Sustainable Remediation of Polluted Soils from the Oil Industry Using Sludge from Municipal Wastewater Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioremediation Experiment of Petroleum-Contaminated Soil Treated with Sewage Sludge
2.2. Physicochemical Analysis of Petroleum-Products-Contaminated Soil Treated with Sewage Sludge
2.3. Enumeration and Isolation of Bacteria from Petroleum-Contaminated Soil Treated with Sewage Sludge
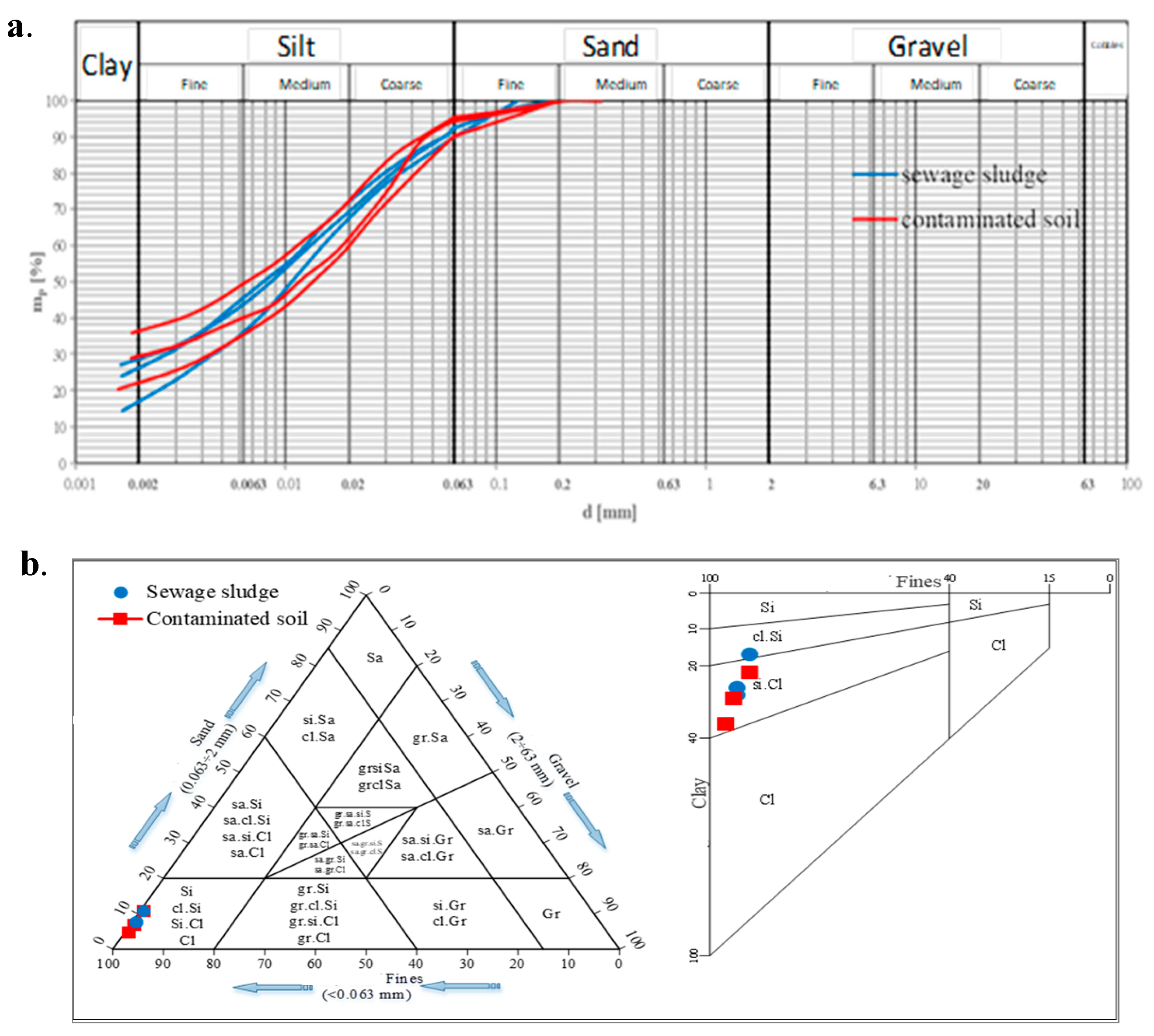
2.4. Geotechnical Tests for Petroleum-Contaminated Soil Treated with Sewage Sludge
3. Results and Discussion
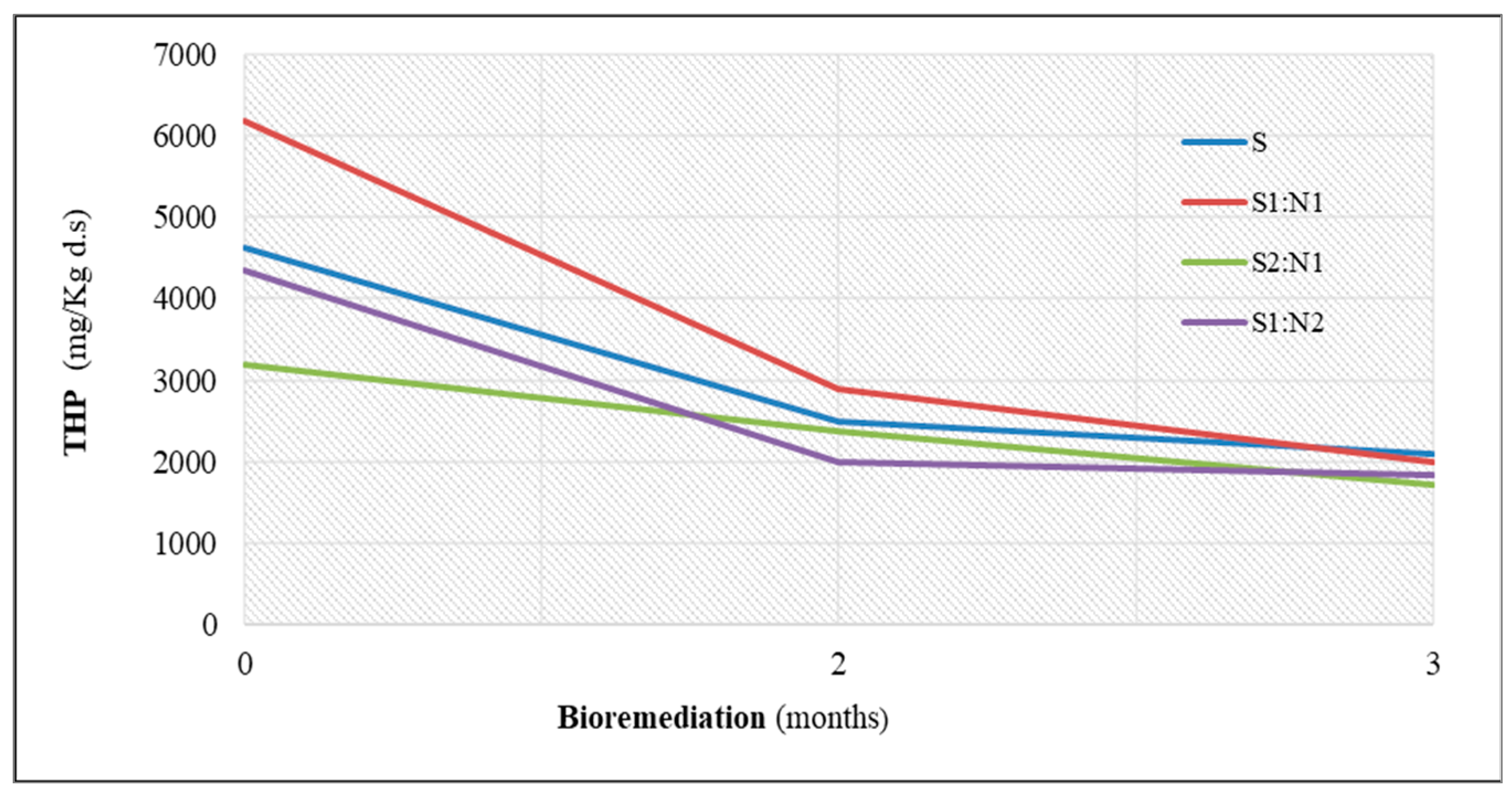
3.1. Bioremediation Experiment of Petroleum-Contaminated Soil Treated with Sewage Sludge
3.2. Physicochemical Analysis of Petroleum-Products-Contaminated Soil Treated with Sewage Sludge
3.3. Enumeration and Isolation of Bacteria from Petroleum-Contaminated Soil Treated with Sewage Sludge
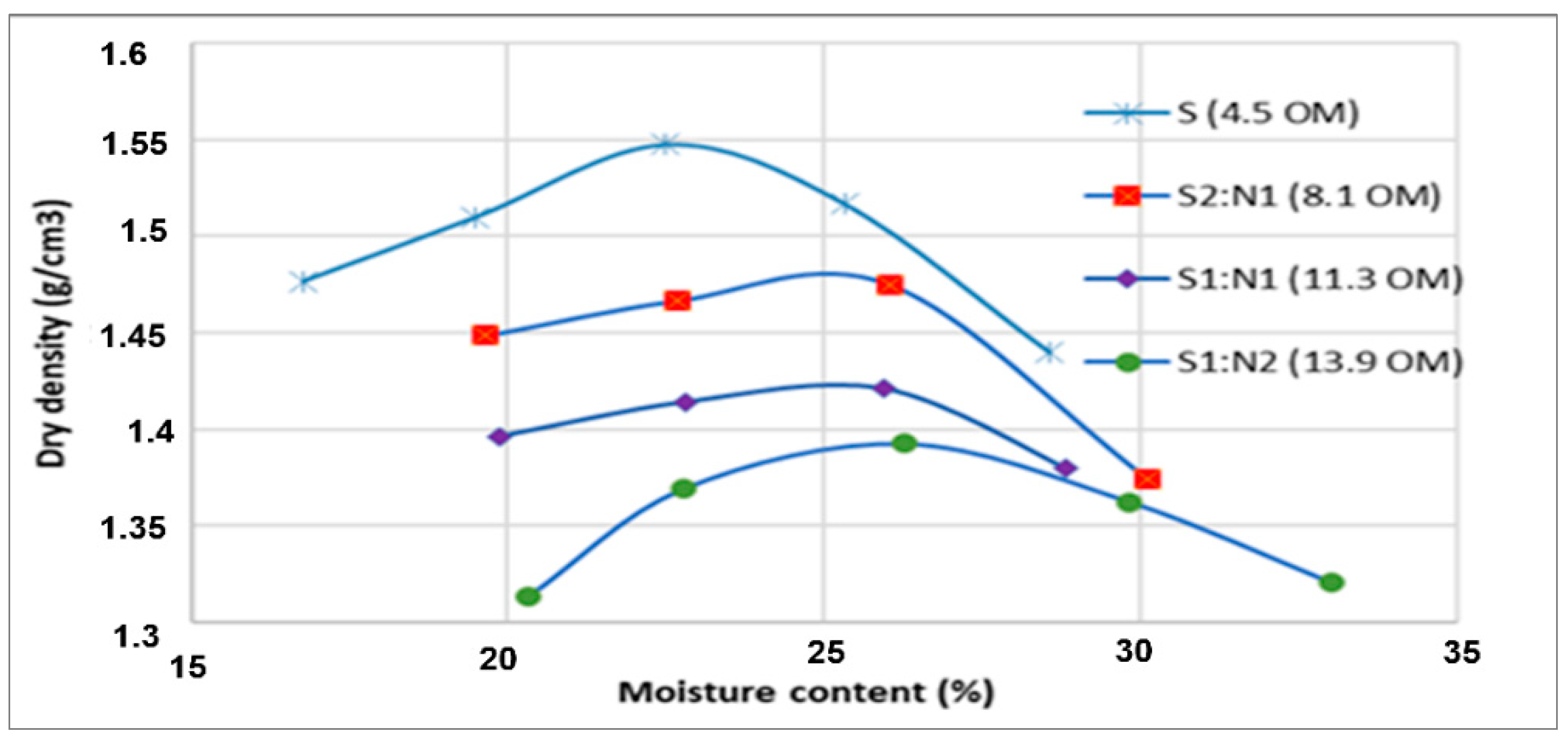
3.4. Geotechnical Tests for Petroleum-Contaminated Soil Treated with Sewage Sludge
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mustafa, A.D.; Juahir, H.; Yunus, K.; Amran, M.A.; Hasnam, C.N.C.; Azaman, F.; Abidin, I.Z.; Azmee, S.H.; Sulaiman, N.H. Oil spill related heavy metal: A review. Malays. J. Anal. Sci. 2015, 19, 1348–1360. [Google Scholar]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Stancu, M.M. Kerosene tolerance in Achromobacter and Pseudomonas species. Ann. Microbiol. 2020, 70, 8. [Google Scholar] [CrossRef]
- Stancu, M.M. Characterization of new diesel-degrading bacteria isolated from freshwater sediments. Int. Microbiol. 2023, 26, 109–122. [Google Scholar] [CrossRef]
- Stancu, M.M. Characterization of a new Pseudomonas aeruginosa strain isolated from petroleum-polluted soil. Waste Biomass Valorization 2024, 1–15. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil–A field study. Int. Biodeterior. Biodegrad. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Dragh, M.A.; Li, S.; Alhujaily, A.; Abbood, H.A.; Zhang, X.; Ma, F. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt. J. Aquat. Res. 2018, 44, 71–76. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- Ravi, A.; Ravuri, M.; Krishnan, R.; Narenkumar, J.; Anu, K.; Alsalhi, M.S.; Devanesan, S.; Kamala-Kannan, S.; Rajasekar, A. Characterization of petroleum degrading bacteria and its optimization conditions on effective utilization of petroleum hydrocarbons. Microbiol. Res. 2022, 265, 127184. [Google Scholar] [CrossRef]
- Jia, W.; Cheng, L.; Tan, Q.; Liu, Y.; Dou, J.; Yang, K.; Yang, Q.; Wang, S.; Li, J.; Niu, G.; et al. Response of the soil microbial community to petroleum hydrocarbon stress shows a threshold effect: Research on aged realistic contaminated fields. Front. Microbiol. 2023, 14, 1188229. [Google Scholar] [CrossRef] [PubMed]
- Abena, M.T.B.; Li, T.; Shah, M.N.; Zhong, W. Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere 2019, 234, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Chicca, I.; Becarelli, S.; Di Gregorio, S. Microbial involvement in the bioremediation of total petroleum hydrocarbon polluted soils: Challenges and perspectives. Environments 2022, 9, 52. [Google Scholar] [CrossRef]
- Mohammadi, L.; Rahdar, A.; Bazrafshan, E.; Dahmardeh, H.; Susan, M.A.B.H.; Kyzas, G.Z. Petroleum hydrocarbon removal from wastewaters: A review. Processes 2020, 8, 447. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge–the current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Kicińska, A.; Kosa-Burda, B.; Kozub, P. Utilization of a sewage sludge for rehabilitating the soils degraded by the metallurgical industry and a possible environmental risk involved. Hum. Ecol. Risk Assess. 2018, 24, 1990–2010. [Google Scholar] [CrossRef]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Cebron, A.; Esposito, G.; van Hullebusch, E.D. Functional potential of sewage sludge digestate microbes to degrade aliphatic hydrocarbons during bioremediation of a petroleum hydrocarbons contaminated soil. J. Environ. Manag. 2021, 280, 111648. [Google Scholar] [CrossRef]
- Satoh, Y.; Ishizuka, S.; Hiradate, S.; Nagano, M.A.A.H.; Koarashi, J. Sequential loss-on-ignition as a simple method for evaluating the stability of soil organic matter under actual environmental conditions. Environ. Res. 2023, 239, 117224. [Google Scholar] [CrossRef]
- EN ISO 14688-1; Geotechnical Research and Testing—Soil Identification and Classification Part 1: Identification and De-Scription. NEN: Delft, The Netherlands, 2018.
- Simionov, I.A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.L.; Faggio, C.; Petrea, Ş.M. Human health risk assessment of potentially toxic elements and microplastics accumulation in products from the Danube River Basin fish market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef]
- Okparanma, R.N.; Mouazen, A.M. Determination of total petroleum hydrocarbon (TPH) and polycyclic aromatic hydrocarbon (PAH) in soils: A review of spectroscopic and non-spectroscopic techniques. Appl. Spectrosc. Rev. 2013, 48, 458–486. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Stancu, M.M.; Grifoll, M. Multidrug resistance in hydrocarbon-tolerant Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2011, 57, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, B.A.; Aragaw, T.A.; Genet, M.B. Bioremediation of petroleum hydrocarbon contaminated soil: A review on principles, degradation mechanisms, and advancements. Front. Environ. Sci. 2024, 12, 1354422. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Calvo, A.R.; Laguna, J.; González-López, J.; Calvo, C. Autochthonous microbial responses and hydrocarbons degradation in polluted soil during biostimulating treatments under different soil moisture. Assay in pilot plant. Int. Biodeterior. Biodegrad. 2016, 108, 91–98. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front. Microbiol. 2016, 7, 1092. [Google Scholar] [CrossRef]
- Onojake, M.C.; Omokheyeke, O.; Osakwe, J.O. Hydrocarbon contamination to environment. Bull. Earth Sci. Thail. 2014, 6, 67–79. [Google Scholar]
- Devatha, C.P.; Vishnu, A.V.; Rao, J.P.C. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Gui, Y.; Zhang, Q.; Qin, X.; Wang, J. Influence of organic matter content on engineering properties of clays. Adv. Civ. Eng. 2021, 2021, 6654121. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter Key to Drought-Resistant Soil and Sustained Food Production; FAO Soils Bulletin 80; FAO: Rome, Italy, 2005. [Google Scholar]
- Ruehlmann, J. Soil particle density as affected by soil texture and soil organic matter: 1. Partitioning of SOM in conceptional fractions and derivation of a variable SOC to SOM conversion factor. Geoderma 2020, 375, 114542. [Google Scholar] [CrossRef]
- Ruehlmann, J.; Körschens, M. Soil particle density as affected by soil texture and soil organic matter: 2. Predicting the effect of the mineral composition of particle-size fractions. Geoderma 2020, 375, 114543. [Google Scholar] [CrossRef]
- Abena, M.T.B.; Chen, G.; Chen, Z.; Zheng, X.; Li, S.; Li, T.; Zhong, W. Microbial diversity changes and enrichment of potential petroleum hydrocarbon degraders in crude oil-, diesel-, and gasoline-contaminated soil. 3 Biotech 2020, 10, 42. [Google Scholar] [CrossRef]
- Gandolfi, I.; Sicolo, M.; Franzetti, A.; Fontanarosa, E.; Santagostino, A.; Bestetti, G. Influence of compost amendment on microbial community and ecotoxicity of hydrocarbon-contaminated soils. Bioresour. Technol. 2010, 101, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Dados, A.; Omirou, M.; Demetriou, K.; Papastephanou, C.; Ioannides, I.M. Rapid remediation of soil heavily contaminated with hydrocarbons: A comparison of different approaches. Ann. Microbiol. 2015, 65, 241–251. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Swain, C.K. Environmental pollution indices: A review on concentration of heavy metals in air, water, and soil near industrialization and urbanization. Discov. Environ. 2024, 2, 5. [Google Scholar] [CrossRef]
- EN ISO 17892-5; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 5: Incremental Loading Oedometer Test. NSAI: Dublin, Ireland, 2017.


| Parameters | Samples | ||
|---|---|---|---|
| Soil | Sludge | ||
| pH | 7.3 | 6.8 | |
| Moisture (W, %) | 19.06 | 305 | |
| Density of the mineral skeleton (ρsmediu, g cm−3) | 2.662 | 2.674 | |
| Organic matter (%) | Loss-on-ignition | 5.2 | 38.2 |
| Oxidation | 5.5 | 39.3 | |
| Heavy metals (mg/kg ds) | Cadmium | <0.8 | <0.8 |
| Cromium | 19.9 | 30.6 | |
| Copper | 21.6 | 107.0 | |
| Nickel | 26.1 | 28.6 | |
| Lead | 12.2 | 27.0 | |
| Zinc | 50.0 | 348.0 | |
| Petroleum hydrocarbons (mg kg−1 ds) | 4630 | 3810 | |
| Number of Bacteria | Samples | ||||
|---|---|---|---|---|---|
| Soil | Soi and Sludge Mixtures (v/v) | ||||
| S1:N1 | S2:N1 | S1:N2 | |||
| Hydrocarbon-tolerant bacteria (cell g−1) | PI | 2.5 × 1011 | 2.5 × 1011 | 2.5 × 1011 | 2.5 × 1011 |
| PII | 1.6 × 1012 | 2.5 × 1011 | 2.5 × 1011 | 2.5 × 1011 | |
| PIII | 3.0 × 1010 | 2.5 × 1011 | 2.5 × 1011 | 2.5 × 1011 | |
| Hydrocarbon-degrading bacteria (cell g−1) | PI | 1.7 × 1010 | 1.7 × 109 | 1.7 × 107 | 9.5 × 104 |
| PII | 2.0 × 1011 | 2.5 × 1011 | 2.5 × 1011 | 2.5 × 1011 | |
| PIII | 3.0 × 105 | 8.0 × 105 | 9.5 × 105 | 1.7 × 105 | |
| Enterobacteria (cfu g−1) | PI | 2.0 × 105 | 3.6 × 105 | 3.5 × 104 | 2.5 × 104 |
| PII | 0, Ff | 0, Ff | 0, Ff | 0, Ff | |
| PIII | 0, Ff | 0, Ff | 0, Ff | 0, Ff | |
| Hydrocarbon-Degrading Bacteria | CI | CII | CIII | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1.1 | C1.3 | C1.4 | C1.5 | C2.1 | C2.3 | C2.4 | C2.5 | C3.1 | C3.3 | C3.4 | C3.5 | ||
| Growth on diesel | Absorbance (OD660 nm) | 0.97 | 0.90 | 0.95 | 0.89 | 0.82 | 0.80 | 0.75 | 0.69 | 0.76 | 0.70 | 0.68 | 0.58 |
| Viability (LB, %) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Viability (LB-diesel, %) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Viability (EMB, %) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Biosurfactants | Emulsification index (E24, %) | 100 | 50 | 10 | 10 | 50 | 50 | 10 | 10 | 10 | 10 | 10 | 10 |
| Diesel overlay | + | + | – | – | + | + | – | – | – | – | – | – | |
| CTAB blue | – | + | – | – | + | + | + | – | – | – | – | + | |
| Diesel biodegradation | Diesel fragmentation | + | + | + | + | + | + | + | + | + | + | + | + |
| CO2 (mg L−1) | 1848 | 1748 | 1660 | 1630 | 1748 | 1748 | 1560 | 1460 | 1460 | 1460 | 1416 | 1416 | |
| Parameters | Samples | |||||
|---|---|---|---|---|---|---|
| Soil | Soil and Sludge Mixtures (v/v) | |||||
| S1:N1 | S2:N1 | S1:N2 | ||||
| Organic matter (%) | Combustion | PI | 5.2 | ND | ND | ND |
| PIII | 4.5 | 11.3 | 8.1 | 13.9 | ||
| Heavy metals (mg kg−1 ds) | Cadmium (Cd) | PI | <0.8 | <0.8 | <0.8 | <0.8 |
| PIII | <0.8 | <0.8 | <0.8 | <0.8 | ||
| Chromium (Cr) | PI | 19.9 | 22.6 | 20.5 | 24.0 | |
| PIII | 23.5 | 24.3 | 25.6 | 24.2 | ||
| Copper (Cu) | PI | 21.6 | 37.9 | 28.5 | 48.1 | |
| PIII | 22.7 | 37.8 | 33.8 | 42.4 | ||
| Nickel (Ni) | PI | 26.1 | 26.7 | 25.2 | 27.2 | |
| PIII | 26.8 | 25.8 | 27.4 | 26.4 | ||
| Lead (Pb) | PI | 12.2 | 16.3 | 17.4 | 17.1 | |
| PIII | 14.2 | 16.8 | 16.3 | 18.0 | ||
| Zinc (Zn) | PI | 50.0 | 110.0 | 74.2 | 141.0 | |
| PIII | 54.8 | 110.0 | 91.4 | 121.0 | ||
| Petroleum hydrocarbons (mg kg−1 ds) | PI | 4630 | 6190 | 3200 | 4350 | |
| PII | 2500 | 2900 | 2000 | 2380 | ||
| PIII | 2100 | 2000 | 1710 | 1840 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorga, C.M.; Georgescu, L.P.; Ungureanu, C.; Stancu, M.M. Sustainable Remediation of Polluted Soils from the Oil Industry Using Sludge from Municipal Wastewater Treatment Plants. Processes 2025, 13, 245. https://doi.org/10.3390/pr13010245
Iorga CM, Georgescu LP, Ungureanu C, Stancu MM. Sustainable Remediation of Polluted Soils from the Oil Industry Using Sludge from Municipal Wastewater Treatment Plants. Processes. 2025; 13(1):245. https://doi.org/10.3390/pr13010245
Chicago/Turabian StyleIorga, Cristian Mugurel, Lucian Puiu Georgescu, Constantin Ungureanu, and Mihaela Marilena Stancu. 2025. "Sustainable Remediation of Polluted Soils from the Oil Industry Using Sludge from Municipal Wastewater Treatment Plants" Processes 13, no. 1: 245. https://doi.org/10.3390/pr13010245
APA StyleIorga, C. M., Georgescu, L. P., Ungureanu, C., & Stancu, M. M. (2025). Sustainable Remediation of Polluted Soils from the Oil Industry Using Sludge from Municipal Wastewater Treatment Plants. Processes, 13(1), 245. https://doi.org/10.3390/pr13010245







