Hydrothermal Carbonization of Water Care Material (WCM) and Analysis of Fuel and Soil Amendment Characteristic of Hydrochar
Abstract
1. Introduction
2. Materials and Methods
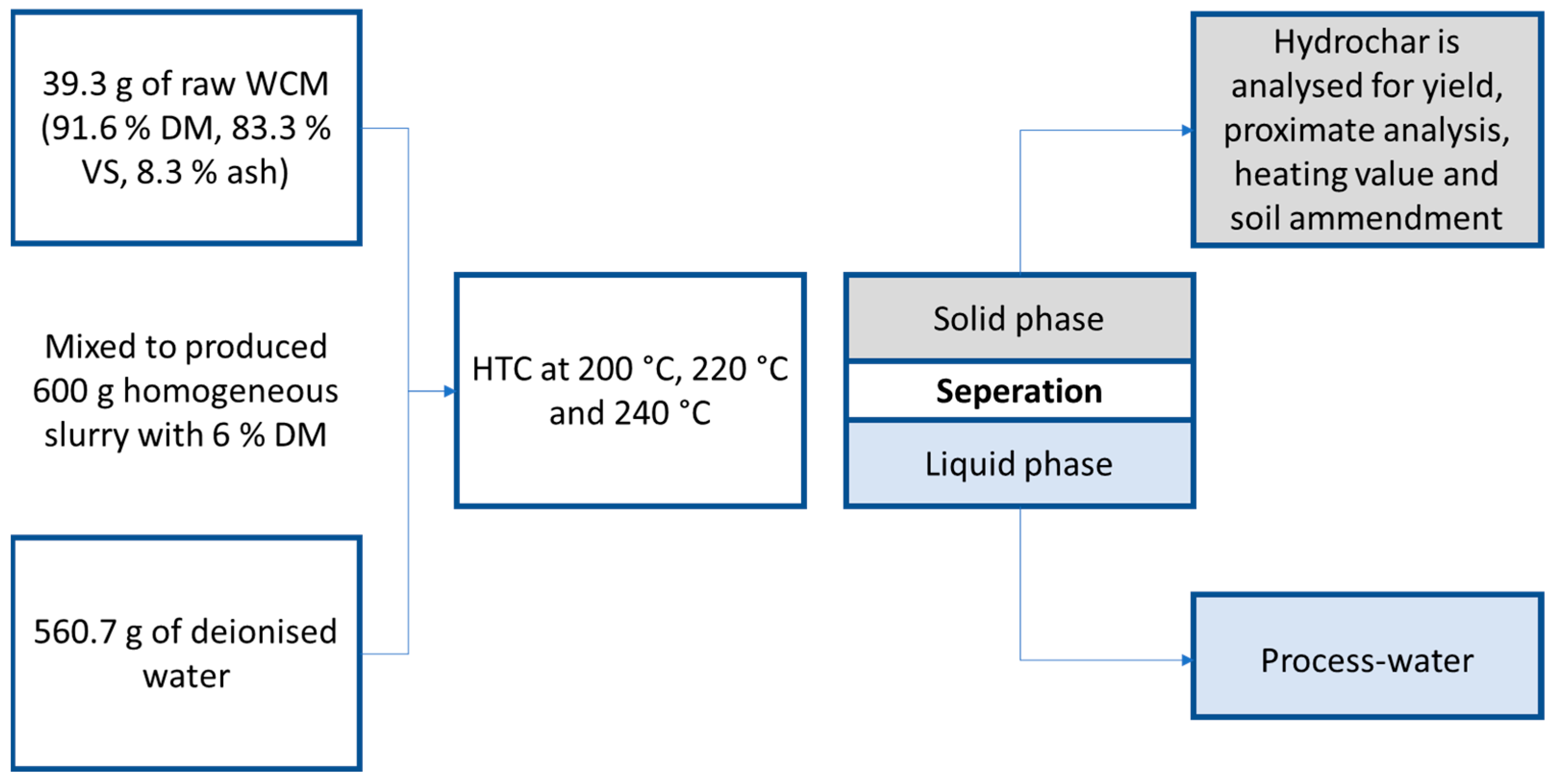
2.1. Feedstock and Experimental Setup
2.2. Hydrothermal Carbonization
2.3. Analysis (Product Recovery and Analysis)
3. Results and Discussion
3.1. Characteristic of the Feedstock Material
3.2. Effect of Operation Condition
3.3. Effects of HTC Reaction Condition on the Dewatering Potential
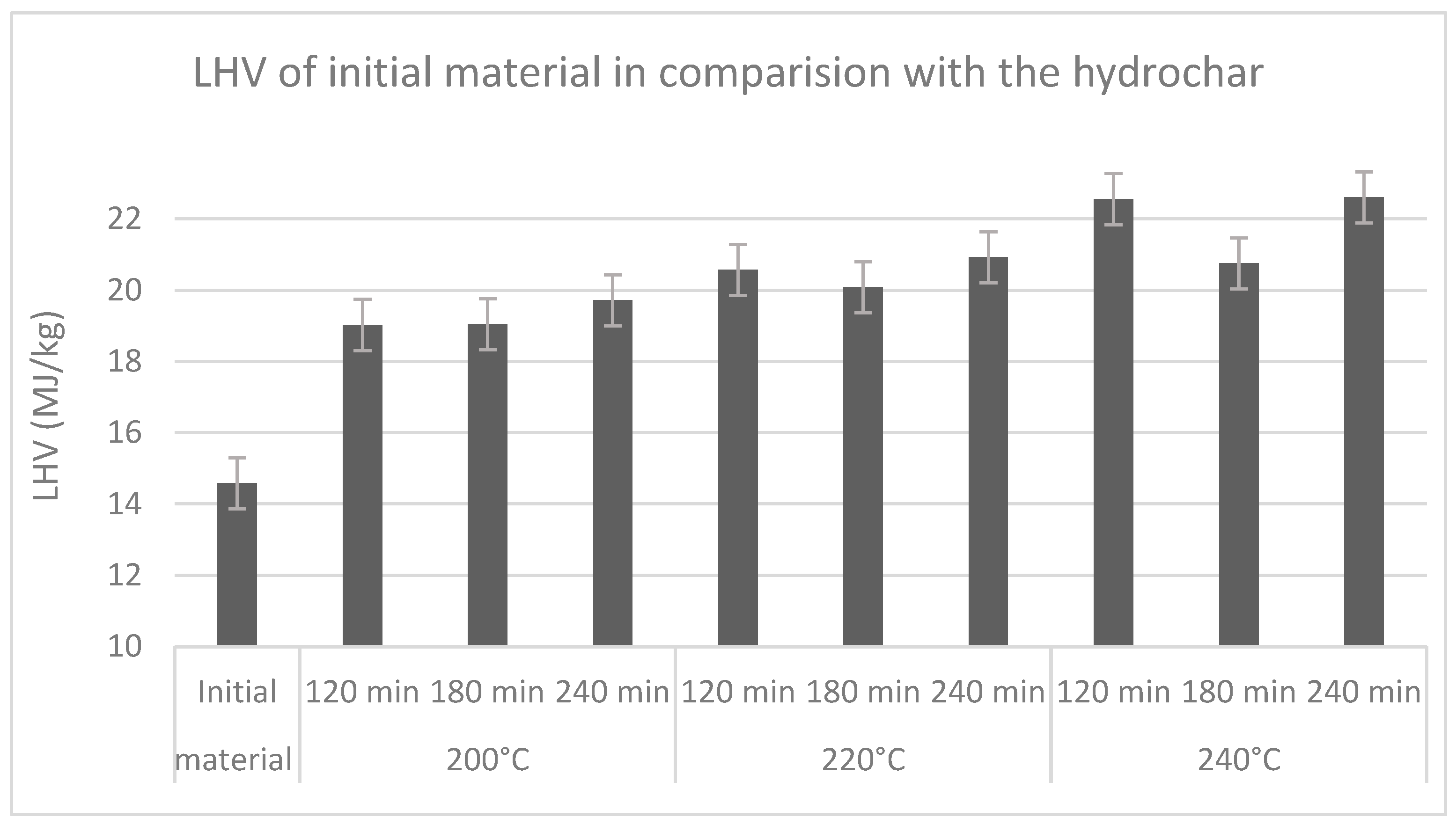
3.4. Effects of Reaction Condition on Heating Value of Water Care Material
3.5. Characteristic of Hydrochar for Its Utilization as Soil Amender
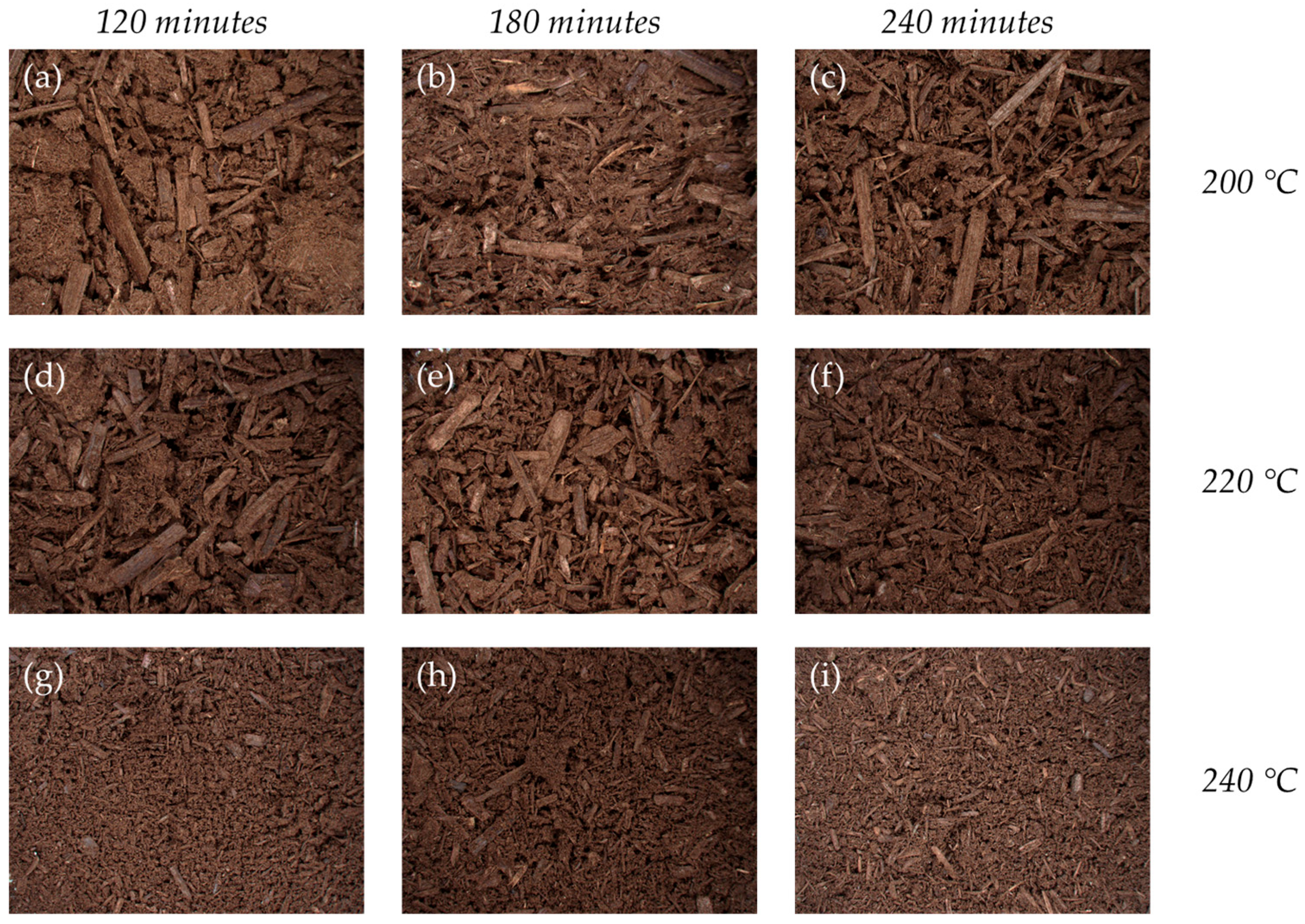
3.5.1. Surface Morphology
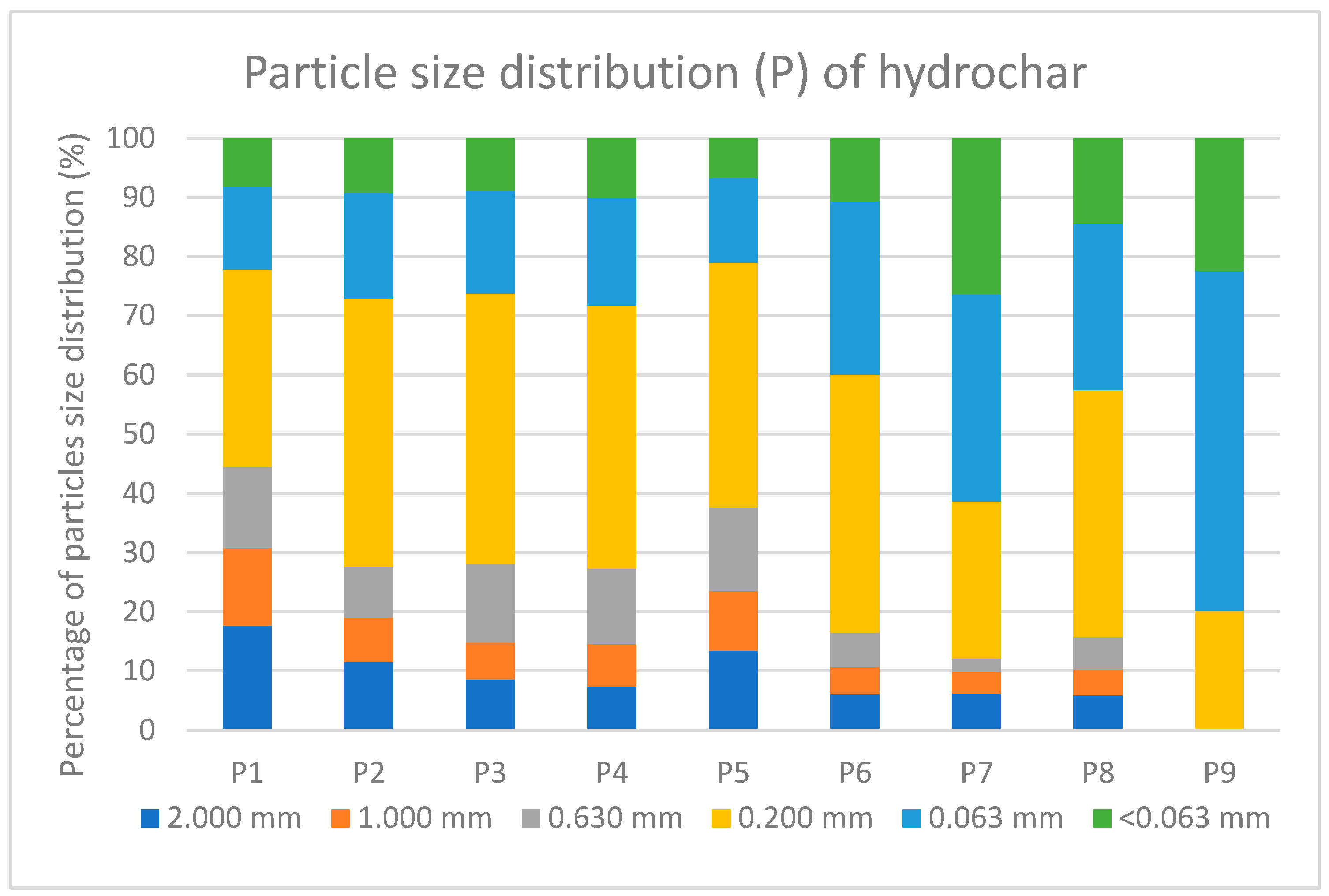
3.5.2. Particle Size
3.5.3. Nutrient Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelles, M.; Angelova, E.; Deprie, K.; Cyffka, K.-F.; Selig, M.; Rensberg, N.; Kornatz, P.; Schaller, S.; Thalheim, T. Stand und Perspektiven der energetishen Verwertung von Biomasse in Deutschland. In Schriftenreihe Umweltingenieurwesen; 18. Rostocker Biomasseforum; Universität Rostock: Rostock, Germany, 2024; pp. 13–38. [Google Scholar]
- Brosowski, A.; Adler, P.; Erdmann, G.; Stinner, W.; Thrän, D.; Mantau, U.; Blanke, C.; Mahro, B.; Hering, T.; Reinhold, G. Biomassepotenziale Von Rest- Und Abfallstoffen, Status quo in Deutschland; FNR: Gülzow-Prüzen, Germany, 2015. [Google Scholar]
- Jering, A.; Klatt, A.; Seven, J.; Ehlers, K.; Günther, J.O.A.; Mönch, L. Globale Landflächen und Biomasse Nachhaltig und Ressourcenschonend Nutzen; Umweltbundesamt: Dessau-Roßlau, Germany, 2012.
- Engler, N.; Schüch, A.; Nelles, M. Abfallbiomasse aus der Lebensmittelindustrie und Landschaftspflege: Biogaserträge und kinetische Kennzahlen. In Schriftenreihe Umweltingenieurwesen; 11. Rostocker Bioenergieforum; Universität Rostock: Rostock, Germany, 2017; pp. 155–168. [Google Scholar]
- Kramer, M.; Umweltmanagement, I. Systemorientierte Zusammenhänge Zwischen Politik, Recht, Management und Technik; GWV Fachverlage GmbH: Wiesbaden, Germany, 2010. [Google Scholar]
- Dahms, T.; Oehmke, C.; Kowatsch, A.; Abel, S.; Wichmann, S.; Wichtmann, W.; Schröder, C. Halmgutartige Festbrennstoffe aus nassen Mooren; Universität Greifswald: Greifswald, Germany, 2017. [Google Scholar]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Nelles, M.; Angelova, E.H.; Glowacki, R. Entwicklung der energetischen Biomassenutzung in Deutschland. In Wasser, Energie und Umwelt: Aktuelle Beiträge aus der Zeitschrift Wasser und Abfall II; Springer: Wiesbaden, Germany, 2022; pp. 591–601. [Google Scholar]
- LUNG. Leitfaden Gewässerentwicklung und -Pflege Maßnahmen als Beitrag zur Umsetzung der Wasserrahmenrichtlinie; Landesamt für Umwelt, Naturschutz und Geologie Mecklenburg-Vorpommern (LUNG M-V): Dessau, Germany, 2022.
- DWA. DWA-M 610—Neue Wege der Gewässerunterhaltung—Pflege und Entwicklung von Fließgewässern; Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall e. V: Hennef, Germany, 2010. [Google Scholar]
- Patt, H.; Jürgung, P.; Kraus, W.; Wasserbau, N. Entwicklung und Gestaltung von Fließgewässern; Springer Fachmedien Wiesbaden GmbH: Wiesbaden, Germany, 2018. [Google Scholar]
- Foth, S. Options for material and energy recovery of water care material (WCM) from the maintenance of water bodies. In Proceedings of the 6th Doctoral Colloquium Bioenergy, Göttingen, Germany, 18–19 September 2023; DBFZ Deutsches Biomasseforschungszentrum Gemeinnützige GmbH: Göttingen, Germany, 2023; pp. 16–17. [Google Scholar]
- Foth, S.; Sprafke, J.; Nelles, M. Utilization of water care material (WCM) in anaerobic digestion. In Schriftenreihe Umweltingenieurwesen; 15. Rostocker Bioenergieforum; Universität Rostock: Rostock, Germany, 2021; pp. 257–272. [Google Scholar]
- Ministerium für Wirtschaft, Arbeit und Tourismus Mecklenburg-Vorpommern. Ministerium für Wirtschaft, Energie aus Abfall in Mecklenburg-Vorpommern; Ministerium für Wirtschaft, Arbeit und Tourismus Mecklenburg-Vorpommern: Schwerin, Germany, 2009.
- DWA. DWA-M 612—Gewässerrandstreifen—Uferstreifen—Gewässerentwicklungskorridore: Grundlagen und Funktionen, Hinweise zur Gestaltung, Beispiele; Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall e. V: Hennef, Germany, 2020. [Google Scholar]
- Osborne, D. The Coal Handbook: Towards Cleaner Production; Woodhead Publishing: Delhi, India, 2013. [Google Scholar]
- Quicker, P.; Weber, K. Eigenschaften von Biomassekarbonisaten. In Biokohle: Herstellung, Eigenschaften und Verwendung von Biomassekarbonisaten; Springer: Wiesbaden, Germany, 2017; pp. 165–212. [Google Scholar]
- Jalilian, M.; Bissessur, R.; Ahmed, M.; Hsiao, A.; He, Q.S.; Hu, Y. A review: Hydrochar as potential adsorbents for wastewater treatment and CO2 adsorption. Sci. Total Environ. 2024, 914, 169823. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Q.; Yin, C.; Su, J.; Cao, C.; Duo, J.; Duan, P.-G. Co-hydrothermal carbonization of biomass: Advances, challenges, and future perspectives in soil remediation applications. Biomass Bioenergy 2025, 202, 108164. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Hagemann, N.; Abächerli, F.; Leifeld, J.; Bucheli, T. Pflanzenkohle in der Landwirtschaft; Agroscope: Zürich, Germany, 2021.
- Zozmann, E.; Bluhm, H.; Lenk, C.; Rupp, J. Ökonomische und sozio-technische Evaluation der Herstellung und Anwendung von Pflanzenkohle als Negativemmissionstechnologie in Deutschland. In Schriftenreihe Umweltingenieurwesen; 18. Biomasseforum; Universität Rostock: Rostock, Germany, 2024; pp. 121–132. [Google Scholar]
- ISO 16948:2015; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- ISO 14918:2018; Thermal Spraying—Qualification Testing of Thermal Sprayers. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- ISO EN 15170:2010; Characterization of Sludge—Determination of Calorific Value. International Organization for Standardization (ISO): Geneva, Switzerland, 2010.
- Demirbas, A. Relationships Proximate Analysis Results and Higher Heating Values of Lignites. Energy Sources, Part A: Recovery. Util. Environ. Eff. 2008, 30, 1876–1883. [Google Scholar]
- Masoumi, S.; Borungadda, V.B.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Wang, H.; Yang, Z.; Li, X.; Liu, Y. Distribution and transformation behaviors of heavy metals and phosphorus during hydrothermal carbonization of sewage sludge. Environ. Sci. Pollut. Res. 2020, 27, 17109–17122. [Google Scholar] [CrossRef]
- Ekanthalu, V.S.; Narra, S.; Sprafke, J.; Nelles, M. Influence of Acids and Alkali as Additives on Hydrothermally Treating Sewage Sludge: Effect on Phosphorus Recovery, Yield, and Energy Value of Hydrochar. Processes 2021, 9, 618. [Google Scholar] [CrossRef]
- Putra, H.E.; Damanhuri, E.; Dewi, K.; Pasek, A.D. Hydrothermal carbonization of biomass waste under low temperature condition. In Proceedings of the 2nd International Conference on Engineering and Technology for Sustainable Development, Taichung, Taiwan, 19–21 October 2018. [Google Scholar]
- Mazumder, S.; Saha, P.; Reza, M.T. Co-hydrothermal carbonization of coal waste and food waste: Fuel characteristics. Biomass Convers. Biorefinery 2020, 12, 3–31. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.; Chang, S. Carbonization temperature and feedstock. Bioresour. Technol. 2021, 330, 124976. [Google Scholar] [CrossRef]
- Omondi, M.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Kamau, S.; Kranja, N.; Ayuke, F.O.; Lehmann, J. Short-term influence of biochar and fertilizer-biochar blends on soil nutrients, fauna and maize growth. Biol. Fertil. Soils 2019, 55, 661–673. [Google Scholar] [CrossRef]
- Lou, S.; Wang, S.; Tian, L.; Li, S.; Li, X. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117–118, 10–15. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Hydochar and biochar: Production, physicochemical properties and techno-economic analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef]
- Sørmo, E.; Silvani, L.; Thune, G.; Gerber, H.; Schmidt, H.; Smebye, A.; Cornelissen, G. Waste timber pyrolysis in a medium-scale unit: Emission budgets and biochar quality. Sci. Total Environ. 2020, 718, 137335. [Google Scholar] [CrossRef] [PubMed]
- Maletić, S.; Isakovski, M.K.; Sigmund, G.; Hofmann, T.; Hüffer, T.; Beljin, J.; Rončević, S. Comparing biochar and hydrochar for reducing the risk of organic contaminants in polluted river sediments used for growing energy crops. Sci. Total Environ. 2022, 843, 157122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.; Lim, J.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.; Ok, Y. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2021, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Sigmund, G.; Hüffer, T. Auswahl und Applikation von Pflanzenkohle für die In-Situ Bodensanierung—Endbericht KOKOSAN II; Department für Umweltgeowissenschaften, Universität Wien: Wien, Austria, 2022. [Google Scholar]
- Justiz, B.D. Düngemittelverordnung (Verordnung über das Inverkehrbringen von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln (DüMV)) vom 5. Dezember 2012 (BGBl. I S. 2482), die zuletzt durch Artikel 1 der Verordnung vom 2. Oktober 2019 (BGBl. I S. 1414) geändert worden ist, 2019.
- Bezerra, J.; Turnhout, E.; Vasquez, I.; Rittl, T.; Arts, B.; Kuyper, T. The promises of the Amazonian soil: Shifts in discourses of Terra Preta and biochar. J. Environ. Policy Plan. 2019, 21, 623–635. [Google Scholar] [CrossRef]
- Frausin, V.; Fraser, J.; Narmah, W.; Lahai, M.; Winnebah, T.; Fairhead, J.; Leach, M. God Made the Soil, but We Made It Fertile: Gender, Knowledge, and Practice in the Formation and Use of African Dark Earths in Liberia and Sierra Leone. Hum. Ecol. 2014, 42, 695–710. [Google Scholar] [CrossRef]
- Kammann, C.; Glaser, B.; Schmidt, H.-P. Combining Biochar and Organic Amendments. In Biochar in European Soils and Agriculture: Science and Practice; Routledge: London, UK, 2016; pp. 136–164. [Google Scholar]
- Kern, J.; Giani, L.; Teixeira, W.; Lanza, G.; Glaser, B. What can we learn from ancient fertile anthropic soil (Amazonian Dark Earths, shell mounds, Plaggen soil) for soil carbon sequestration? CATENA 2019, 172, 104–112. [Google Scholar] [CrossRef]



| Parameters | Units | Value |
|---|---|---|
| Moisture content | %OS | 1 |
| Total solids | %OS | 99 |
| Volatile solids | %OS | 92.5 |
| Ash (815 °C) | %OS | 6.9 |
| Ash (550 °C) | %OS | 7.5 |
| Fixed carbon (FC) | %OS | 0.6 |
| XF | % DM | 37.77 |
| NDF | % DM | 78.52 |
| ADF | %DM | 50.84 |
| HHV | MJ/kg | 15.3847 |
| LHV | MJ/kg | 14.5827 |
| Carbon | % DM | 49.394 |
| Nitrogen | % DM | 1.3124 |
| Sulfur | % DM | 0.30576 |
| Total phosphorus | Mg/kg | 1724.69593 |
| Hydrogen | % DM | 6.5 |
| Oxygen | % DM | 42.48784 |
| Potassium | g/kg DM | 7.7 |
| Calcium | g/kg DM | 5.1 |
| Sodium | g/kg DM | 0.2 |
| Magnesium | g/kg DM | 0.8 |
| Copper | mg/kg DM | 5.2 |
| Arsenic | mg/kg DM | 0.504 |
| Cadmium | mg/kg DM | 0.027 |
| Lead | mg/kg DM | 4.26 |
| Temp. | Residence Time | Mass Yield | High Heating Value (HHV) | Energy Yield |
|---|---|---|---|---|
| °C | % | MJ/kg | % | |
| 200 °C | 120 | 61.22% | 20.0734 | 79.88% |
| 180 | 61.75% | 20.0903 | 80.64% | |
| 240 | 59.69% | 20.7969 | 80.69% | |
| 220 °C | 120 | 59.17% | 21.6985 | 83.45% |
| 180 | 58.75% | 21.1897 | 80.92% | |
| 240 | 47.69% | 22.0736 | 68.43% | |
| 240 °C | 120 | 43.17% | 23.7969 | 66.77% |
| 180 | 53.83% | 21.8902 | 76.60% | |
| 240 | 41.39% | 23.8445 | 64.15% |
| Temp. | Residence Time | Proximate Analysis (wt% Fresh Basis) | Lower Heating Value (LHV) | ||||
|---|---|---|---|---|---|---|---|
| Dry Matter | Organic Dry Matter | Ash 550 °C | Ash 815 °C | Fixed Carbon | |||
| °C | minute | % OS | % OS | % OS | % OS | % OS | MJ/kg |
| 200 °C | 120 | 96.60 | 84.70 | 15.30 | 14.80 | 0.50 | 19.03 |
| 180 | 96.50 | 87.60 | 12.40 | 11.80 | 0.60 | 19.04 | |
| 240 | 96.40 | 91.70 | 8.30 | 7.90 | 0.40 | 19.71 | |
| 220 °C | 120 | 96.90 | 91.00 | 9.00 | 8.60 | 0.40 | 20.57 |
| 180 | 96.60 | 92.70 | 7.30 | 6.90 | 0.40 | 20.09 | |
| 240 | 97.00 | 86.90 | 13.10 | 12.50 | 0.60 | 20.92 | |
| 240 °C | 120 | 97.80 | 86.40 | 13.60 | 13.20 | 0.40 | 22.56 |
| 180 | 97.10 | 91.10 | 8.90 | 8.50 | 0.40 | 20.75 | |
| 240 | 98.10 | 82.00 | 18.00 | 17.60 | 0.40 | 22.60 | |
| Sample ID | C | N | S | P Total |
|---|---|---|---|---|
| % | % | % | mg/kg | |
| P1-200-120 | 48.72 | 0.91 | 0.30 | 2775.05 |
| P2-200-180 | 49.16 | 0.91 | 0.24 | 2192.81 |
| P3-200-240 | 50.50 | 0.91 | 0.29 | 2778.12 |
| P4-220-120 | 52.00 | 0.99 | 0.25 | 2800.04 |
| P5-220-180 | 51.17 | 0.89 | 0.23 | 2365.68 |
| P6-220-240 | 52.97 | 1.19 | 0.28 | 3448.45 |
| P7-240-120 | 56.76 | 1.43 | 0.26 | 3631.69 |
| P8-240-180 | 53.42 | 1.14 | 0.22 | 3094.04 |
| P9-240-240 | 59.67 | 1.62 | 0.31 | 3369.81 |
| 146-GPM-21 | 49.394 | 1.3124 | 0.30576 | 1724.69593 |
| Sample ID | K | Ca | Na | Mg | Cu | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| g/kg | g/kg | g/kg | g/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| 146-GPM-21 | 7.7 | 5.1 | 0.2 | 0.8 | 5.2 | 0.504 | 0.027 | 4.26 |
| P9-240-240 | 1.2 | 5.6 | 0.1 | 0.6 | 26.1 | 0.57 | 0.043 | 7.84 |
| Sample ID | C% | H% | O% | H/C | O/C | C/N |
|---|---|---|---|---|---|---|
| P1-200-120 | 48.72 | 5.27 | 44.81 | 0.92 | 0.11 | 53.83 |
| P2-200-180 | 49.16 | 5.41 | 44.28 | 0.90 | 0.11 | 54.02 |
| P3-200-240 | 50.50 | 5.38 | 42.92 | 0.85 | 0.11 | 55.49 |
| P4-220-120 | 52.00 | 5.23 | 41.54 | 0.80 | 0.10 | 52.79 |
| P5-220-180 | 51.17 | 5.62 | 42.10 | 0.82 | 0.11 | 57.49 |
| P6-220-240 | 52.97 | 5.40 | 40.17 | 0.76 | 0.10 | 44.70 |
| P7-240-120 | 56.76 | 5.09 | 36.47 | 0.64 | 0.09 | 39.83 |
| P8-240-180 | 53.42 | 5.60 | 39.62 | 0.74 | 0.10 | 46.86 |
| P9-240-240 | 59.67 | 4.91 | 33.49 | 0.56 | 0.08 | 36.83 |
| 146-GPM | 49.39 | 6.50 | 42.49 | 0.13 | 0.86 | 37.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foth, S.; Shettigondahalli Ekanthalu, V.; Jansen, F.; Nelles, M. Hydrothermal Carbonization of Water Care Material (WCM) and Analysis of Fuel and Soil Amendment Characteristic of Hydrochar. Processes 2025, 13, 3398. https://doi.org/10.3390/pr13113398
Foth S, Shettigondahalli Ekanthalu V, Jansen F, Nelles M. Hydrothermal Carbonization of Water Care Material (WCM) and Analysis of Fuel and Soil Amendment Characteristic of Hydrochar. Processes. 2025; 13(11):3398. https://doi.org/10.3390/pr13113398
Chicago/Turabian StyleFoth, Sebastian, Vicky Shettigondahalli Ekanthalu, Florian Jansen, and Michael Nelles. 2025. "Hydrothermal Carbonization of Water Care Material (WCM) and Analysis of Fuel and Soil Amendment Characteristic of Hydrochar" Processes 13, no. 11: 3398. https://doi.org/10.3390/pr13113398
APA StyleFoth, S., Shettigondahalli Ekanthalu, V., Jansen, F., & Nelles, M. (2025). Hydrothermal Carbonization of Water Care Material (WCM) and Analysis of Fuel and Soil Amendment Characteristic of Hydrochar. Processes, 13(11), 3398. https://doi.org/10.3390/pr13113398






