Abstract
Silver sulfide quantum dots (Ag2S QDs) are promising nanomaterials for biomedical applications due to their near-infrared emission and biocompatibility. In this study, Ag2S QDs were synthesized using bovine serum albumin (BSA) as a stabilizing and reducing agent to assess their potential in targeted photothermal therapy. The QDs showed an average size of 1.06 ± 0.38 nm by DLS and 4.42 nm by TEM. Conjugation to an anti-PSG1 monoclonal antibody was performed via EDC/Sulfo-NHS chemistry and confirmed by FTIR spectroscopy, a decrease in zeta potential, and a redshift in emission. The conjugate exhibited an average size of 22.82 ± 9.7 nm and a zeta potential of +85.7 mV, indicating high colloidal stability. Fluorescence studies showed that the conjugate emits at 590 nm when excited at 560 nm, whereas the BSA-Ag2S QDs (non-conjugated) emit at 480 nm upon excitation at 400 nm, reflecting changes in optical properties due to conjugation. Thermal imaging under 808 nm laser irradiation revealed efficient photothermal conversion, with temperature increases up to 13.6 °C at 200 μg/mL and a conversion efficiency of 11.41 ± 0.04%. The conjugate was non-cytotoxic to fibroblasts but induced selective cytotoxicity in HeLa cells after laser exposure, with a selectivity index of 3.0. These findings suggest that Ag2S-BSA QDs conjugated with anti-PSG1 represent promising candidates for further investigation in cancer nanotheranostics.
1. Introduction
In recent years, the development of conjugates has gained significant importance in the field of cancer nanomedicine, particularly for use in photothermal therapy (PTT). This technique is characterized by short treatment times, ease of application, and rapid recovery. It works by using heat generated from materials that absorb near-infrared (NIR) light to destroy cancer cells while sparing healthy tissue, making it a minimally invasive treatment. Moreover, these conjugates can integrate functional components such as nanoparticles, biomolecules, and therapeutic agents into a single system [1,2,3]. These hybrid systems enable the selective targeting of compounds to tumor cells, thereby improving therapeutic efficacy and reducing systemic side effects. Various platforms have been used as the basis for developing these conjugates, including metallic nanoparticles, polymers, liposomes, and quantum dots [4]. These platforms can be functionalized with antibodies, aptamers, peptides, or glycoproteins to achieve cell-specific targeting [5].
Among these platforms, silver sulfide quantum dots (Ag2S QDs) have attracted considerable attention due to their low toxicity, high biocompatibility, and ability to emit light in the near-infrared (NIR) region [6]. These properties make them a promising alternative for biomedical applications, particularly in in vivo bioimaging and photothermal therapy [7]. Furthermore, the conjugation of specific biomolecules with nanomaterials has been explored for the detection, labeling, and treatment of various cancers, yielding promising results [8]. Recent studies have demonstrated a variety of successful applications of Ag2S QDs in in vivo biomedical settings. For instance, Ag2S QDs functionalized with affibodies have been developed for targeted photoacoustic imaging of tumors overexpressing the epidermal growth factor receptor (EGFR), enabling highly specific detection through near-infrared (NIR) fluorescence. In a murine model, this system allowed real-time visualization of tumors with a tumor-to-background fluorescence ratio greater than 4:1, demonstrating high selectivity and accumulation at the tumor site within hours post-injection [9]. Similarly, platforms targeting the enzyme carbonic anhydrase IX (CA IX) have combined NIR-II emission with photothermal therapy, achieving both diagnostic imaging and localized treatment in hypoxic tumor models in vivo. In these studies, after laser irradiation at 808 nm, tumor temperatures increased above 55.1 °C, resulting in significant tumor ablation with minimal damage to surrounding tissues [10]. Their utility has also been demonstrated in photothermal immunotherapy involving long-term cell tracking, where Ag2S QDs served as dual-function tracers and therapeutic agents. In breast cancer-bearing mice, the nanoplatform enabled the visualization of tumor-associated immune cells for up to 90 days through NIR-II fluorescence imaging, induced a significant reduction in tumor volume, and resulted in a survival rate of 83% following repeated photothermal sessions [11].
Among these biomolecules, the monoclonal antibody anti-PSG1 (pregnancy-specific β-1 glycoprotein 1) has attracted growing interest as a targeting agent because it is specifically overexpressed in solid tumors, including breast, lung, colon, and ovarian cancers [12,13], while showing limited expression in healthy tissues. This selective expression pattern positions PSG1 as a biomarker of significant clinical relevance, with high specificity and strong binding affinity. In addition, PSG1 has been implicated in tumor-promoting processes such as angiogenesis, immune evasion, and uncontrolled cell proliferation, further reinforcing its role as a suitable target for active targeting strategies in cancer therapy and diagnosis. Conjugates incorporating anti-PSG1 monoclonal antibodies as molecular recognition elements offer the potential to enhance tumor selectivity and improve the efficacy of theranostic systems. Notably, the integration of this molecular specificity with the unique optical and photothermal properties of Ag2S QDs opens new opportunities for the development of dual-function platforms capable of both imaging and treating tumors with high precision and minimal off-target effects.
In this context, the present study proposes the design and characterization of a novel conjugate based on silver sulfide quantum dots functionalized with bovine serum albumin (Ag2S-BSA QDs), further conjugated with the anti-PSG1 monoclonal antibody. The aim is to evaluate the optical behavior, photothermal response, and cytotoxicity of this system in vitro. As a preliminary investigation, this work seeks to lay the foundation for future development of PSG1-targeted nanomaterials with potential applications in cancer diagnosis and therapy.
2. Materials and Methods
2.1. Synthesis of BSA-Stabilized Ag2S Quantum Dots (Ag2S-BSA QDs)
Initially, 5 mL of a 40 mg/mL aqueous BSA solution was prepared in a round-bottom flask and placed in an oil bath at 37 °C under constant magnetic stirring to ensure complete protein hydration and solubilization. The initially viscous and slightly opalescent solution became transparent after approximately 10 min of stirring, indicating complete dissolution of BSA. Subsequently, 5 mL of a 0.01 M aqueous AgNO3 solution was added dropwise to the BSA solution over a 10 min period to ensure thorough homogenization. Afterward, 1 mL of a 1 M NaOH solution was added to the reaction mixture, raising the pH to approximately 10. This alkaline environment promotes the formation of coordinate bonds between Ag+ ions and electronegative atoms such as oxygen and nitrogen present in the peptide bonds along the amino acid chain of BSA, which acts as a Lewis base. Several minutes are required to ensure that most of the silver ions have bonded with BSA; therefore, the mixture was stirred for an additional 10 min. Nucleation of Ag2S QDs was initiated by adding 1 mL of a 0.0615 M aqueous Na2S solution to the reaction mixture. A slight brown coloration appeared immediately and gradually darkened over 13 h at 37 °C under continuous stirring, indicating the formation and growth of Ag2S nanocrystals within the BSA matrix. The resulting colloidal suspension was purified to remove unbound BSA and residual salts through repeated centrifugation and filtration cycles using Amicon® Ultra-4 (100 kDa (Sigma-Aldrich, Monterrey, Mexico) filters at 10,000 rpm for 5 min with Milli-Q water. The retained fraction, containing BSA-stabilized QDs, was either stored as a colloidal suspension at 4–8 °C or lyophilized for further characterization. All reagents were obtained from Sigma-Aldrich (Merck, Monterrey, Mexico), unless otherwise specified.
2.2. Conjugation of Ag2S-BSA QDs with Monoclonal Antibody PSG1 Using EDC/Sulfo-NHS Chemistry
For the preparation of the conjugate, it was necessary to exchange the original aqueous medium (Milli-Q water) in which the Ag2S-BSA QDs (1 mg/mL) were dispersed with a 0.1 M MES buffer solution (pH 5.5). This exchange was performed by centrifugation at 3500× g for 10 min at 4 °C using an Amicon® ultrafiltration concentrator system (30 kDa), replacing the supernatant with fresh MES buffer at each cycle. The process was repeated three times, and the pH was adjusted to 5.5 using 1 M NaOH. Subsequently, 1.44 mg/mL of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) was added directly to the solution and incubated for 2 min at room temperature with gentle agitation. EDC reacts with the carboxyl groups present on the BSA, forming an unstable acylisourea ester intermediate. Then, 3.25 mg/mL of N-hydroxysulfosuccinimide (Sulfo-NHS) was added to the mixture and incubated for 10 min in the dark at 400 rpm, allowing the formation of a stable NHS-ester intermediate, which enhances coupling efficiency with primary amines. To remove excess unreacted EDC and Sulfo-NHS, the mixture was centrifuged again at 3500× g for 10 min at 4 °C in the dark, using an Amicon® ultrafiltration concentrator system (30 kDa). The supernatant was replaced with PBS buffer (pH 7.4), and the process was repeated three times. After activation of the carboxyl groups, conjugation with the monoclonal anti-PSG1 antibody (this product is preservative-free, ThermoFisher Scientific, Monterrey, Mexico) was carried out by adding 5 µL of a 1 µg/µL antibody solution. The mixture was incubated for 2 h at room temperature under constant agitation (400 rpm) in the dark to facilitate the formation of covalent bonds between the antibody’s amine groups and the NHS-activated carboxyl groups on BSA. To minimize nonspecific cross-linking between BSA and other proteins, a blocking step with 1 M glycine was performed by incubating the mixture for 15 min in the dark. Finally, the mixture was centrifuged at 16,873× g for 10 min using the Amicon® ultrafiltration concentrator system (100 kDa) to remove unbound antibody, glycine, and residual cross-linking reagents. The final conjugate was stored at 4 °C in the dark until further use.
2.3. Material Characterization
The characterization of the Ag2S-BSA QDs involved multiple analytical techniques. The morphology and size of the QDs were assessed via transmission electron microscopy (TEM) using a JEOL 2200 CS/UHR instrument (Akishima, Tokyo, Japan) operating at 200 kV. The hydrodynamic diameter of Ag2S-BSA QDs was determined with a Microtrac Nanosizer, utilizing a sample concentration of 50 µg/mL.
Zeta potential measurements were carried out with a NanoTrac Wave II analyzer (Montgomeryville, PA, USA) on samples adjusted to pH 7.4 and 4.0. These measurements were performed following particle size analysis by dynamic light scattering, maintaining consistent experimental conditions. Zeta potential values were monitored over 60 s.
Fluorescence properties were evaluated using a Fluorolog-3 spectrofluorometer (Horiba Scientific, Jobin Yvon Inc., Piscataway, NJ, USA). The samples were diluted to 10% with distilled water and analyzed at room temperature. The excitation wavelength was set at 340 nm, with a 5 nm slit width, and emission spectra were recorded across the 180–550 nm range. Luminescence imaging was additionally performed using an OmniCure® LX40 UV LED (Excelitas Technologies, Ontario, Canada) spot curing system.
Fourier Transform Infrared spectra (FTIR) were collected using a Nicolet 6700 spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA), scanning in the wavenumber range of 4000 to 450 cm−1.
X-ray diffraction (XRD) analysis was conducted on a Panalytical X’Pert powder diffractometer (Malvern Panalytical, Almelo, Overijssel, The Netherlands) to evaluate the crystalline structure of the samples, covering a 2θ range from 10° to 80°.
Finally, UV–Visible absorption spectra were recorded with a Varioskan™ LUX multimode microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The QDs were resuspended in distilled water and analyzed at room temperature across a wavelength range of 280 to 800 nm.
Turbidimetry Stability Index
The stability of Ag2S-BSA QDs was assessed through Turbidimetry Stability Index (TSI) measurements conducted over 10 days. For this analysis, the QDs were prepared at a concentration of 50 µg/mL. The assay involved measuring the light transmitted through 200 µL of colloidal suspension at 340 nm using a Benchmark Plus microplate spectrophotometer (Bio-Rad, CA, USA). The TSI was then determined by evaluating the correlation between the incident light intensity and the light detected after passing through the sample, following the procedure described in Equation (1).
where xi represents the absorbance recorded at each time point, xBs denotes the mean absorbance across all measurements, and n corresponds to the total number of scans conducted with the instrument.
Cell viability was evaluated using the MTT assay in primary fibroblasts derived from Balb/c mice through enzymatic digestion with collagenase (Research Ethics Committee approval number: CEI-2025-1-31). The cells were maintained in DMEM culture medium supplemented with heat-inactivated fetal bovine serum and antibiotics and subsequently exposed to various concentrations of Ag2S-BSA quantum dots and their conjugated form. The treatments were incubated for 24 and 48 h at 37 °C in a humidified environment containing 5% CO2. Untreated cells served as the control group. After incubation, MTT solution (5 mg/mL) was added to each well, and the cells were incubated for an additional hour. The resulting formazan crystals were then solubilized in DMSO. Absorbance was recorded at 570 nm using a Benchmark Plus microplate spectrophotometer, and cell viability was calculated as described by Equation (2).
where ODt and ODc denote the optical densities of the treated and control samples, respectively.
The photothermal effect of the sample conjugate was evaluated using an 808 nm near-infrared (NIR) diode laser system (Q-BAIHE) with an output power of 2.4 W. In each experiment, 1 mL of the aqueous sample dispersion was irradiated with the NIR laser for 10 min. The resulting temperature increase was measured using a Fluke 51II precision digital thermometer equipped with a thermocouple, which was immersed in the dispersion during irradiation. Thermographic images were captured at 0, 5, and 10 min using a thermographic camera (Keysight U5857A). As a negative control, 1 mL of tri-distilled water (J.T. Baker) was used. The photothermal conversion efficiency (η) was calculated using Roper’s method and Equation (3), under a controlled temperature of 37 °C.
where h represents the heat transfer coefficient, A is the surface area of the container, ΔTmax corresponds to the maximum temperature change observed in the bioconjugate solution, I denotes the power density of the NIR laser, A808 is the absorbance of the conjugate solution at 808 nm, and Qs refers to the heat generated due to light absorption by water. The product hA was determined by analyzing the linear relationship between −ln θ and time, which was derived from the cooling curve shown in Figure 4B.
For the photothermal experiments, HeLa cells were plated in 24-well plates at a density of 40,000 cells per well. After a 24 h incubation period, the cells were washed twice with PBS, and the conjugate solution (200 μg/mL in DMEM) was added to each well. The experimental controls included untreated HeLa cells, as well as fibroblasts treated and untreated with the conjugate at the same concentration. After 4 h of exposure to the conjugate, the cells were subjected to laser irradiation at 808 nm with a power output of 2.4 W for a duration of 3 min per well. After irradiation, the cells were cultured for an additional 2 h, washed with PBS, and then treated with MTT solution for 1 h to assess cell viability. The selectivity index (SI) was calculated according to Equation (4).
All quantitative experiments were conducted in triplicate (n = 3), and the results are presented as mean ± standard deviation. Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test, with a confidence level of 95% (p < 0.05).
3. Results and Discussion
The size distribution of the obtained Ag2S-BSA QDs showed an average size of 1.06 ± 0.38 nm (Figure 1B), which enables photoluminescence in the near-infrared (NIR) range. This effect is attributed to the quantum confinement characteristic of Ag2S QDs [14]. Additionally, TEM analysis revealed an average nanoparticle size of 4.42 nm (Figure 1A), confirming their nanoscale dimensions. These results are consistent with those reported by Arrieta [15].
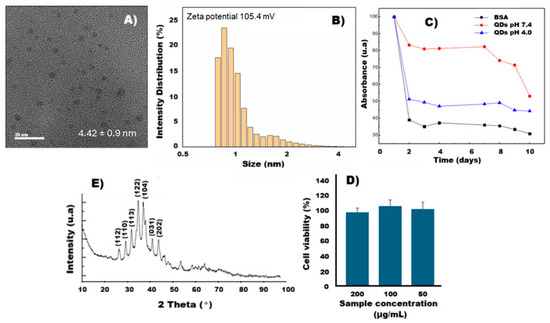
Figure 1.
(A) TEM image of Ag2S-BSA QDs, (B) size of Ag2S-BSA QDs, (C) absorbance percentage of BSA and Ag2S-BSA QDs at different pH, (D) X-ray diffraction patterns, and (E) cell viability percentage of fibroblast cells against Ag2S-BSA QDs at 200, 100, and 50 µg/mL for 24 h.
The Ag2S-BSA QDs exhibited good colloidal stability over a period of 10 days, as assessed by TSI (Figure 1C and Table 1). TSI values of 60.79 were recorded for free BSA and 49.21 and 43.13 for the Ag2S-BSA QDs at pH 4.0 and 7.4, respectively. These results indicate that the QDs possess greater colloidal stability at pH 7.4, which is physiological pH, and moderate stability under acidic conditions (pH 4.0). The lower TSI values observed for the QD systems compared to free BSA suggest an effective interaction between the protein and the nanocrystal surface, contributing to improved colloidal stabilization. This behavior can be explained by the formation of a protein corona around the nanocrystal surface. At pH 7.4, BSA is negatively charged due to the deprotonation of acidic residues, which enhances electrostatic repulsion between QDs and prevents aggregation [16]. Additionally, the adsorbed BSA layer contributes steric stabilization, acting as a physical barrier that limits interparticle interaction. Together, these effects provide electrosteric stabilization, which is highly favorable for dispersion in physiological media. In contrast, at acidic pH values closer to BSA’s isoelectric point (4.7), the protein carries a reduced net charge, diminishing electrostatic repulsion and allowing moderate nanoparticle aggregation [17,18]. The observed TSI values support this interpretation and align with previous studies that describe the pH-dependent behavior of BSA-coated nanomaterials. This pH-sensitive colloidal stability is particularly relevant for biomedical applications, as it implies stable behavior in physiological environments.

Table 1.
TSI of BSA and Ag2S-BSA QDs at different pH values.
Silver sulfide exhibits several allotropic forms, among which the most prominent are α-Ag2S (acanthite, monoclinic), stable below 178 °C, and β-Ag2S (argentite, cubic bcc), stable in the approximate range of 178–600 °C. The XRD pattern of the calcined Ag2S-BSA QDs sample at 200 °C (Figure 1D) showed peaks at 2θ = 31.9°, 32.2°, 32.4°, 34.1°, 38.1°, and 44.3°, corresponding to the crystallographic planes of the monoclinic α-Ag2S phase (acanthite, JCPDS-14-0072), indexed as (110), (112), (113), (122), (104), (003), and (202). These results confirm the predominance of the monoclinic phase, which is the thermodynamically stable form of Ag2S at room temperature. No significant peaks were observed at 44.3°, 64.4°, or 77.5° that would indicate the presence of face-centered cubic metallic silver (Ag fcc), nor were any signals associated with Ag2O detected, ruling out the presence of major crystalline impurities. A broad halo between 15–25° (2θ) was observed, attributed to amorphous residues derived from BSA calcination, likely carbon or other organic compounds. These findings indicate that calcination did not cause significant grain growth or structural alteration, and the monoclinic phase of Ag2S QDs was preserved at room temperature.
The Ag2S-BSA QDs exhibited high cell viability (>95%) across all tested concentrations (50, 100, and 200 µg/mL), indicating low cytotoxicity under the evaluated conditions (Figure 1D). No significant differences were observed between the tested concentrations.
The cross-linking between Ag2S-BSA QDs and the anti-PSG1 monoclonal antibody, achieved through a chemical reaction with EDC/Sulfo-NHS (Figure 2A), revealed the presence of intermediate functional groups in the FTIR spectrum shown in Figure 2B [19]. The purple spectrum (Figure 2(B1)) corresponds to BSA, where the absorption peaks at 1628 cm−1 and 1546 cm−1 are attributed to the vibrational modes of amide I and amide II, respectively, characteristic of protein structures such as BSA [15]. The spectrum of Ag2S-BSA QDs (Figure 2(B2)) displays the same peaks, with no additional bands, indicating preservation of the protein structure. Upon addition of the EDC/Sulfo-NHS cross-linking agents (Figure 2(B3)), a new peak appears at 1054 cm−1, corresponding to the N–O bond of the intermediate succinimide ester, along with an increase at 1024 cm−1, associated with C=O functional groups from the cross-linkers [20]. Finally, after conjugation with the anti-PSG1 monoclonal antibody (Figure 2(B4)), the 1054 cm−1 peak disappears, indicating the consumption of the succinimide intermediate and the formation of an amide bond, thus confirming successful conjugation with the antibody.
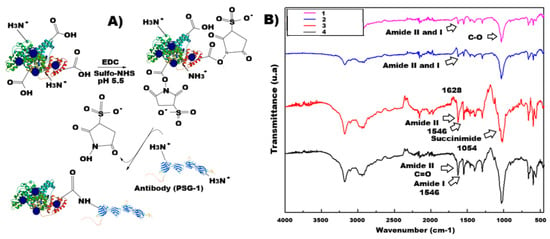
Figure 2.
(A) Schematic of the conjugation procedure; (B) FTIR spectra of (B1) BSA, (B2) Ag2S-BSA QDs, (B3) Ag2S-BSA QDs/EDC/Sulfo NHS, and (B4) conjugate.
The conjugate exhibited an average particle size of 22.82 ± 9.7 nm (Figure 3A), which is suitable for avoiding rapid renal clearance, a process that primarily affects nanoparticles smaller than 10 nm and enables prolonged circulation in the bloodstream [21]. This size range is also within the ideal window for facilitating passive accumulation in tumor tissues through the Enhanced Permeability and Retention (EPR) effect [22]. Moreover, following conjugation of the Ag2S-BSA QDs with the anti-PSG1 monoclonal antibody, the zeta potential decreased from +105.4 mV to +85.7 mV (Figure 1B and Figure 3A). The decrease in zeta potential observed after conjugation of the Ag2S-BSA QDs with the anti-PSG1 monoclonal antibody can be attributed to the physicochemical characteristics of the antibody. As a glycoprotein, the antibody contains a variety of functional groups on its surface, including amine, carboxyl, hydroxyl, and thiol groups. When it is conjugated to the QD surface, it forms a protein corona-like shell that partially shields the original surface charge of the nanoparticle, resulting in a lower measured zeta potential [23]. This effect is well documented in nanoparticle/biomolecule conjugates, where the adsorption or covalent attachment of macromolecules leads to surface modification, changes in electrostatic interactions, and charge redistribution [23]. In this case, the antibody may also introduce steric hindrance, further stabilizing the colloid through a combination of electrostatic and steric repulsion. Despite the reduction in surface charge, the final zeta potential remains high enough (+85.7 mV) to ensure strong electrostatic repulsion and good colloidal stability under physiological conditions [24]. Conjugation with anti-PSG1 monoclonal antibody is expected to facilitate active targeting, since glycoprotein PSG1 can recognize and bind to receptors expressed on tumor cells of the cervix, breast, colon, ovary, and other tissues, thereby promoting specific internalization of the conjugate [12]. This active targeting mechanism is complemented by passive accumulation due to the nanoparticle’s appropriate size.
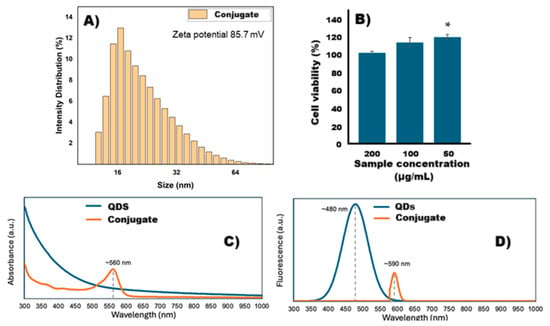
Figure 3.
(A) Size of nanoparticles, (B) cell viability, (C) Uv–vis spectra, and (D) fluorescence spectra of conjugate. ∗: significant difference compared to 200 µg/mL sample.
To evaluate the cytotoxicity of the conjugate, fibroblast cells were exposed to various concentrations, and in vitro cytotoxicity was assessed using the MTT assay after 24 h. As shown in Figure 3B, no cytotoxicity was observed in fibroblasts, and cell viability remained consistent across concentrations of 200, 100, and 50 μg/mL. These results support the conjugate’s biocompatibility and its potential for safe use in photothermal therapy.
The fluorescence emission spectra are shown in Figure 3D. When excited at 560 nm, only the conjugate exhibits a fluorescence emission band, centered at approximately 590 nm. In contrast, the BSA-Ag2S QDs do not show detectable fluorescence under this excitation condition, suggesting that conjugation with PSG1 alters the optical properties of the QDs, possibly by modifying surface states or energy levels. To further explore the fluorescence behavior of BSA-Ag2S QDs, a shorter excitation wavelength of 400 nm was employed, guided by literature reports indicating typical Ag2S QD absorption bands in the 400–600 nm range [25,26,27]. Under this excitation, the BSA-Ag2S QDs exhibit an emission band at approximately 480 nm, which is consistent with the quantum confinement effect, where shorter excitation wavelengths correspond to higher bandgap energies a characteristic property of quantum dots [28,29,30,31]. The absence of a distinct QD absorption band in the UV–Vis spectra of BSA-Ag2S QDs may be attributed to spectral overlap (Figure 3C). The high concentration of BSA produces a strong, broad absorption band around 280 nm, which may obscure the weaker Ag2S QD absorbance signal due to their relatively lower concentration. This overlap could explain why the QD excitation band is not visually apparent in the UV–Vis spectrum. Additionally, the fluorescence intensity of the BSA-Ag2S QDs (excited at 400 nm) is notably higher than that of the conjugate (excited at 560 nm). This difference is likely due to the dilution step required during PSG1 conjugation, which reduces the overall QD concentration in the final sample.
During the photothermal analysis of the conjugate composed of Ag2S-BSA QDs and the anti-PSG1 monoclonal antibody (Figure 4), efficient conversion of NIR light into thermal energy was observed at different concentrations (200, 100, and 50 µg/mL). Conversely, the control (triple-distilled water) showed only a minimal rise in temperature. After 10 min of irradiation, starting from an initial temperature of 37 °C, the conjugate reached temperatures of 50.6 °C, 46.5 °C, and 41.8 °C for concentrations of 200, 100, and 50 µg/mL, respectively. These increases correspond to ΔT values of 13.6, 9.5, and 4.8 °C (Figure 4A), which are considered sufficient to induce thermal damage in cancer cells. [8,32]. These results confirm that conjugation of the Ag2S-BSA QDs with the monoclonal antibody did not impair their NIR absorbance. The infrared thermal images of the conjugate suspensions (Figure 4C) visually confirm the rapid temperature rise upon NIR irradiation. To assess photothermal stability, the conjugate at a concentration of 200 µg/mL was subjected to three irradiation–cooling cycles using an 808 nm laser for 10 min, followed by natural cooling (Figure 4B). No degradation or loss of heating capacity was observed during the cycles, highlighting the conjugate’s excellent photothermal stability and potential as a photothermal agent. Figure 4D shows the thermal response of the conjugate after 5 min of irradiation, demonstrating a concentration-dependent temperature increase, reaching up to 46.9 °C at 200 µg/mL. This behavior confirms the photothermal efficiency of the nanoparticles.
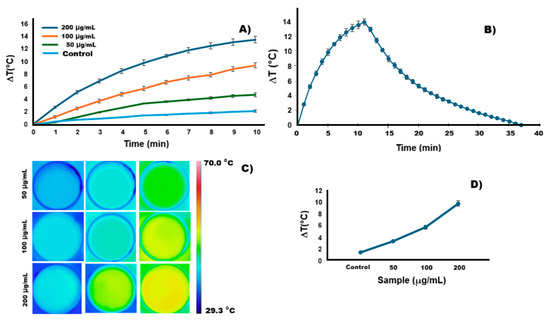
Figure 4.
(A) Heating curves of the conjugate suspension at different concentrations and of the control sample under 808 nm laser irradiation. (B) Photothermal heating curve of the conjugate (200 µg/mL) during irradiation and the corresponding cooling curve after the laser was turned off. (C) Infrared thermal images of the bioconjugate suspension at different concentrations and the control sample, obtained under 808 nm laser irradiation. (D) The temperature change (ΔT) was measured during 5 min of irradiation for various concentrations of the conjugate and the control sample.
Based on the model proposed by Roper in 2007 [33] and considering a laser power of 2.4 W and an absorbance of 0.34, the photothermal conversion efficiency (PCE) of the conjugate system was estimated to be 11.41 ± 0.0435%. Although this value is moderate and lower than that reported for other systems with higher absorbance [2], it is essential to note that our experiments were conducted at 37 °C, under physiologically relevant conditions. In contrast, most studies in the literature calculate PCE starting from room temperature (~25 °C), where a greater temperature differential (ΔT) can increase the apparent efficiency. Despite this, our conjugate reached therapeutically relevant temperatures of 50.6 °C, starting from 37 °C at a relatively low concentration (200 µg/mL), demonstrating effective photothermal performance. For example, Ag2S-based nanoprobes modified with PEG have been reported to have PCE values up to 45.17%. Still, these results were obtained starting from lower initial temperatures and at higher concentrations (1 mg/mL), which contribute to higher ΔT values [10]. Our system, although not PEGylated, shows excellent photothermal stability, biocompatibility, and selective cytotoxicity upon irradiation, making it a promising candidate for photothermal therapy. Future optimization strategies such as PEGylation, particle size tuning, or surface engineering may further enhance the photothermal efficiency and broaden the applicability of the conjugate.
The results obtained from the photothermal therapy assay (Figure 5) indicate that the conjugate alone (without irradiation) did not induce significant cytotoxicity in either HeLa cells or fibroblasts. In fact, it showed an apparent increase in cell viability, reaching 110% in HeLa cells and 102.46% in fibroblasts. This increase suggests that, in the absence of irradiation, the conjugate may exert a proliferative effect or be non-toxic under the experimental conditions used, requiring irradiation to become activated. These findings support biocompatibility and suitability for biomedical applications. Upon irradiation, a significant reduction in HeLa cell viability was observed (48.25% viability, equivalent to 51.75% cytotoxicity), while fibroblasts showed only a slight decrease in viability to 82.7% (17.3% cytotoxicity). This behavior indicates that the cytotoxic activity of the conjugate is dependent on irradiation, a feature characteristic of photoactivatable therapeutic strategies such as photodynamic therapy, light-activated chemotherapy, or photoresponsive conjugates [34]. This selective activation provides a key therapeutic advantage by enabling the targeted destruction of tumor cells while sparing healthy tissues.
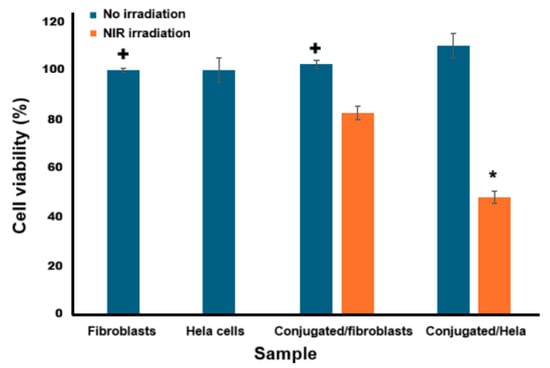
Figure 5.
Cell viability of the HeLa cells after being incubated with the conjugate (200 μg/mL) without and with 3 min NIR irradiation and then cultured in fresh DMEM for an additional 2 h. +: significant difference compared to conjugated/Hela sample, ∗: A significant difference was observed compared to the conjugated/fibroblast sample under NIR irradiation (p = 0.05, comparison is made between samples).
The calculated selectivity index (SI) was 3.0, indicating that the conjugate is three times more toxic to cancer cells than to normal cells under irradiation. This level of selectivity is considered both clinically acceptable and promising, as it allows the therapeutic effect to be focused on tumor tissue while minimizing collateral damage. In general, an SI ≥ 3 is regarded as a benchmark for good selectivity, reinforcing the therapeutic potential of the conjugate for oncological applications [8]. The selective cytotoxicity observed in HeLa cells aligns with the reported overexpression of PSG1 and suggests potential targeting by the conjugate. However, direct evidence of PSG1-specific binding was not obtained in this study. Additional experiments, such as immunostaining, Western blotting, or receptor-blocking assays, are needed to confirm the mechanism of cell recognition and uptake.
Despite the promising results observed in HeLa cells, this study is limited to a single cancer cell line. Therefore, the assertion of cancer-specific applicability should be interpreted with caution. Planned future work will include a broader panel of cancer cell lines, such as breast, ovarian, and colon cancer models, to mimic the tumor microenvironment better and validate the conjugate’s therapeutic potential across different oncological contexts. These additional evaluations will be essential to establish the bioconjugate’s selectivity and translational relevance more comprehensively.
Although mechanistic assays such as apoptosis/necrosis discrimination or ROS detection were not performed, prior studies on Ag2S quantum dots and similar photothermal nanomaterials provide insights into possible mechanisms of action. Upon irradiation, Ag2S-based nanoparticles have been reported to induce localized hyperthermia, leading to protein denaturation and membrane disruption, which can trigger apoptotic or necrotic pathways [11]. In some instances, the photothermal effect also promotes the generation of reactive oxygen species (ROS), contributing to oxidative stress and mitochondrial dysfunction [35,36]. Moreover, antibody conjugation, such as with PSG1 in this case, may enhance selective uptake via receptor-mediated endocytosis, increasing intracellular accumulation and localized damage in cancer cells expressing the target antigen [10]. These proposed mechanisms are consistent with the selective cytotoxicity observed in HeLa cells under irradiation. Nonetheless, future studies are required to clarify the specific pathways involved.
4. Conclusions
The Ag2S-BSA QDs conjugated with Anti-PSG1 Monoclonal Antibodies resulted in a stable, biocompatible, and photoactivatable nanomaterial with potential for targeted photothermal therapy. The conjugate showed strong NIR photoluminescence and selective cytotoxicity upon 808 nm irradiation, significantly reducing HeLa cell viability (48.25%) while sparing fibroblasts (82.7%). In the absence of irradiation, the conjugate was non-toxic and even enhanced cell viability, confirming its safety under physiological conditions. A selectivity index of 3.0 indicates preferential toxicity toward cancer cells, aligning with therapeutic benchmarks. Although PSG1-specific targeting is suggested by HeLa cell response, further studies are needed to confirm receptor-mediated uptake. Future work will include testing on additional cancer models and mechanistic assays to validate its clinical potential.
Author Contributions
Conceptualization, D.M.-O., M.S. and I.O.-A.; methodology, D.M.-O., I.O.-A., S.-A.M.-E., M.E.M.-D., A.V.-R., C.C.-G. and P.E.-R.; formal analysis, M.E.M.-D., A.V.-R., S.-A.M.-E. and F.J.-V.; investigation, D.M.-O., I.O.-A., P.E.-R., M.F.A.-G. and L.E.V.-G.; resources, I.O.-A.; data curation, D.M.-O.; writing—original draft preparation, D.M.-O.; writing—review and editing, I.O.-A.; visualization, F.J.-V. and M.S.; project administration, I.O.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures involving animals were conducted in accordance with institutional and international guidelines for the care and use of laboratory animals. Primary fibroblasts were isolated from Balb/c mice following approval by the Research Ethics Committee of Universidad Autonoma de Ciudad Juarez, under protocol number CEI-2025-1-31. The permit was granted on 25 February 2025. The monoclonal antibody against PSG1 (catalog # MA5-30794, Thermo Fisher Scientific) was purchased from a certified commercial supplier. No animals or human subjects were involved in the production of this antibody.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
Daniel Martínez Osuna expresses his gratitude to the SECIHTI for their support of the Ph.D. Program in Materials Sciences. He also thanks the working group at the Universidad Autónoma de Ciudad Juárez for providing the facilities necessary for this research, particularly the Departments of Physics and Mathematics, Chemistry, and Biology. The authors also wish to acknowledge the Laboratorio Nacional de Nanotecnología (Nanotech), with special appreciation to Raúl Armando Ochoa Gamboa and Marco Antonio Ruiz Esparza Rodríguez for their technical assistance in acquiring the TEM images.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| QDs | Quantum Dots |
| PSG1 | Pregnancy-specific β-1 glycoprotein 1 |
| BSA | Bovine serum albumin |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide |
References
- Feng, J.; Chen, L.; Xia, Y.; Xing, J.; Li, Z.; Qian, Q.; Wang, Y.; Wu, A.; Zeng, L.; Zhou, Y. Bioconjugation of Gold Nanobipyramids for SERS Detection and Targeted Photothermal Therapy in Breast Cancer. ACS Biomater. Sci. Eng. 2017, 3, 608–618. [Google Scholar] [CrossRef]
- Tao, W.; Ji, X.; Xu, X.; Islam, M.A.; Li, Z.; Chen, S.; Saw, P.E.; Zhang, H.; Bharwani, Z.; Guo, Z.; et al. Antimonene quantum dots: Synthesis and application as near-infrared photothermal agents for effective cancer therapy. Angew. Chem. 2017, 129, 11896–11900. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, A.; Chatterjee, M.; Singh, G. Present scenario of bioconjugates in cancer therapy: A review. Int. J. Mol. Sci. 2019, 20, 5243. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Khawar, M.B.; Liang, J.; Sun, H. Bio-Conjugated Quantum Dots for Cancer Research: Detection and Imaging. Front. Oncol. 2021, 11, 749970. [Google Scholar] [CrossRef]
- Krishna, R.H.; Chandraprabha, M.N.; Monika, P.; Br, T.; Chaudhary, V.; Manjunatha, C. Biomolecule conjugated inorganic nanoparticles for biomedical applications: A review. Biotechnol. Genet. Eng. Rev. 2024, 40, 3611–3652. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Zhao, Y.-W.; Zhang, Z.-Y.; Xiong, H.-M.; Yu, S.-N. One-pot synthesis of water-dispersible Ag2S quantum dots with bright fluorescent emission in the second near-infrared window. Nanotechnology 2013, 24, 55706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, M.; Liu, H.; Zhang, S. Low-toxic Ag2S quantum dots for photoelectrochemical detection glucose and cancer cells. Biosens. Bioelectron. 2014, 56, 307–312. [Google Scholar] [CrossRef]
- Hu, X.L.; Kwon, N.; Yan, K.C.; Sedgwick, A.C.; Chen, G.R.; He, X.P.; James, T.D.; Yoon, J. Bio-Conjugated Advanced Materials for Targeted Disease Theranostics. Adv. Funct. Mater. 2020, 30, 1907906. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, N.; Qin, Y.; Wu, F.; Xu, Z.; Lan, T.; Liu, H. Affibody-functionalized Ag2S quantum dots for photoacoustic imaging of epidermal growth factor receptor overexpressed tumors. Nanoscale 2018, 10, 16581–16590. [Google Scholar] [CrossRef]
- Cui, X.; Hu, Z.; Li, R.; Jiang, P.; Wei, Y.; Chen, Z. CA IX-targeted Ag2S quantum dots bioprobe for NIR-II imaging-guided hypoxia tumor chemo-photothermal therapy. J. Pharm. Anal. 2024, 14, 100969. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Wu, Y.; Jiang, X.; Ji, Y.; Braeckmans, K.; Xu, X. Long-term in vivo immune tracking nanoplatform based on Ag2S quantum dots for the photothermal immunotherapy of breast cancer. BMC Biol. 2025, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Esquivel, M.; Romero-Morelos, P.; Taniguchi-Ponciano, K.; Mendoza-Rodriguez, M.; Marrero-Rodriguez, D.; Bandera-Delgado, A.; Huerta-Padilla, V.; Serna-Reyna, L.; Gómez-Gutiérrez, G.; Gómez-Virgilio, L.; et al. Expression of Pregnancy Specific β-1 Glycoprotein 1 in Cervical Cancer Cells. Arch. Med. Res. 2020, 51, 504–514. [Google Scholar] [CrossRef]
- Horne, C.H.; Reid, I.N.; Milne, G.D. Prognostic significance of inappropriate production of pregnancy proteins by breast cancers. Lancet 1976, 2, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. Near-Infrared Photoluminescent Ag2S Quantum Dots from a Single Source Precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Sandoval, N.; Estrada Rojas, P.; Olivas-Armendáriz, I.; Valencia Gómez, L.E.; Hernández Paz, J.F.; Monarrez Cordero, B.E.; Rodríguez González, C.A. Effect of Ag2S-BSA nanoparticle size on 3T3 fibroblast cell line cytotoxicity. J. Nanoparticle Res. 2020, 22, 106. [Google Scholar] [CrossRef]
- Al-Jawad, S.M.; Taha, A.A.; Al-Halbosiy, M.M.; Al-Barram, L.F. Synthesis and characterization of small-sized gold nanoparticles coated by bovine serum albumin (BSA) for cancer photothermal therapy. Photodiagnosis Photodyn. Ther. 2018, 21, 201–210. [Google Scholar] [CrossRef]
- Siddiq, A.M.; Murugan, D.; Srivastava, R.; Alam, M.S. Influence of pH on interaction of silver nanoparticles-protein: Analyses by spectroscopic and thermodynamic ideology. Colloids Surf. B Biointerfaces 2019, 184, 110524. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Yu, B.; Browne, C.; Garnier, G. Reversible pH responsive bovine serum albumin hydrogel sponge nanolayer. Front. Bioeng. Biotechnol. 2020, 8, 573. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Analytical Techniques for Characterizing Tumor-Targeted Antibody-Functionalized Nanoparticles. Life 2024, 14, 489. [Google Scholar] [CrossRef]
- Widyasari, D.A.; Kristiani, A.; Randy, A.; Manurung, R.V.; Dewi, R.T.; Andreani, A.S.; Yuliarto, B.; Jenie, S.A. Optimized antibody immobilization on natural silica-based nanostructures for the selective detection of E. coli. RSC Adv. 2022, 12, 21582–21590. [Google Scholar] [CrossRef]
- Buzun, K.; Kryshchyshyn-Dylevych, A.; Senkiv, J.; Roman, O.; Gzella, A.; Bielawski, K.; Bielawska, A.; Lesyk, R. Synthesis and Anticancer Activity Evaluation of 5-[2-Chloro-3-(4-nitrophenyl)-2-propenylidene]-4-thiazolidinones. Molecules 2021, 26, 3057. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomedicine 2015, 11, 1893–1907. [Google Scholar] [CrossRef]
- Lee, N.K.; Wang, C.P.; Lim, J.; Park, W.; Kwon, H.K.; Kim, S.N.; Park, C.G. Impact of the conjugation of antibodies to the surfaces of polymer nanoparticles on the immune cell targeting abilities. Nano Converg. 2021, 8, 24. [Google Scholar] [CrossRef]
- Amezaga Gonzalez, M.F.; Ramirez-Reyes, A.; Mendoza-Duarte, M.E.; Vega-Rios, A.; Martinez-Ozuna, D.; Rodriguez-Gonzalez, C.A.; Martel-Estrada, S.A.; Olivas-Armendariz, I. Stability of Carbon Quantum Dots for Potential Photothermal and Diagnostic Applications. C 2025, 11, 56. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, H.; Yang, H.; Zhu, L.; Ding, C.; Zhang, G.; Bi, J.; Yan, S.; Liu, G.; Hou, H. UV-Vis-NIR-light-driven Ag2O/Ag2S/CuBi2O4 double Z-scheme configuration for enhanced photocatalytic applications. Mater. Sci. Semicond. Process. 2021, 126, 105668. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, J.; Li, C.; Zhou, G.; Yang, W.; Wang, D.; Zheng, H.; Du, Y.; Li, X.; Li, Q. Near-infrared-emitting colloidal Ag2S quantum dots excited by an 808 nm diode laser. J. Mater Sci. 2017, 52, 9424–9429. [Google Scholar] [CrossRef]
- Tubtimtae, A.; Wu, K.L.; Tung, H.Y.; Lee, M.W.; Wang, G.J. Ag2S quantum dot-sensitized solar cells. Electrochem. Commun 2010, 12, 1158–1160. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Mao, S.; Wang, Y.; Shen, W.; Wang, W.; Huang, L.; Li, H.; Tang, J. Recent progress in synthetic methods and applications in solar cells of Ag2S quantum dots. Mater. Res. Bull. 2018, 106, 113–123. [Google Scholar] [CrossRef]
- Soltani, N.; Gharibshahi, E.; Saion, E. Band gap of cubic and hexagonal cds quantum dots-experimental and theoretical studies. Chalcogenide Lett. 2012, 9, 321–328. [Google Scholar]
- Baskoutas, S.; Terzis, A.F. Size-dependent band gap of colloidal quantum dots. J. Appl. Phys. 2006, 99, 013708. [Google Scholar] [CrossRef]
- Lin, K.F.; Cheng, H.M.; Hsu, H.C.; Lin, L.J.; Hsieh, W.F. Band gap variation of size-controlled ZnO quantum dots synthesized by sol-gel method. Chem. Phys. Lett. 2005, 409, 208–211. [Google Scholar] [CrossRef]
- Yao, X.; Tian, Z.-F.; Liu, J.; Zhu, Y.; Hanagata, N. Mesoporous Silica Nanoparticles Capped with Graphene Quantum Dots for Potential Chemo-Photothermal Synergistic Cancer Therapy. Langmuir 2016, 33, 591–599. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]
- Wong, C.H.; Khor, B.-K.; Murugaiyah, V.; Chear, N.J.-Y.; Yam, W.S. Cytotoxicity Cell Line Selectivity and Proapoptotic Activity of New Anticancer Agents Derived From N,N′-Functionalised Benzimidazolium Salts and Their Silver(I)-N-Heterocyclic Carbene Complexes. Drug Dev. Res. 2025, 86, e70100. [Google Scholar] [CrossRef] [PubMed]
- Hashemkhani, M.; Celikbas, E.; Khan, M.; Sennaroglu, A.; Acar, H.Y. ALA/Ag2S/MnO2 hybrid nanoparticles for near-infrared image-guided long-wavelength phototherapy of breast cancer. ACS Biomater. Sci. Eng. 2023, 9, 4126–4137. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Liu, X.; Wang, J.; Qiu, X.; Wei, W.; Zhao, J. Peptide-mediated Aqueous Synthesis of NIR-II Emitting Ag2S Quantum Dots for Rapid Photocatalytic Bacteria Disinfection. Angew. Chem. 2023, 135, e202300085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).