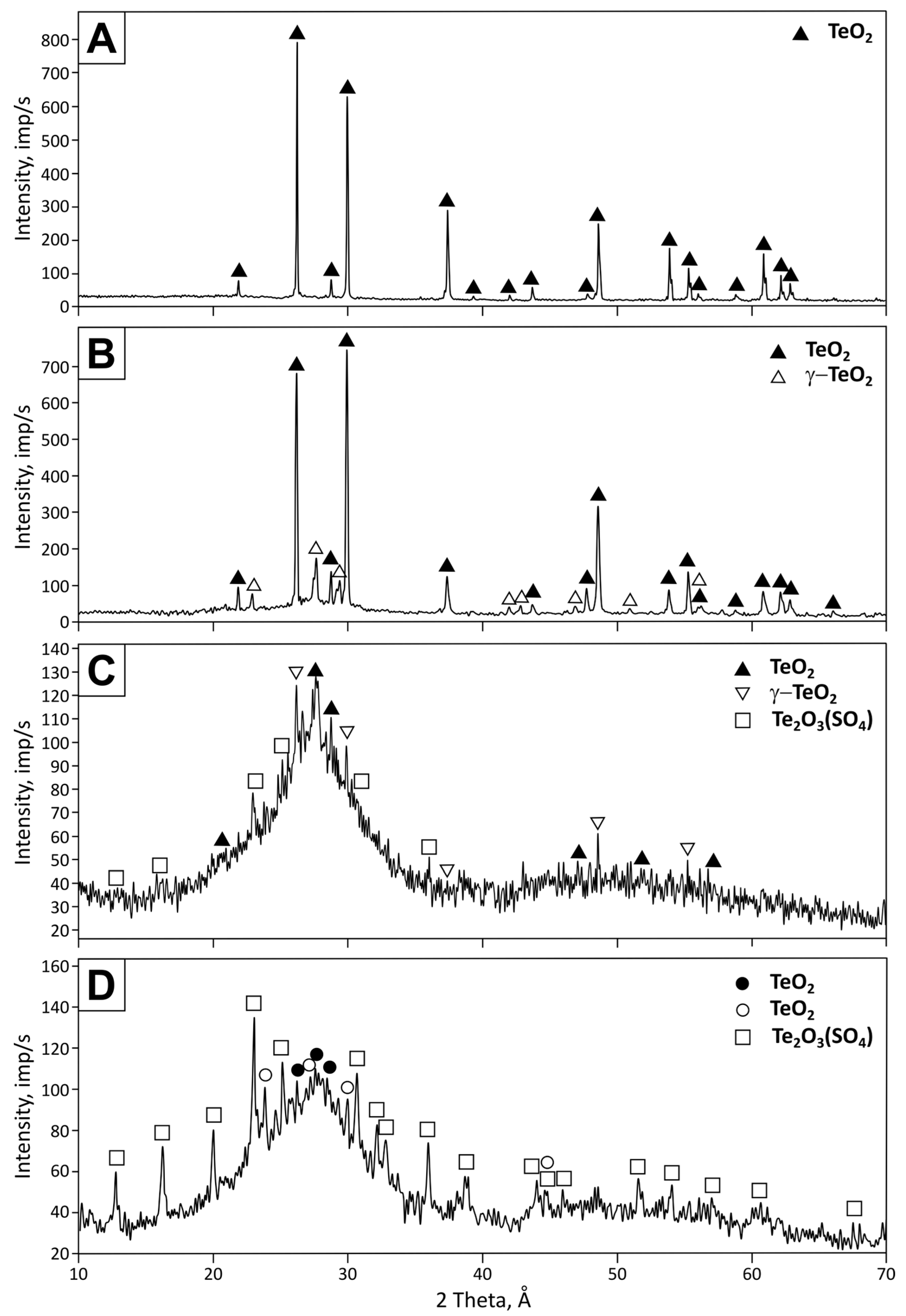





| Phase | Name | PDF Card | Deposition Temperature, °C | |||
|---|---|---|---|---|---|---|
| 600–450 | 450–350 | 350–250 | 250–100 | |||
| TeO2 | paratellurite | 00-042-1365 | 100 | 89.6 | 38.3 | – |
| γ-TeO2 | tellurium oxide | 00-052-0795 | – | 10.4 | 46.8 | – |
| TeO2 | tellurium oxide | 00-041-0945 | – | – | – | 46.1 |
| TeO2 | tellurite | 01-074-1131 | – | – | – | 37.4 |
| Te2O3(SO4) | dittellurium trioxide sulfate | 01-070-0135 | – | – | 14.9 | 16.5 |
| No. | Possible Reactions | Temperature, K/°C | ||||||
|---|---|---|---|---|---|---|---|---|
| 573/ 300 | 673/ 400 | 773/ 500 | 873/ 600 | 973/ 700 | 1073/800 | 1173/900 | ||
| ΔGT, kJ | ||||||||
| 1 | TeO2 + C = Te + CO2 (g) | −174.59 | −192.26 | −210.92 | −230.69 | −250.36 | −267.90 | −284.09 |
| 2 | TeO2 + 2C = Te + 2CO (g) | −103.66 | −139.20 | −175.66 | −213.13 | −250.40 | −285.45 | −319.06 |
| 3 | 2TeO2 + 2C = Te2 (g) + 2CO2 (g) | −276.61 | −326.96 | −376.59 | −425.63 | −474.13 | −518.16 | −559.29 |
| 4 | 2TeO2 + 4C = Te2 (g) + 4CO (g) | −134.70 | −220.79 | −306.01 | −390.45 | −474.16 | −553.21 | −629.18 |
| 5 | Te + O2 (g) = TeO2 | −220.48 | −203.03 | −184.56 | −164.95 | −145.43 | −128.01 | −111.92 |
| Factors | Levels | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| X1 | Temperature, C | 450 | 500 | 550 | 600 | 625 | |
| X2 | Pressure, Pa | 10.64 | 39.9 | 66.5 | 93.1 | 133 | |
| X3 | Time, min | 60 | 75 | 90 | 105 | 120 | |
| X4 | Coal consumption | % | 50 | 80 | 100 | 120 | 150 |
| g | 0.075 | 0.12 | 0.15 | 0.18 | 0.22 | ||
| Experimental Conditions | Tellurium Extraction Rate, % | SN Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| t, °C | P, Pa | τ, min | C, % | α1 | α2 | α3 | αav | |
| 450 | 10.64 | 60 | 50 | 5.33 | 5.49 | 5.03 | 5.28 | 14.44 |
| 450 | 66.5 | 90 | 100 | 18.86 | 18.75 | 18.91 | 18.84 | 25.50 |
| 450 | 39.9 | 75 | 80 | 18.87 | 15.31 | 16.43 | 16.87 | 24.45 |
| 450 | 133 | 120 | 150 | 31.25 | 30.97 | 30.78 | 31.00 | 29.83 |
| 450 | 93.1 | 105 | 120 | 24.12 | 24.96 | 23.20 | 24.09 | 27.63 |
| 550 | 10.64 | 90 | 80 | 50.82 | 51.40 | 50.74 | 50.99 | 34.15 |
| 550 | 66.5 | 75 | 150 | 69.18 | 69.28 | 69.21 | 69.22 | 36.81 |
| 550 | 39.9 | 120 | 120 | 77.10 | 74.83 | 73.11 | 75.01 | 37.49 |
| 550 | 133 | 105 | 50 | 28.45 | 30.11 | 30.83 | 29.80 | 29.47 |
| 550 | 93.1 | 60 | 100 | 45.27 | 44.19 | 46.43 | 46.30 | 33.31 |
| 500 | 10.64 | 75 | 120 | 44.02 | 43.55 | 44.75 | 44.11 | 32.89 |
| 500 | 66.5 | 120 | 50 | 18.86 | 18.99 | 19.41 | 19.09 | 25.61 |
| 500 | 39.69 | 105 | 100 | 37.74 | 36.89 | 37.51 | 37.38 | 31.45 |
| 500 | 133 | 60 | 80 | 25.16 | 25.16 | 25.47 | 25.26 | 28.05 |
| 500 | 93.1 | 90 | 150 | 50.31 | 50.39 | 50.48 | 50.39 | 34.05 |
| 625 | 10.64 | 120 | 100 | 94.34 | 94.20 | 94.48 | 94.34 | 39.49 |
| 625 | 66.5 | 105 | 80 | 69.18 | 69.03 | 69.29 | 69.17 | 36.79 |
| 625 | 39.9 | 60 | 150 | 90.05 | 90.21 | 90.85 | 90.37 | 39.12 |
| 625 | 133 | 90 | 120 | 94.34 | 95.03 | 94.41 | 94.59 | 39.52 |
| 625 | 93.1 | 75 | 50 | 37.74 | 38.00 | 37.58 | 37.77 | 31.54 |
| 600 | 10.64 | 105 | 150 | 98.77 | 99.47 | 98.53 | 98.92 | 39.91 |
| 600 | 66.5 | 60 | 120 | 88.05 | 88.32 | 87.84 | 88.07 | 38.89 |
| 600 | 39.9 | 90 | 50 | 39.44 | 39.36 | 39.47 | 39.42 | 31.92 |
| 600 | 133 | 75 | 100 | 81.76 | 80.89 | 81.23 | 81.29 | 38.20 |
| 600 | 93.1 | 120 | 80 | 50.31 | 50.29 | 50.44 | 50.35 | 34.04 |
| Coeff | Std Err | t Stat | p-Value | Lower | Upper | |
|---|---|---|---|---|---|---|
| Intercept | −177.78 | 16.57 | −10.72 | 3.35 × 10−10 | −212.15 | −143.41 |
| Temperature, C | 0.34 | 0.03 | 11.91 | 4.58 × 10−10 | 0.28 | 0.4 |
| Coal content, % (by stoichiometry) | 0.44 | 0.05 | 8.15 | 4.35 × 10−8 | 0.33 | 0.55 |
| Influencing Factor | Dependency Equation | R | tR |
|---|---|---|---|
| Temperature, C | αt = −129.9 + 0.33 · t | 0.99 | 537.08 |
| Pressure, Pa | αP = 57 − 0.17 · (P − 20)1/3 | 0.86 | 5.83 |
| Time, min | ατ = 45 + 0.06 · τ | 0.78 | 3.50 |
| Coal consumption, % | αv = 7.69 + 0.44 · C | 0.95 | 18.54 |
| Conditions | Tellurium Extraction Rate, % | |||||
|---|---|---|---|---|---|---|
| t, °C | P, Pa | τ, min | C, % | Forecast | Experiment | |
| Formula (4) | Formula (6) | |||||
| 625 | 10.64 | 60 | 130 | 94.19 | 97.45 | 95.38 |
| 600 | 10.64 | 60 | 150 | 94.44 | 98.84 | 98.01 |
| 625 | 133 | 60 | 150 | 94.50 | 100 | 97.22 |
| 625 | 93.1 | 60 | 150 | 97.26 | 100 | 96.51 |
| 625 | 66.5 | 60 | 150 | 99.10 | 100 | 98.74 |
| Element | Te | Al | Si | S | Se | Sn |
|---|---|---|---|---|---|---|
| Content, wt. % | 99.497 | 0.024 | 0.132 | 0.011 | 0.014 | 0.322 |
| Uncertainty, wt. % | 0.077 | 0.001 | 0.008 | 0.001 | 0.001 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitsenko, A.; Linnik, X.; Volodin, V.; Burabayeva, N.; Trebukhov, S. Production of Elemental Tellurium During Processing of Tellurium-Containing Middling. Processes 2025, 13, 3711. https://doi.org/10.3390/pr13113711
Nitsenko A, Linnik X, Volodin V, Burabayeva N, Trebukhov S. Production of Elemental Tellurium During Processing of Tellurium-Containing Middling. Processes. 2025; 13(11):3711. https://doi.org/10.3390/pr13113711
Chicago/Turabian StyleNitsenko, Alina, Xeniya Linnik, Valeriy Volodin, Nurila Burabayeva, and Sergey Trebukhov. 2025. "Production of Elemental Tellurium During Processing of Tellurium-Containing Middling" Processes 13, no. 11: 3711. https://doi.org/10.3390/pr13113711
APA StyleNitsenko, A., Linnik, X., Volodin, V., Burabayeva, N., & Trebukhov, S. (2025). Production of Elemental Tellurium During Processing of Tellurium-Containing Middling. Processes, 13(11), 3711. https://doi.org/10.3390/pr13113711






