Abstract
Dual-ion batteries (DIBs) were demonstrated as a promising technology for large-scale energy storage due to their low cost, recyclability, and impressively fast charge capability. Graphite as a commonly used cathode material in DIBs, however, suffers from poor compatibility with commercial Li-ion electrolytes and graphite anodes, making it difficult to directly utilize the well-established infrastructure for Li-ion batteries. Herein, we report a small aromatic amine molecule 4,4′,4″-tris(diphenylamino)triphenylamine (N4) functioning as a compatible anion host in the EC-containing Li-ion electrolyte. With an average discharge voltage of 3.6 V (vs. Li+/Li), the N4 electrode delivers a reversible specific capacity of 108 mAh/g, which is much higher than 29 mAh/g for the graphite cathode at the same condition. The high capacity retention of 91.3% was achieved after 500 cycles at 1 A/g. The N4 electrode also exhibited good rate performance. Via different characterization techniques like Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy, the energy storage mechanism of N4 was revealed as a conversion between amine and quaternary amine cations, accompanied by PF6− (de-)insertion. As consequences, the assembled N4||graphite DIB w showed a high discharge capacity of 90 mAh/g within 1.5–4.1 V, and good cycling stability with a 98% capacity retention after 40 cycles. Decent rate performance was achieved in the N4||graphite DIB as well. This work provides new insights into designing a compatible anion host for affordable DIBs.
1. Introduction
Lithium-ion batteries (LIBs) are the dominant energy storage technology for digital products, electric vehicles, and grid-level renewable energy utilization [1,2,3,4,5]. However, the rare metal elements in the commercial cathode materials for LIBs, such as Co and Ni, have aggravated concerns on their sustainable development [6,7,8,9]. It is of significance to develop sustainable battery systems. Among different technologies, dual-ion batteries (DIBs) have emerged as a potential candidate, and have garnered widespread attention due to their low cost, environmental friendliness, and high working voltage [10,11,12,13]. Different from the rocking-chair Li+ transporting mechanism between the cathode and anode in LIBs, anions uptake in cathodes and Li+ being inserted into an anode occur simultaneously in DIBs [14,15,16,17].
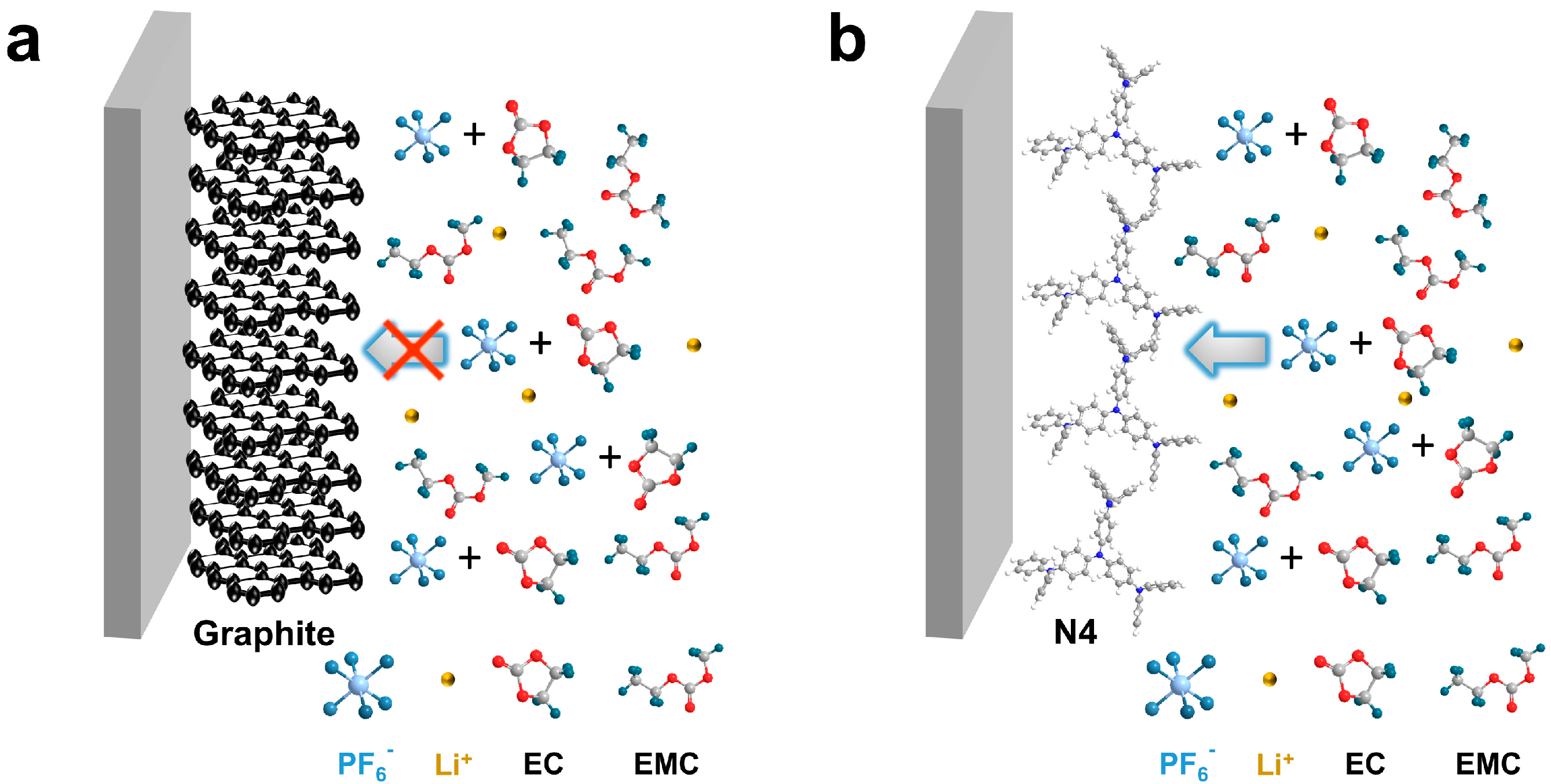
Various anions such as hexafluorophosphate (PF6−), bis(trifluoromethanesulfonyl) imide (TFSI−), perchlorate (ClO4−), and bis(fluorosulfonyl)imide (FSI−) can be electrochemically intercalated into graphite to form graphite intercalation compounds (GICs) [18,19,20,21]. It was reported that solvents in electrolytes have largely influenced the electrochemical performance to graphite cathodes. Generally, graphite delivers a high specific capacity of ~100 mAh/g in a LiPF6-ethyl methyl carbonate (EMC) electrolyte, but a much lower capacity (<30 mAh/g) in a high dielectric constant solvent like ethylene carbonate (EC) [22]. The strong interaction between EC and PF6− inhibits PF6− from becoming desolvated for intercalation into graphite (Scheme 1a). Unfortunately, EC is an indispensable component in commercial Li-ion electrolytes, and acts as a solid electrolyte interphase (SEI)-forming agent for graphite anodes, which is very important for stable operation of LIBs [23,24,25,26]. The incompatibility hinders the direct use of graphite cathodes in commercial Li-ion electrolytes, sacrificing manufacturing convenience of modern Li-ion battery production infrastructures.
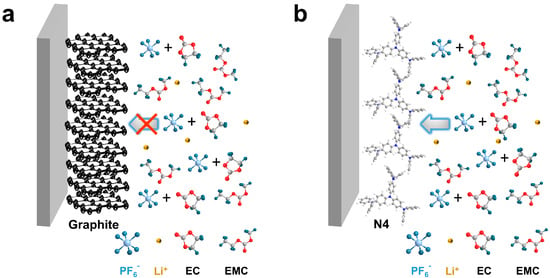
Scheme 1.
Schematic illustration of (a) graphite cathode and (b) N4 cathode working in commercial EC-containing Li-ion electrolyte.
One alternative solution is replacing graphite cathodes with other anion hosts that are insensitive to EC-containing electrolytes. Organic materials composing of nature-abundance C, H, O, N, S, etc., elements stand out due to their rich synthetic paths, recyclability, and tailorable structure for target properties [27,28]. More importantly, organic materials with “soft” lattice may allow structure rearrangement for anion insertion without complete desolvation. Several organic materials were applied as cathode materials in DIBs in the presence of EC. For instance, Obrezk et al. have demonstrated the stable operation of polydiphenylamine PDPA in 1M LiPF6-EC/dimethyl carbonate (1:1, v/v), showing a reversible capacity of 145 mAh/g [29]. Similarly, a polymer PTO-2CZ electrochemically synthesized by carbazole (CZ) and pyrene-4,5,9,10-tetraone (PTO) exhibited fast reaction kinetics and outstanding electrochemical performance in 1 M LiPF6-EC/EMC (3:7, v/v), with a reversible capacity of 202 mAh/g at 0.2 A/g [30]. However, few small organic molecules have been explored as cathodes for DIBs.
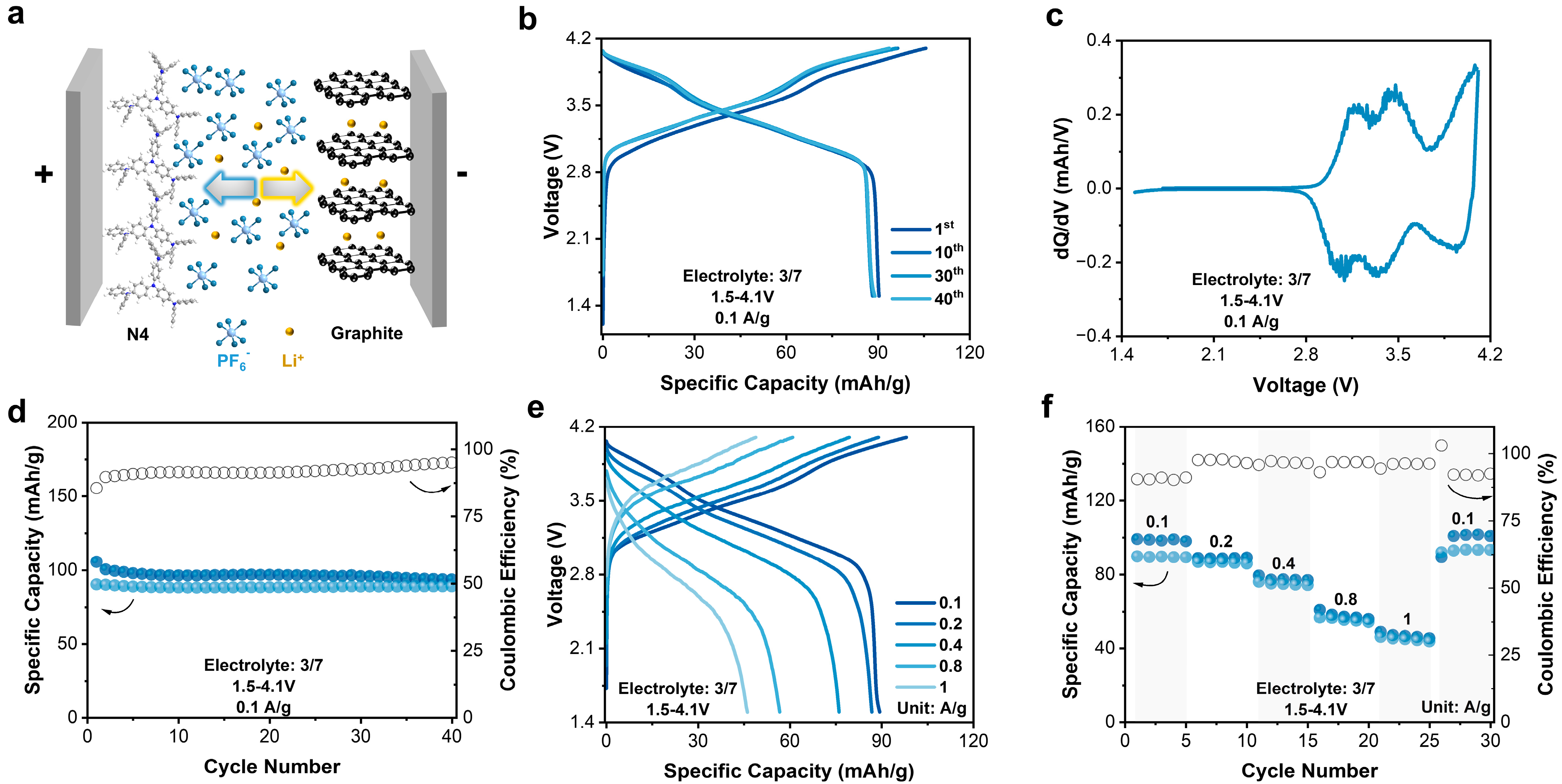
Here, we report a small aromatic amine molecule, 4,4′,4″-tris(diphenylamino)triphenylamine (N4), as a compatible cathode material for Li+-based DIBs in the commercial EC-containing electrolyte (Scheme 1b). The N4 electrode delivered a specific capacity of 108 mAh/g at 0.1 A/g and output an average working potential of 3.6 V vs. Li+/Li in the half cells. However, graphite cathodes can only achieve a low capacity of 29 mAh/g. When the current density increased by forty times from 0.1 to 4 A/g, the discharge-specific capacity of N4 decreased from 108 to 67 mAh/g, demonstrating a fairly good rate performance. Regarding the energy storage mechanism, in the charge process, the amine (NR3) moieties in the N4 molecule were oxidized into quaternary amine radical cations (NR4•+), forming quinone diiminium dynamically. To keep the charge neutrality, PF6− was inserted into the N4 electrode. Because of the high compatibility and redox activity of N4, a N4||graphite full cell was assembled in 2 M LiPF6-EC/EMC (3:7) electrolyte. The DIB displayed an initial discharge capacity of 90 mAh/g at 0.1A/g and maintained a 98.5% capacity after 40 cycles. Our work proves the feasibility of small organic molecules as stable cathode materials for DIBs in commercial EC-containing electrolytes.
2. Materials and Methods
2.1. Materials
Lithium hexafluorophosphate (LiPF6, purity ≥ 97%) was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ethylene carbonate (EC, purity ≥ 99.95%) and ethyl methyl carbonate (EMC, purity ≥ 99.98%) solvents were provided by DoDoChem Technology Co., Ltd. (Suzhou, China). Sodium alginate (Alg, viscosity 1000 mPa.s) was purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Polyvinylidene difluoride (PVDF) was supplied by Arkema (Colbert Essennak, France). N-Methyl-2-pyrrolidone (NMP, purity ≥ 99.95%) was obtained from Aladdin. 4,4′,4″-tris(diphenylamino)triphenylamine (N4, purity 97.0%) was purchased from TCI Chemicals (Shanghai, China). The conductive carbon black (Super P) and mesocarbon microbeads (MCMB-N) were sourced from Dongguan Kelude New Energy Technology Co., Ltd. (Dongguan, China), and acetylene black was provided by Alfa Aesar (Shanghai, China).
2.2. Preparation of Electrolytes
The electrolytes were prepared by dissolving LiPF6 salt in pure EMC and mixed EC/EMC. The LiPF6 electrolyte concentration was controlled at 2 M due to the balanced electrochemical properties in the dual-ion battery [31]. All electrolyte preparations were carried out in an argon-filled glovebox (O2 < 0.1 ppm, H2O < 0.1 ppm).
2.3. Preparation of Electrodes
2.3.1. Preparation of N4 Cathode
The N4 cathode was prepared by mixing N4 (active material), Super P (conductive carbon black), and Alg (binder) in a mass ratio of 70:20:10, with water as the solvent to form a homogeneous slurry. The slurry was uniformly coated onto aluminum foil and dried at 80 °C. The dried electrode was then punched into circular disks with a diameter of 10 mm. The average loading of the active material was approximately 2 mg/cm2.
2.3.2. Preparation of Graphite Cathode
The graphite cathode was prepared by mixing graphite powder (MCMB), acetylene black (conductive additive), and Alg (binder) in a mass ratio of 80:10:10. The slurry was uniformly coated onto aluminum foil and dried at 80 °C. The average loading of the active material was around 2 mg/cm2.
2.3.3. Preparation of Graphite Anode
The graphite anode was prepared by mixing graphite powder (MCMB), Super P (conductive carbon black), and PVDF (binder) in a mass ratio of 80:10:10, using NMP as the solvent. The slurry was coated onto copper foil and vacuum-dried at 120 °C. The dried electrodes were then punched into 10 mm diameter circular disks. The average loading of the active material was approximately 2 mg/cm2.
2.4. Cell Assembly
Using lithium metal with a diameter of 10 mm and a thickness of 2 mm as the counter electrode, half cells were assembled in a coin configuration in an argon-filled glovebox. After activating both the N4 cathode and MCMB anode for ten cycles in half cells, the electrodes were extracted and assembled into a full cell. The loading of the active material for the cathode was approximately 2.5 mg/cm2, and the loading of graphite for the anode was 0.9 mg/cm2.
2.5. Electrochemical Testing
Cyclic voltammetry (CV) was conducted using a CHI 660B electrochemical workstation (CH Instruments, Shanghai, China). The charge–discharge tests were performed on a LAND CT3002A battery system (Wuhan Land Electronics, Wuhan, China).
2.6. Material Characterization
To thoroughly understand the surface chemistry and morphological changes in the N4 cathode, cycled cells were disassembled in an argon-filled glovebox. The electrodes were rinsed multiple times with a dimethyl carbonate (DMC) solvent to remove residual salts and subsequently vacuum-dried in the glovebox chamber. Fourier transform infrared spectroscopy (FTIR, NICOLET 6700, Thermo, Waltham, MA, USA) was used to characterize the functional groups in N4. The morphological changes in N4 were observed using a scanning electron microscope (SEM, S4800, Hitachi, Tokyo, Japan). The crystalline structure of the samples was analyzed using X-ray powder diffraction (XRD, D8 ADVANCE DAVINCI, BRUKER, Karlsruhe, Germany). Chemical information during the charge–discharge process was characterized by X-ray photoelectron spectroscopy (XPS, AXIS SUPRA, Kratos, Manchester, UK). Raman spectroscopy (Renishaw inVia Reflex, Renishaw, London, UK) was used to analyze the sample structure.
2.7. Electrochemical Tests
2.7.1. Kinetics Analysis
Theoretically, the current (i) dependence of an electrode-active material on the scan rate (v) follows the power law i = avb. The b-value was used to evaluate the kinetics properties of redox reactions based on the following equation:
A b-value of 0.5 indicates purely diffusion-controlled processes, and a value of 1 represents purely capacitive behavior. When lies between 0.5 and 1, it indicates a pseudocapacitive contribution.
2.7.2. Pseudocapacitive Contribution Test
The proportion of diffusion and adsorption is based on the following equation:
where is the peak current in the CV curves; and is the scan rate. Among them, and represent the current occupied by capacitive and diffusion, respectively.
2.7.3. Galvanostatic Intermittent Titration Technique (GITT)
The GITT test was used to evaluate the carrier diffusion speed based on the following equation:
where (g) and (g·mol−1) are active mass and molecular weight; (s) is the constant current pulse time (3 min); (cm3mol−1) is the molar volume; A (cm2) is the surface area of the electrode (0.25 cm2); and (V) and (V) are assigned to the potential change between the two relaxations and the potential change caused by the pulse recorded by the LAND system. The pulse current density for the N4 electrodes is 100 mA/g, with a relax time of 60 min.
3. Results and Discussion
3.1. Electrochemical Performance of Graphite Cathodes in Commercial Electrolytes
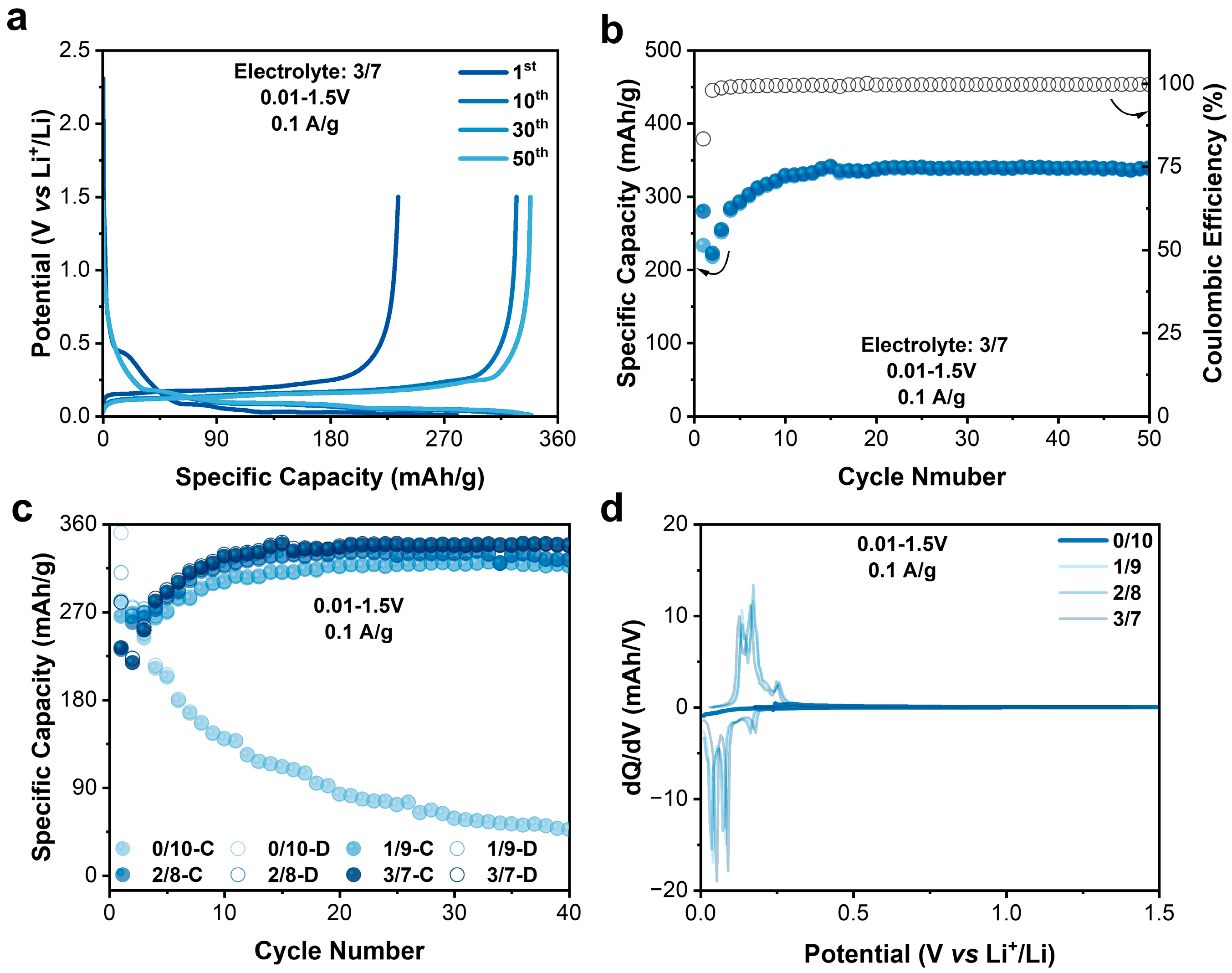
Under reasonably low cost, the commercial electrolytes for LIBs possess balanced properties among ionic conductivity, electrochemical window, interphase forming, and compatibility. Developing new energy storage devices based on these mature electrolytes will potentially reduce the manufacturing cost and increase the technology readiness level. In commercial Li-ion electrolytes, EC is a key component to form SEI on graphite anodes. It, however, plays an adverse effect on graphite cathodes in DIBs. Zhang et al. studied the effect of solvents on the insertion of PF6− into graphite and drawn the conclusion that with the interlayer distance between the adjacent graphene layers, EC-solvated PF6− was much larger than that in the pristine graphite, making it difficult for intercalation to occur [32]. To investigate the effect of a commercial LiPF6 EC/EMC electrolyte on a graphite cathode, a Li–graphite half cell was built with mesocarbon microbeads (MCMBs) as the graphite source (Figure S1). Four electrolytes with different EC ratios were prepared accordingly: 2 M LiPF6-EMC, 2M LiPF6-EC/EMC (1:9, v/v), 2M LiPF6-EC/EMC (2:8), and 2M LiPF6-EC/EMC (3:7, referred to 0/10, 1/9, 2/8, 3/7 in the following text). The MCMB cathodes were tested at a low current density of 0.1 A/g at 3–5 V vs. Li+/Li. For the MCMB cathodes, when the EC ratio increased, there was a significant decrease in capacity during the cycling test (Figure S2a). The stable capacities achieved were 83, 57, 29, and 29 mAh/g for the 0/10, 1/9, 2/8, 3/7 electrolytes. From the dQ/dV curves (Figure S2b), it was clear that the addition of EC increased the onset intercalation potential of PF6− into the MCMBs from 4.3 to 4.4 to 4.6 V, resulting in a decreased capacity.
3.2. Characterization and Electrochemical Performance of N4 Electrodes
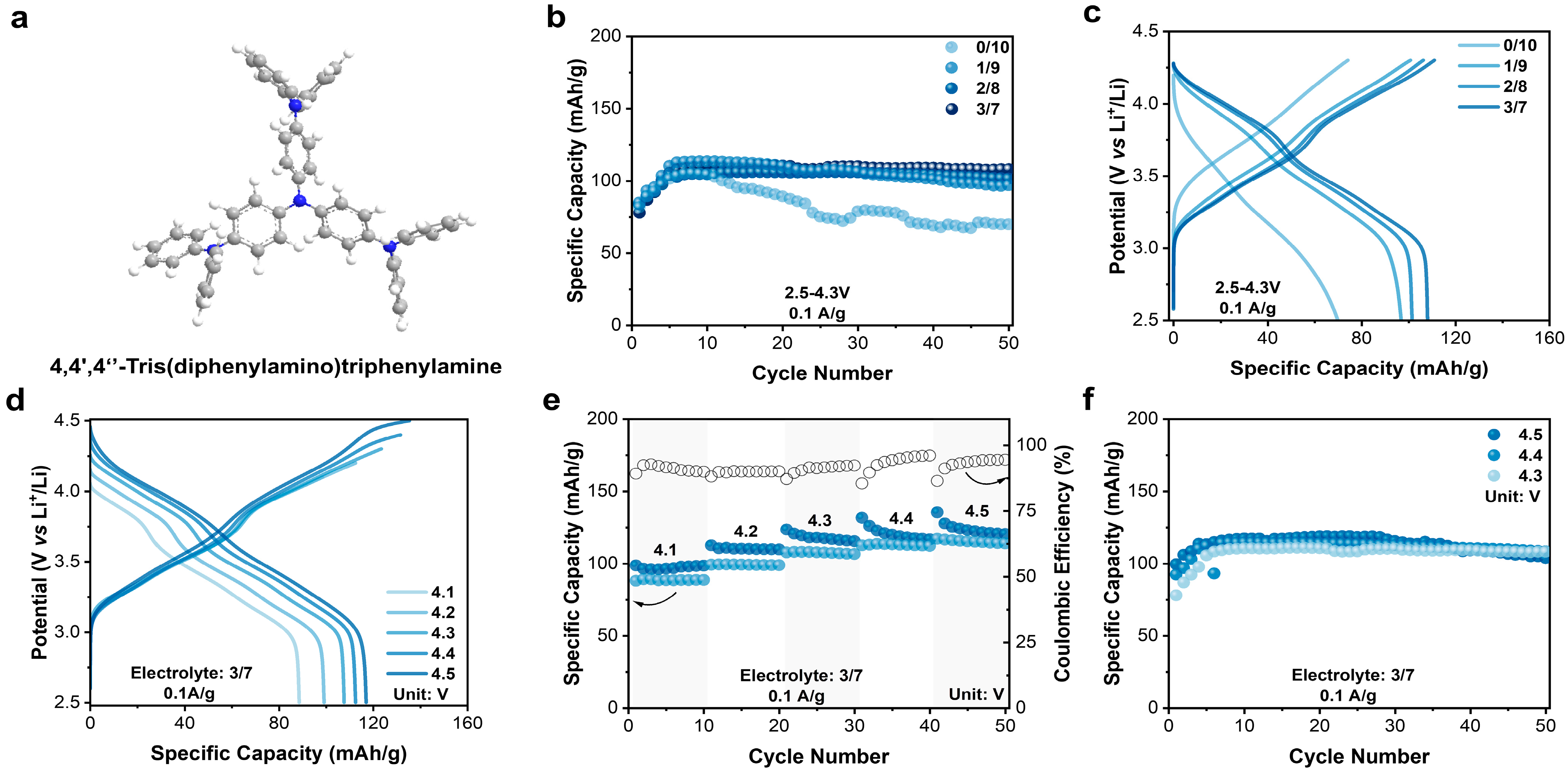
Organic materials have unique advantages for applications in batteries, benefitting from the low cost and structure flexibility. Generally, organic materials for anion storage in DIBs possess p-type redox active centers. Quaternization chemistry of amine was frequently used for anion storage at high potential [33]. Here, a small aromatic amine molecule, 4,4′,4″-tris(diphenylamino)triphenylamine (N4) (Figure 1a), consisting of four amino (NR3) groups and nine aromatic rings, was applied as the cathode material in the EC-containing electrolyte for DIBs. The four NR3 groups in N4 would ensure multi-electron transfer during the quaternization reaction, leading to a high theoretical capacity of 143 mAh/g; the aromatic rings may help stabilize the resulting N radical cation by electron delocalization. Further, N4 has a 3D conformation in which each N center is well separated and accessible. In addition, the relatively large molecular weight (Mr = 746.94 g/mol) and high conjugation may reduce its solubility in electrolytes, even under a fully charged/oxidized state. In the Fourier transform infrared (FTIR) spectrum of N4 (Figure S3a), the absorption peak at 1265, 1489, and 1587 cm−1 are attributing to the stretching vibration of the C-N single bond, C-C single bonds, and aromatic rings, respectively [29,34,35]. Additionally, the characteristic peaks at 995, 1158, and 1282 cm−1 in the Raman spectrum (Figure S3b) represent C-H, C-N, and C-C bonds, respectively [36]. Meanwhile, the X-ray diffraction (XRD) pattern (Figure S3c) and SEM measurement (Figure S3d) reveal that the N4 molecule exhibits certain crystallinity with a rod-like morphology.
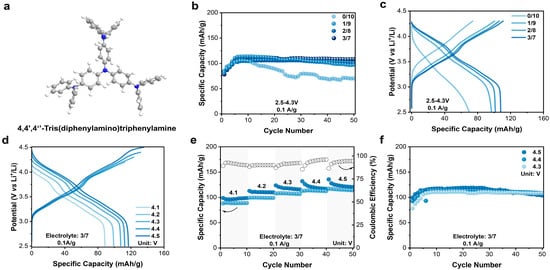
Figure 1.
(a) Chemical structure of N4. (b) Cycling comparison of N4 in different electrolytes. (c) Charge–discharge curve of N4 in different electrolytes. (d) Charge–discharge curves for different cutoff potentials. (e) Comparison of charge–discharge capacities at different cutoff potentials. (f) Cycling comparison of N4 electrodes at different upper cutoff potentials.
The electrochemical performance of the N4 electrodes in the electrolytes with different EC content was evaluated in coin-type half cells. The galvanostatic measurement was conducted at a current density of 0.1 A/g within 2.5–4.3 V. From the charge–discharge curves of the N4 electrodes (Figure S4), the initial charge–discharge curves significantly differed from the subsequent ones, implying a necessary activation process. Then, N4 gradually stabilized in the subsequent cycles (Figure 1b). And higher EC content resulted in enhanced cycling stability (Figure 1b,c). When the EC content reached 30%, a maximum reversible capacity of 108 mAh/g (75.3% of theoretical value) was obtained after 50 cycles. EC may help form a solid electrolyte interface on the surface of the N4 electrode, thereby slowing down capacity decay as observed for the EC-free electrolyte.
After that, the impact of upper cutoff potential, varying from 4.1 to 4.5 V, on cycling performance was also investigated in the 3/7 electrolyte (Figure 1d,e). Once the cutoff potential increased, the reversible capacity was simultaneously improved, achieving 88, 99, 108, 113, and 117 mAh/g, respectively. However, cycling performance varied significantly when prolonging the cycling period. As shown in Figure 1f, the N4 electrode at the cutoff potential of 4.3 V shows a better cycling stability than that at 4.4 and 4.5 V, indicating 4.3 V as an optimum cutoff potential. After 50 cycles, the specific capacity remained 108 mAh/g, 108 mAh/g, and 103 mAh/g, leading to capacity retention of 98.2%, 94.7%, and 86.6%, respectively. Overall, N4 exhibited a high capacity and good stability in the 3/7 electrolyte within 2.5–4.3 V. Additionally, a long-term cycling test was conducted as well at 1 A/g within the optimized window (Figure S5). The N4 electrode displayed an initial capacity of 92 mAh/g, and maintained 84 mAh/g after 500 cycles, demonstrating a high cycling stability.
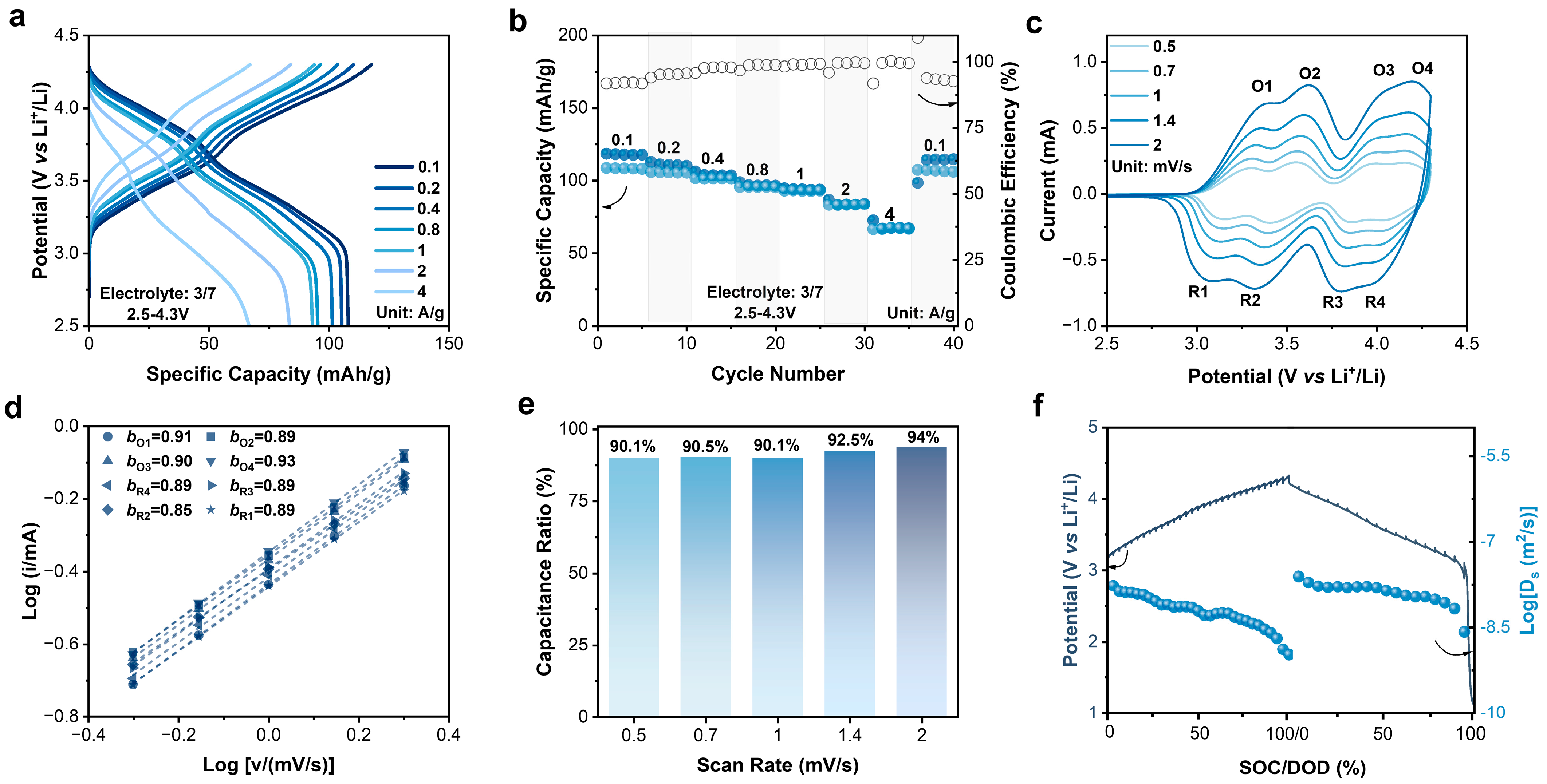
Organic electrode materials exhibit high rate performance and fast kinetics characteristics. Wang et al. reported an organic material CuTAPc with a capacity of 109 mAh/g at even 20 A/g, a capacity retention rate of 46.1% compared to that at 50 mA/g [37]. Obrezkov et al. reported PDPAPZ with a capacity of 82 mAh/g at 20 A/g, and 76.6% of that at 0.1 A/g [35]. The rate capability of N4 was also evaluated at different rates. When the current density increased by forty times from 0.1 to 4 A/g, the discharge-specific capacity decreased from 108 to 67 mAh/g with a retention rate of 62%. When the current density returned to 0.1 A/g, the capacity recovered, indicating an excellent reversibility (Figure 2a,b). The high rate performance implies fast redox kinetics of N4. The b-values of redox peaks of N4 in cyclic voltammetry (CV) curves were linear fitted to quantify the kinetics characteristics (Figure 2c,d).
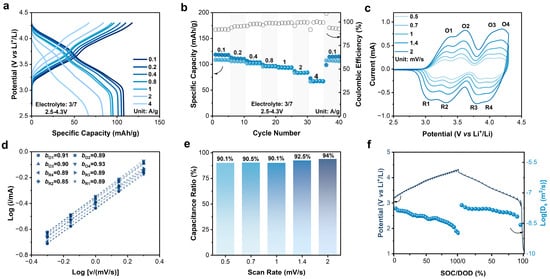
Figure 2.
(a) Charge–discharge curves of N4 electrode at different current rates. (b) Rate performance of N4 electrode at different current rates. (c) CV curves at different scan rates. (d) b-values obtained at different scan rates. (e) Pseudocapacitive contribution of N4 electrode at different scan rates. (f) Diffusion coefficients determined by GITT.
In the CV measurement of the N4-Li half cells, four pairs of redox peaks could be clearly distinguished at 3.33/3.11 V, 3.58/3.38 V, 4.01/3.82 V, and 4.18/4.01 V (1 mV/s), representing the step-by-step reactions of the four redox active NR3 centers in the N4 molecule [33]. Basically, the polymerization or oligomerization of the N4 molecule was excluded because neither new redox peaks nor peak shifts were observed. The b-values of the four pairs of the redox peaks are bO1 = 0.91; bO2 = 0.89; bO3 = 0.90; bO4 = 0.93; bR1 = 0.89; bR2 = 0.89; bR3 = 0.89; bR4 = 0.89, individually. Such high b-values suggest a dominant pseudocapacitive feature of the N4 redox process. As shown in Figure 2e, the ratio of the pseudocapacitive contribution was calculated approximately 90% even at a low scan rate of 0.2 mV/s, which increased to 94% at 2 mV/s. Furthermore, the diffusion coefficient (Ds) of the charge carrier was investigated via the galvanostatic intermittent titration technique (GITT, Figure 2f). During charging, Ds ranged from 10−7.8 to 10−9.0 m2/s, and from 10−7.6 to 10−8.6 m2/s once discharging.
3.3. Energy Storage Mechanism of N4
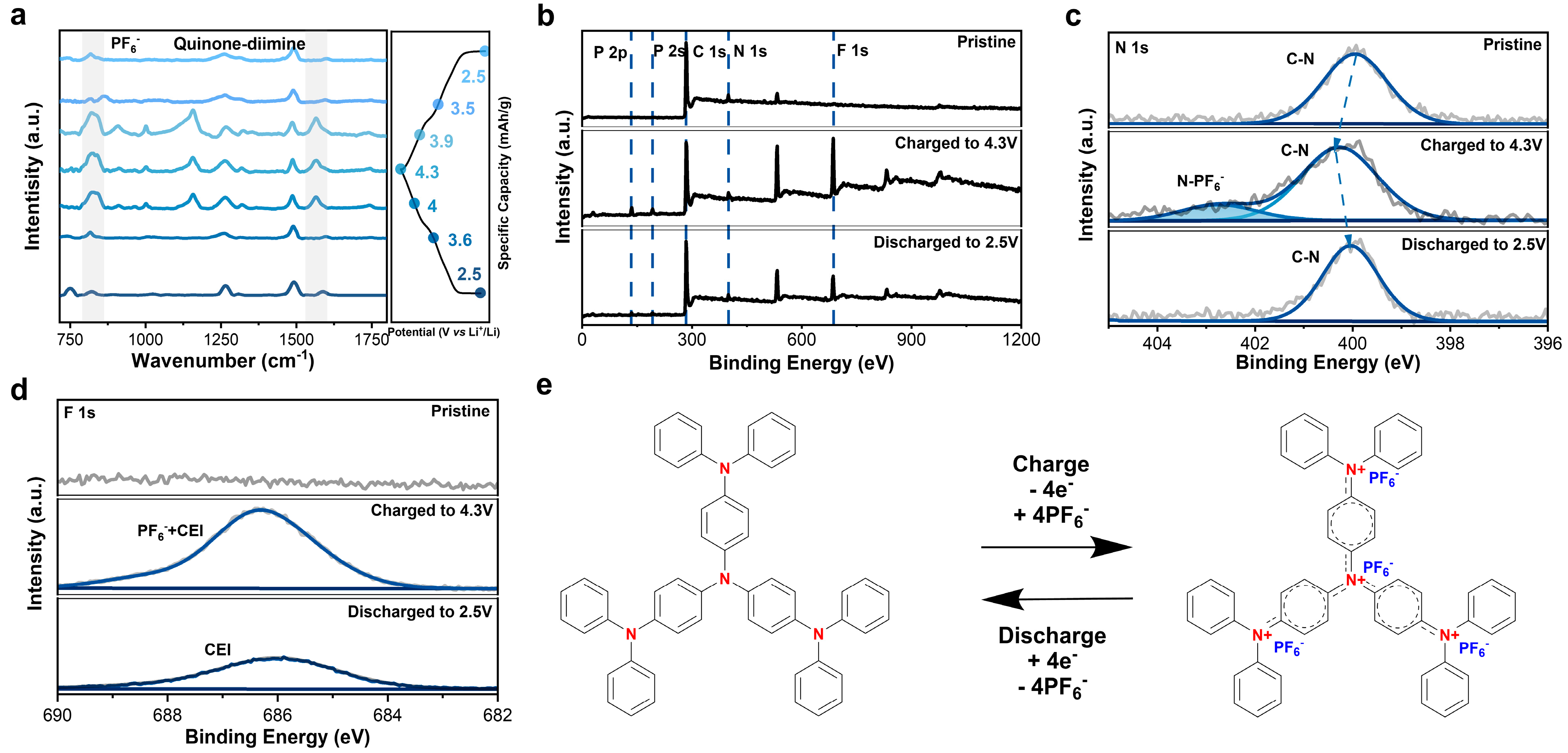
The energy storage mechanism and structure evolution of N4 were investigated via Fourier transform infrared spectroscopy (ex situ FTIR), X-ray photoelectron spectra (XPS), scanning electron microscope (SEM), and X-ray powder diffraction (XRD) at different charge/discharge states. Upon charging, the intensity of the peak at 830 cm−1 in the FTIR spectra increased, which was attributed to PF6− (Figure 3a) [36,38], while it gradually decreased when discharging, indicating the reversible (de-)insertion of PF6− into/from N4 electrode. Additionally, changes in the peak at 1568 cm−1 were related to the transition from a phenylenediamine structure to a quinone-diimine structure [29,39], elucidating the redox reaction of nitrogen atoms in N4.
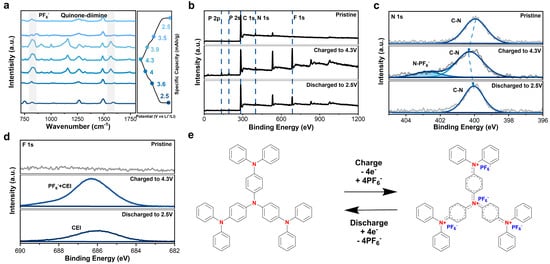
Figure 3.
(a) FTIR spectra of N4 electrode under different charge/discharge states. (b) XPS of N4 electrode under different charge/discharge conditions. (c) High-resolution N 1s spectra under different states. (d) High-resolution F 1s spectra und different states. (e) Proposed electrochemical energy storage mechanism.
The XPS of N4 at different states also exhibits similar changes (Figure 3b,d). No F or P signals were detected in the pristine N4 electrode. Upon charging to 4.3 V, the signals with binding energy at 135, 192, and 686 eV greatly increased, which belong to P 2p, P 2s, and F 1s, respectively, representing the insertion of PF6−. After fully discharging to 2.5 V, the intensity of the F and P signals decreased much, indicating that PF6− were removed from N4. The weak signal of F and P may derive from PF6− trapped in the electrode or the formed inorganic-rich interphase. High-resolution XPS for N 1s and F 1s (Figure 3c,d) provided a clear view of the redox process. The binding energy of N 1s at the charged state shifted to a higher value compared to the pristine and discharged ones, indicating an electron deficient state of the N4 electrode at 4.3 V and a sign of quarternary amine cation (NR4+). At the same time, a new peak located at 403 eV appeared, which belongs to the interaction of N-PF6−. In terms of F 1s, a stronger PF6− signal was detected at 687 eV at a fully charged state, and the residual F signal is noticed, too. Overall, the electrochemical energy storage mechanism is illustrated in Figure 3e, where four N centers (NR3) are oxidized to form quaternary amine radical cations (NR4•+). The four NR4•+ will further dynamically form quinone diiminium from a low-energy perspective.
During electrochemical cycling, the morphology of the N4 electrodes experienced significant changes. As shown in Figure S6a, in the pristine electrode, rod-like N4 was covered by carbon black. After one cycle, some rod-like N4 still existed but no rod-like structures could be seen after 50 cycles (Figure S6b,c). Furthermore, the XRD patterns of the cycled N4 electrode only showed peaks from the PP separator and carbon black rather than from N4 (Figure S7), while the N4 powder and pristine electrodes exhibited clear diffraction peaks. After cycling, they all disappeared, indicating an amorphization structure of the cycled sample. Additionally, we found that N4 would detach from the aluminum current collector and stick to the PP separator (Figure S8). It implies that partial dissolution–sedimentation behavior may have occurred.
3.4. Electrochemical Performance of MCMB Graphite Anodes
As is well known, EC can be used as a film-forming agent to participate in the in situ generation of a solid electrolyte interphase (SEI) on graphite anodes to improve their cycling stability. Yu et al. offer a novel perspective on the longstanding EC–PC disparity in Li-ion batteries by examining the graphite–electrolyte interface and its role in SEI formation. They reveal that in EC-based electrolytes, the enrichment of the interface with EC molecules increases local stiffness/viscosity, thereby hindering co-intercalation of solvated Li+ into graphite. This results in minimal graphite expansion and the formation of a stable, protective SEI [24]. Herein, the electrochemical performance of MCMB anodes in the 3/7 electrolyte was also tested in half cells within a potential range of 0.01–1.5 V vs. Li+/Li (Figure 4a,b). As expected, the MCMB anode demonstrated a highly reversible capacity of 340 mAh/g with the CE maintaining > 99%. When reducing the EC content in the electrolyte, the reversible capacity decreases. And when the EC content was zero, the capacity rapidly decayed. After 40 cycles, the specific capacity was only 50 mAh/g with no dQ/dV peaks (Figure 4c,d). Clearly, the addition of EC is crucial for the stable high capacity performance of MCMB anodes. The rate performance of the MCMB anodes were also evaluated, as shown in Figure S9. When the current density increased by forty times from 0.1 to 1 A/g, the discharge-specific capacity decreased from 340 to 97 mAh/g. Apparently, the rate performance of the MCMB anode was not comparable to that of the N4 cathode.
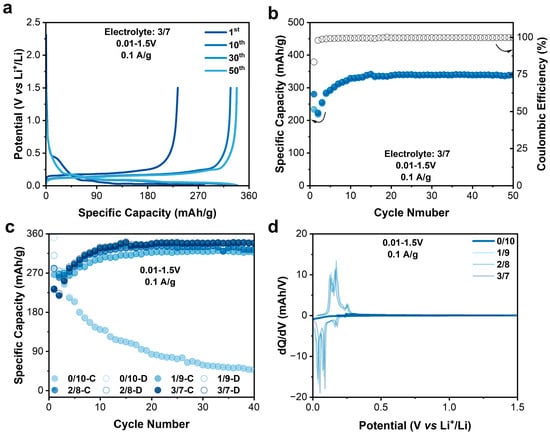
Figure 4.
(a) Charge–discharge profile of MCMB anode. (b) Specific capacity and Coulombic efficiency of graphite anode. (c) Comparison of charge and discharge capacities of graphite anodes in electrolytes with different EC contents. (d) Fortieth cycle dQ/dV of graphite anode in electrolytes with different EC contents.
3.5. Electrochemical Performance of N4||MCMB DIBs
The stable electrochemical performance of N4 and MCMB electrodes in the commercial 3/7 electrolyte encourages us to build an N4||MCMB DIB. The N4||MCMB DIB was assembled under a positive/negative (P/N) ratio of 2.7 and was evaluated within a voltage window of 1.5–4.1 V (Figure 5a). As shown in Figure 5b,c, this DIB exhibited similar slope-type charge–discharge curves as that of the N4 electrodes (Figure 5b). In the corresponding dQ/dV curves (Figure 5c), inheriting from the N4 electrodes, the apparent redox peaks disclosed the activity of N4 in N4||MCMB DIBs. Operating at 0.1 A/g, the N4||MCMB DIBs exhibited an initial discharge capacity of 90 mAh/g with a CE of 85%. After 40 cycles, the capacity retention rate was 98.5%, displaying a good cycling stability. Additionally, the N4||MCMB DIB had a small polarization: the average discharge voltage was 3.38 V, and the average charge voltage was 3.56 V, delivering an energy efficiency of 85.86%. When the current density increased by ten times from 0.1 to 1 A/g, the discharge-specific capacity decreased from 89 to 46 mAh/g, respectively, with a retention rate of 53%. The rate performance of the N4||MCMB DIB was inferior to that of the N4 half cells, which was mainly controlled by the rate-determining MCMB anode (Figure 5e,f). Moreover, a long-term cycling at 1 A/g was conducted (Figure S10), showing an initial capacity of 46 mAh/g and a reversible capacity of 33 mAh/g after 100 cycles, with a capacity retention of 71.7%.
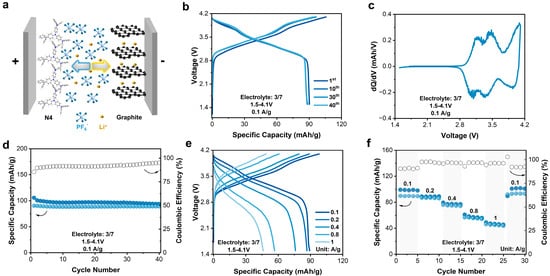
Figure 5.
(a) Schematic diagram of full cell. (b) Charge–discharge profile of full cell. (c) dQ/dV of full cell. (d) Specific capacity and Coulombic efficiency of full cell. (e) Charge–discharge curves at different current rates for full cell. (f) Specific capacity and Coulombic efficiency at different current rates for full cell.
4. Conclusions
In conclusion, we have demonstrated a small organic molecule N4 as a compatible cathode material replacing graphite cathodes in EC-containing commercial Li-ion electrolytes. The anion PF6− storage mechanism was proved as the conversion between the phenylenediamine and quinone-diimine-like compounds. In the Li half cells, N4 exhibited a high capacity of 108 mAh/g (75.3% of the theoretical value) via the unique four-electron redox mechanism and thus a novel N4||MCMB DIB was constructed through PF6− uptake into the N4 molecules and inserting Li+ into graphite. The working voltage of the full battery is 1.5–4.1 V, and it exhibited an initial discharge capacity of 90 mAh/g. After 40 cycles, the capacity retention rate was 98.53%. Our work verified the feasibility of small organic molecules as cathode materials for stable DIBs, solving the incompatibility of graphite cathodes in EC-containing electrolytes, which has great significance for developing novel electrode materials for DIBs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13010232/s1. Figure S1. (a) XRD spectrum. (b) Microscopic morphology of MCMB; Figure S2. (a) Cycling performance comparison of graphite cathodes in electrolytes with different EC contents. (b) 50th cycle dQ/dV of graphite cathode in electrolytes with different EC contents. (c) 1st cycle charge discharge curves of graphite cathode in electrolytes with different EC contents. (d) 50th cycle charge discharge curves of graphite cathode in electrolytes with different EC contents; Figure S3. (a) FITR spectrum, (b) Raman spectrum, (c) XRD pattern and (d) SEM image of N4; Figure S4. Charge discharge curves of N4 cathode in (a) 0/10 electrolyte, (b) 1/9 electrolyte, (c) 2/8 electrolyte and (d) 3/7 electrolyte. The irreversible capacity in initial cycles may come from electrolyte decomposition and interphase formation, which is necessary for stable cycling of N4 electrodes; Figure S5. Cycling performance of N4 cathode at 1 A/g; Figure S6. SEM images of N4 electrode (a) at pristine state, (b) after one cycle and (c) after 50 cycles; Figure S7. XRD patterns of N4 powder, SP, PP separator, pristine N4 electrode and cycled N4 electrodes; Figure S8. A photo of N4 electrode after cycling; Figure S9. (a) Charge discharge curves of MCMB anode at different charge discharge rates. (b) of the rate performance of MCMB anodes at different rates; Figure S10. (a) Cycling performance of Full battery at 1A/g. (b) Charge-discharge curves at different cycles.
Author Contributions
Investigation, J.C.; Visualization, J.C.; Writing—Original Draft, J.C.; Funding Acquisition, G.W.; Writing—Review and Editing, J.C., J.Z. and G.W.; Formal Analysis, Q.L.; Methodology, J.Y. (Jiayuan Yu) and Y.Y. Project Administration, G.W.; Validation, L.L., Z.L. and J.Y. (Jiahui Ye). All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Hundred Talents Programs of Chinese Academy of Sciences (Chinese Academy of Sciences), the Ningbo major Research and Development Plan Project (Ningbo science & technology bureau, 2023Z111), and the Ningbo Yongjiang Talent Programme (Ningbo science & technology bureau, 2022A-091-G).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
J.C. acknowledges Lianjiang Deng and Weiping Xie (Public technology center, NIMTE, CAS) for FTIR measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- He, P.; Tang, Y.; Tan, Z.; Lei, C.; Qin, Z.; Li, Y.; Li, Y.; Cheng, Y.; Wu, F.; He, Z.; et al. Solid-state batteries encounter challenges regarding the interface involving lithium metal. Nano Energy 2024, 124, 109502. [Google Scholar] [CrossRef]
- Mathiyalagan, K.; Shin, D.; Lee, Y.-C. Difficulties, strategies, and recent research and development of layered sodium transition metal oxide cathode materials for high-energy sodium-ion batteries. J. Energy Chem. 2024, 90, 40–57. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Versaci, D.; Colombo, R.; Montinaro, G.; Buga, M.; Cortes Felix, N.; Evans, G.; Bella, F.; Amici, J.; Francia, C.; Bodoardo, S. Tailoring cathode materials: A comprehensive study on LNMO/LFP blending for next generation lithium-ion batteries. J. Power Sources 2024, 613, 234955. [Google Scholar] [CrossRef]
- Wang, X.; Feng, W.; Zhou, Z.; Zhang, H. Design of sulfonimide anions for rechargeable lithium batteries. Chem. Commun. 2024, 60, 11434–11449. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, Z.; Wang, Y.; Li, J.-T.; Fu, H. Recent advances in preferentially selective Li recovery from spent lithium-ion batteries: A review. J. Environ. Chem. Eng. 2024, 12, 112903. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Prasada Rao, R.; Dalapati, G.K.; Adams, S.; Ramakrishna, S. Sustainable Materials and Decarbonization Prospects in Battery Technologies. ACS Appl. Energy Mater. 2024, 7, 3018–3020. [Google Scholar] [CrossRef]
- Shi, R.; Wang, B.; Tang, D.; Wei, X.; Zhou, G. Towards High Value-Added Recycling of Spent Lithium-Ion Batteries for Catalysis Application. Electrochem. Energy Rev. 2024, 7, 28. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Ruan, J.; Li, Q.; Ding, J.; Fang, F.; Wang, F. Research progress in non-aqueous low-temperature electrolytes for sodium-based batteries. Sci. China Chem. 2024, 67, 4063–4084. [Google Scholar] [CrossRef]
- Morag, A.; Chu, X.; Marczewski, M.; Kunigkeit, J.; Neumann, C.; Sabaghi, D.; Zukowska, G.Z.; Du, J.; Li, X.; Turchanin, A.; et al. Unlocking Four-electron Conversion in Tellurium Cathodes for Advanced Magnesium-based Dual-ion Batteries. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401818. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yao, W.; Zhou, L.; Zhang, F.; Zheng, Y.; Lee, C.S.; Tang, Y. Secondary Amines Functionalized Organocatalytic Iodine Redox for High-Performance Aqueous Dual-Ion Batteries. Adv. Mater. 2024, 36, e2314247. [Google Scholar] [CrossRef]
- He, F.; Zhou, Y.; Chen, X.; Wang, T.; Zeng, Y.; Zhang, J.; Chen, Z.; Liu, W.; Gao, P. A bipolar pyridine-functionalized porphyrin with hybrid charge-storage for dual-ion batteries. Chem. Commun. 2023, 59, 2787–2790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tang, M.; Lan, H.; Zhu, Q.; Wang, G.; Yang, G.; Yang, J.; Zhou, W.; Wang, H. An anode-free sodium dual-ion battery. Energy Storage Mater. 2024, 70, 103480. [Google Scholar] [CrossRef]
- Dai, H.; Chen, P.; Chen, Y.; Xu, W.; Ding, B.; Xia, X.; Liu, H. Dual Energy Storages by Sequential “Rocking Chair” and “Dual Ion” Processes of LiFe0.6Mn0.4PO4/Carbon Modified Graphite Flakes. ACS Appl. Energy Mater. 2024, 7, 2671–2680. [Google Scholar] [CrossRef]
- Jayan, P.; Anjali, A.; Park, S.; Lee, Y.S.; Aravindan, V. Controlled Synthesis of SnO2 Nanostructures as Alloy Anode via Restricted Potential Toward Building High-Performance Dual-Ion Batteries with Graphite Cathode. Small 2023, 20, e2305309. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, S.; Wang, H.; Li, L.; Fang, Y.; Zhang, F.; Gao, X.; Zhang, Z.; Yuan, W. A Review of Anode Materials for Dual-Ion Batteries. Nano-Micro Lett. 2024, 16, 252. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Zhang, F.; Lee, C.-S.; Tang, Y. Anion-hosting cathodes for current and late-stage dual-ion batteries. Sci. China Chem. 2024, 67, 1485–1509. [Google Scholar] [CrossRef]
- Li, G.; Shi, X.-J.; Dong, T.; Yu, Q.; Mao, Z.-F.; Liu, X.-H.; Wang, R.; He, B.-B.; Jin, J.; Gong, Y.-S.; et al. Binder-induced ultrafast PF6−-intercalation toward a high-voltage, high-power and long-cycling zinc–graphite dual-ion battery. Rare Met. 2024, 43, 5017–5029. [Google Scholar] [CrossRef]
- Cheng, Z.; Dong, Q.; Pu, G.; Song, J.; Zhong, W.; Wang, J. A Durable and High-Voltage Mn–Graphite Dual-Ion Battery Using Mn-Based Hybrid Electrolytes. Small 2024, 20, e2400389. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, X.; Ueno, H.; Deng, T.; Zheng, W. Manipulating Multivalent Anion Intercalation for High-Energy Aqueous Zn–Graphite Dual-Ion Batteries. Adv. Energy Mater. 2024, 14, 2401914. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, K.; Yu, D.Y.W. Tuning Electrolyte Solvation Structure and CEI Film to Enable Long Lasting FSI−-Based Dual-Ion Battery. Adv. Funct. Mater. 2023, 33, 2300305. [Google Scholar] [CrossRef]
- Fan, H.; Qi, L.; Yoshio, M.; Wang, H. Hexafluorophosphate intercalation into graphite electrode from ethylene carbonate/ethylmethyl carbonate. Solid State Ion. 2017, 304, 107–112. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, B.K.; Kang, S.; Yang, S.J.; Choi, S.H.; Yoo, D.J.; Kim, K.J. Bespoke Dual-Layered Interface Enabled by Cyclic Ether in Localized High-Concentration Electrolytes for Lithium Metal Batteries. Adv. Funct. Mater. 2024, 34, 2408365. [Google Scholar] [CrossRef]
- Luchkin, S.Y.; Pazhetnov, E.M. Explaining the EC–PC disparity in Li-ion batteries: How interface stiffness governs SEI formation on graphite. J. Mater. Chem. A 2024, 12, 29795–29801. [Google Scholar] [CrossRef]
- Martins, M.; Haering, D.; Connell, J.G.; Wan, H.; Svane, K.L.; Genorio, B.; Farinazzo Bergamo Dias Martins, P.; Lopes, P.P.; Gould, B.; Maglia, F.; et al. Role of Catalytic Conversions of Ethylene Carbonate, Water, and HF in Forming the Solid-Electrolyte Interphase of Li-Ion Batteries. ACS Catal. 2023, 13, 9289–9301. [Google Scholar] [CrossRef]
- Zhang, S.S.; Jow, T.R.; Amine, K.; Henriksen, G.L. LiPF6–EC–EMC electrolyte for Li-ion battery. J. Power Sources 2002, 107, 18–23. [Google Scholar] [CrossRef]
- Ikhe, A.B.; Seo, J.Y.; Park, W.B.; Lee, J.-W.; Sohn, K.-S.; Pyo, M. 3-V class Mg-based dual-ion battery with astonishingly high energy/power densities in common electrolytes. J. Power Sources 2021, 506, 230261. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, K.; Liu, P.; Li, H.; Jiao, L. Optimized Cathode for High-Energy Sodium-Ion Based Dual-Ion Full Battery with Fast Kinetics. Adv. Funct. Mater. 2021, 31, 2107830. [Google Scholar] [CrossRef]
- Obrezkov, F.A.; Shestakov, A.F.; Vasil’ev, S.G.; Stevenson, K.J.; Troshin, P.A. Polydiphenylamine as a promising high-energy cathode material for dual-ion batteries. J. Mater. Chem. A 2021, 9, 2864–2871. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Zhang, C.; Guo, Y.; Yu, A.; Mei, S.; Yao, C.J. Electropolymerization of Donor–Acceptor Conjugated Polymer for Efficient Dual-Ion Storage. Adv. Sci. 2024, 11, e2310239. [Google Scholar] [CrossRef]
- Guan, D.; Wang, W.; Chen, B.; Wu, J.; Hu, G.; Peng, Z.; Cao, Y.; Wen, L.; Du, K. Does Salt Concentration Matter? New Insights on the Intercalation Behavior of PF6− into Graphite Cathode for the Dual-Ion Battery. Adv. Funct. Mater. 2023, 33, 2215113. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Suppression of PF6− intercalation into graphite by small amounts of ethylene carbonate in activated carbon/graphite capacitors. Chem. Commun. 2010, 46, 1544–1546. [Google Scholar] [CrossRef]
- Wang, G.; Dmitrieva, E.; Kohn, B.; Scheler, U.; Liu, Y.; Tkachova, V.; Yang, L.; Fu, Y.; Ma, J.; Zhang, P.; et al. An Efficient Rechargeable Aluminium–Amine Battery Working Under Quaternization Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202116194. [Google Scholar] [CrossRef]
- Chen, X.; Duan, X.; Oh, W.-D.; Zhang, P.-H.; Guan, C.-T.; Zhu, Y.-A.; Lim, T.-T. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: Maneuverable N-B bonding configurations and oxidation pathways. Appl. Catal. B Environ. 2019, 253, 419–432. [Google Scholar] [CrossRef]
- Obrezkov, F.A.; Somova, A.I.; Fedina, E.S.; Vasil’ev, S.G.; Stevenson, K.J.; Troshin, P.A. Dihydrophenazine-Based Copolymers as Promising Cathode Materials for Dual-Ion Batteries. Energy Technol. 2020, 9, 2000772. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Amzil, S.; Qiu, T.; Xu, W.; Jiang, F.; Fang, Z.; Huang, J.; Dai, G. Tetraphenylbiphenyldiamine: Insight into anion storage mechanism as a cathode in dual ion batteries. Appl. Surf. Sci. 2021, 542, 148581. [Google Scholar] [CrossRef]
- Wang, H.G.; Wang, H.; Si, Z.; Li, Q.; Wu, Q.; Shao, Q.; Wu, L.; Liu, Y.; Wang, Y.; Song, S.; et al. A Bipolar and Self-Polymerized Phthalocyanine Complex for Fast and Tunable Energy Storage in Dual-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2019, 58, 10204–10208. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Wang, W.; Xiong, P.; Li, B.; Li, M.; Yang, J.; Xu, Y. In Situ Electropolymerization Enables Ultrafast Long Cycle Life and High-Voltage Organic Cathodes for Lithium Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 11992–11998. [Google Scholar] [CrossRef] [PubMed]
- Obrezkov, F.A.; Fedina, E.S.; Somova, A.I.; Akkuratov, A.V.; Stevenson, K.J. Facile Method for Cross-Linking Aromatic Polyamines to Engender beyond Lithium Ion Cathodes for Dual-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 11827–11835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).