A Glycopeptide from Agaricus balchaschensis Mitigates Cadmium Damage in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
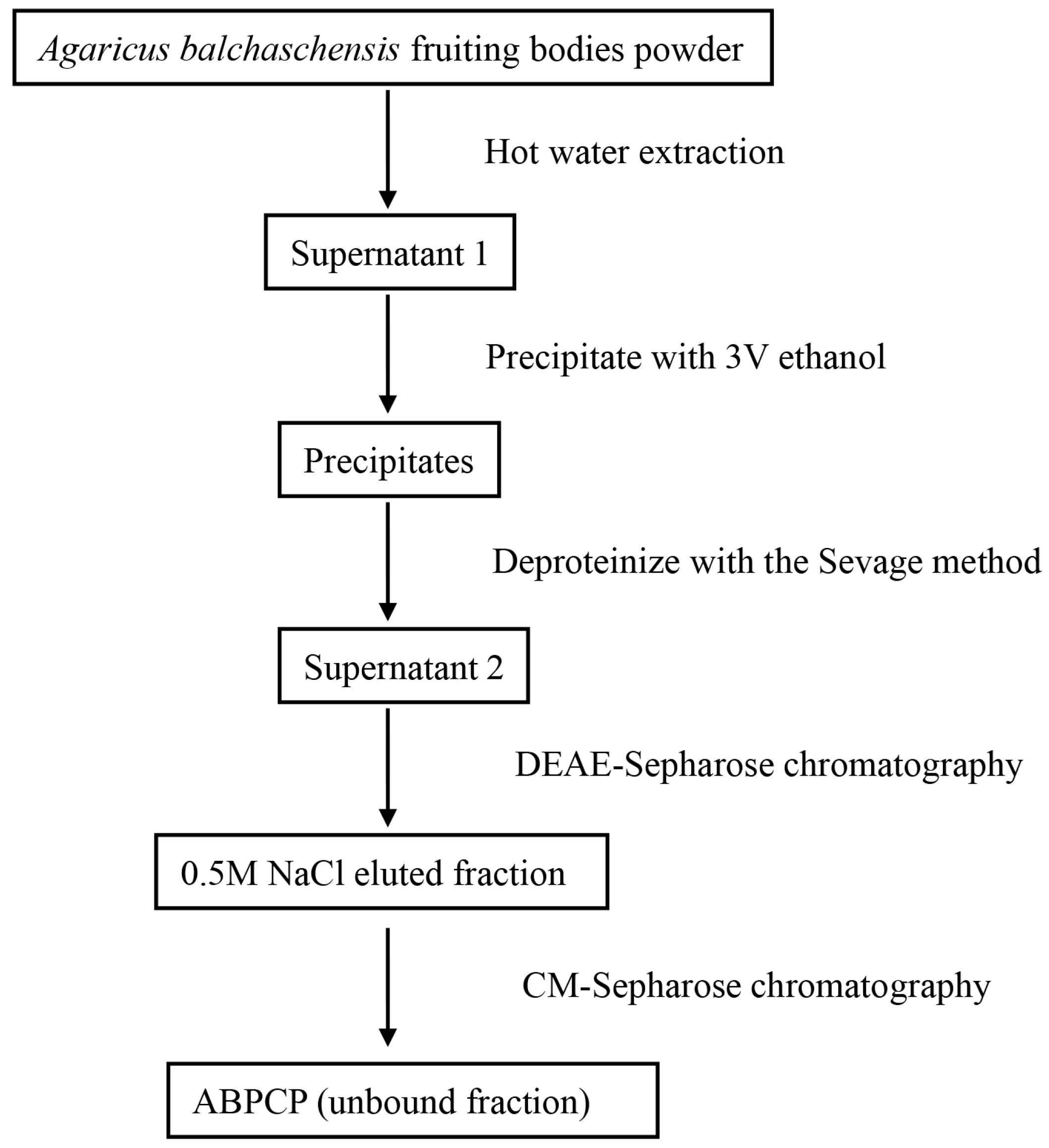
2.2. Preparation of Polysaccharide
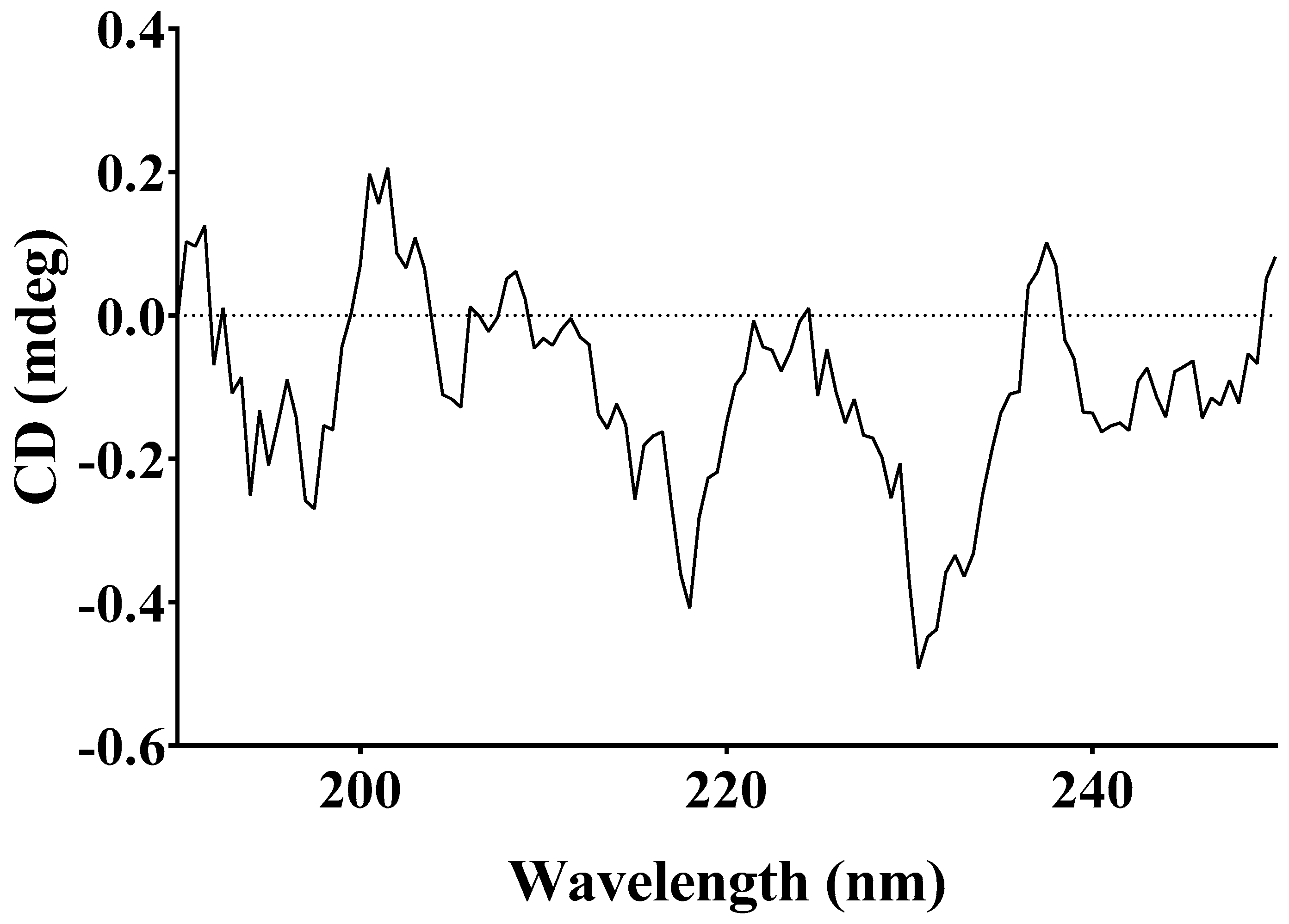
2.3. FTIR and NMR Analysis
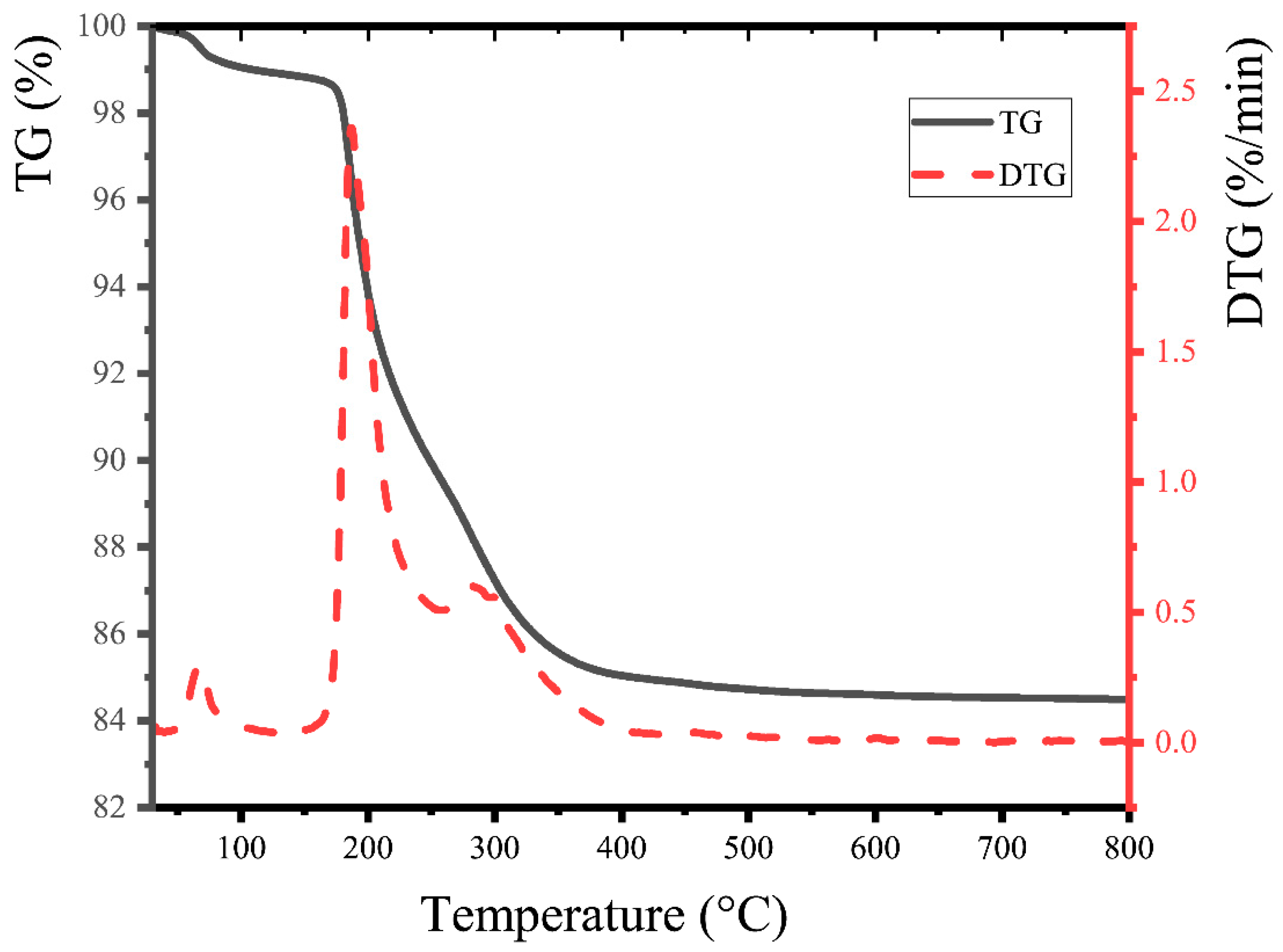
2.4. Thermogravimetric Analysis
2.5. Amino Acid Analysis
2.6. Reduction of the Toxicity of Heavy Metals In Vivo
2.6.1. Animal Experiments
2.6.2. Biochemical Analysis
2.6.3. Organ Cadmium Content Analysis
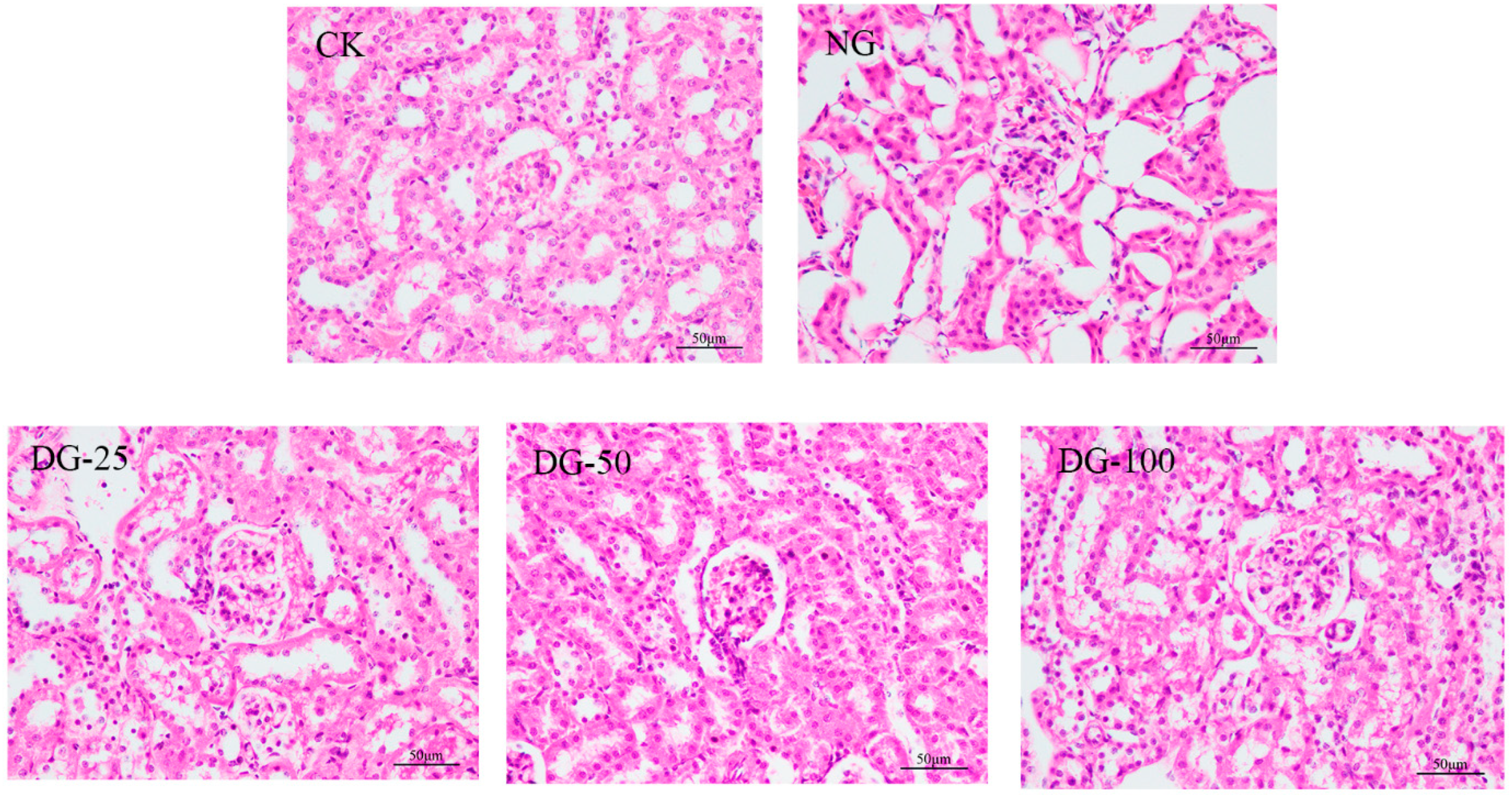
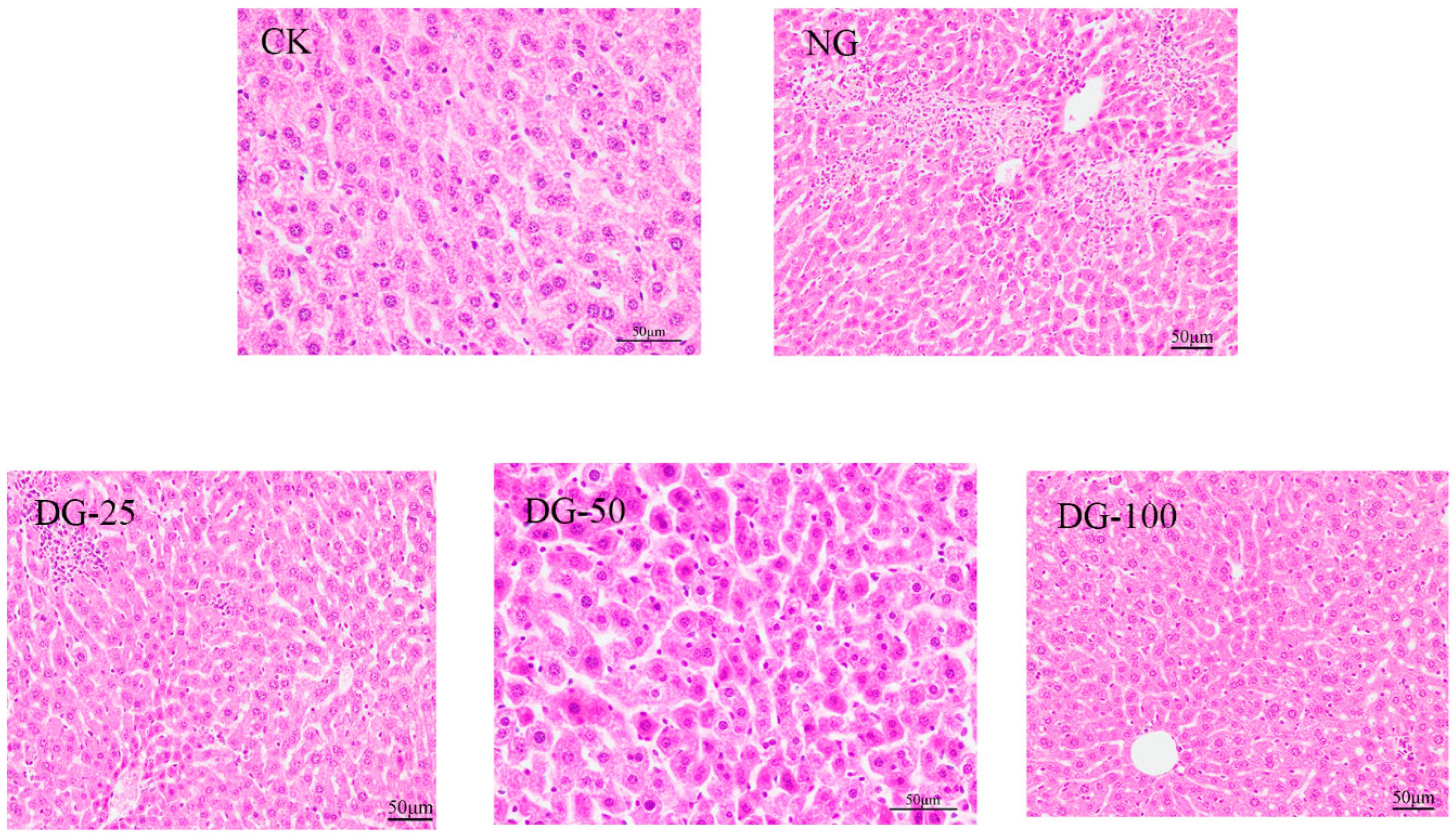
2.6.4. Histopathological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. NMR Analysis
3.3. Thermogravimetric (TG) Analysis of ABPCP
3.4. Amino Acid Composition Analysis
3.5. The Ability of ABPCP to Reduce Heavy Metals Toxicity In Vivo
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Ye, Y.; Xu, D.; Ji, J.; Sun, J.; Xu, M.; Xia, B.; Shen, H.; Xia, R.; Shi, W.; et al. Structural characterization and anti-inflammatory activity of a novel neutral polysaccharide isolated from Smilax glabra Roxb. Int. J. Biol. Macromol. 2023, 234, 123559. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-P.; Lin, W.; Ma, J.-X.; Li, N.; Wang, J.-X.; Li, J.-M. The physiochemical characteristics of Cyclocarya paliurus polysaccharides and in vitro anti-diabetic effects of their different fractions. Ind. Crops Prod. 2024, 211, 118188. [Google Scholar] [CrossRef]
- Chen, S.K.; Wang, X.; Guo, Y.Q.; Song, X.X.; Yin, J.Y.; Nie, S.P. Exploring the partial degradation of polysaccharides: Structure, mechanism, bioactivities, and perspectives. Compr. Rev. Food. Sci. Food Saf. 2023, 22, 4831–4870. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.S.; Taherishirazi, M.; Niazi, A.; Afsharifar, A.; Moghadam, A. Structure guided design and cloning of peptide inhibitors targeting CDK9/cyclin T1 protein protein interaction. Front. Pharmacol. 2024, 15, 1327820. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.S.; Niazi, A.; Mirzapour-Kouhdasht, A.; Pereira, E.C.; Garcia-Vaquero, M. Enhancing cyclotide bioproduction: Harnessing biological synthesis methods and various expression systems for large-scale manufacturing. Crit. Rev. Biotechnol. 2024, 7, 1–23. [Google Scholar] [CrossRef]
- Olech, M.; Cybulska, J.; Nowacka-Jechalke, N.; Szpakowska, N.; Maslyk, M.; Kubinski, K.; Martyna, A.; Zdunek, A.; Kaczynski, Z. Novel polysaccharide and polysaccharide-peptide conjugate from Rosa rugosa Thunb. pseudofruit-Structural characterisation and nutraceutical potential. Food Chem. 2023, 409, 135264. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-C.; Guo, W.-L.; Li, L.; Yu, X.-D.; Liu, B. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods 2019, 57, 48–58. [Google Scholar] [CrossRef]
- Fang, H.; Li, X.; Lin, D.; Wang, L.; Yang, T.; Yang, B. Inhibition of intrarenal PRR-RAS pathway by Ganoderma lucidum polysaccharide peptides in proteinuric nephropathy. Int. J. Biol. Macromol. 2023, 253, 127336. [Google Scholar] [CrossRef]
- Meng, M.; Wang, L.; Yao, Y.; Lin, D.; Wang, C.; Yao, J.; Sun, H.; Liu, M. Ganoderma lucidum polysaccharide peptide (GLPP) attenuates rheumatic arthritis in rats through inactivating NF-kappaB and MAPK signaling pathways. Phytomedicine 2023, 119, 155010. [Google Scholar] [CrossRef]
- Xie, J.; Lin, D.; Li, J.; Zhou, T.; Lin, S.; Lin, Z. Effects of Ganoderma lucidum polysaccharide peptide ameliorating cyclophosphamide-induced immune dysfunctions based on metabolomics analysis. Front. Nutr. 2023, 10, 1179749. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Ma, A.; Zhang, S.; Lin, D.; Lin, S.; Li, M.; Zhou, H.; Yang, B. Ganoderma lucidum Polysaccharide Peptide attenuates post myocardial infarction fibrosis via down-regulating TGF-β1/SMAD and relieving oxidative stress. Pharmacol. Res.-Mod. Chin. Med. 2022, 4, 100152. [Google Scholar] [CrossRef]
- Nauroze, T.; Ali, S.; Kanwal, L.; Ara, C.; Akbar Mughal, T.; Andleeb, S. Ameliorative effect of Nigella sativa conjugated silver nanoparticles against chromium-induced hepatotoxicity and renal toxicity in mice. Saudi J. Biol. Sci. 2023, 3, 103571. [Google Scholar] [CrossRef]
- Ghosh, P.; Dey, T.; Majumder, R.; Datta, M.; Chattopadhyay, A.; Bandyopadhyay, D. Insights into the antioxidative mechanisms of melatonin in ameliorating chromium-induced oxidative stress-mediated hepatic and renal tissue injuries in male Wistar rats. Food Chem. Toxicol. 2023, 173, 113630. [Google Scholar] [CrossRef]
- Wongmekiat, O.; Peerapanyasut, W.; Kobroob, A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn-Schmiedeberg’s Arch Pharmacol. 2018, 391, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Dastan, D.; Karimi, S.; Larki-Harchegani, A.; Nili-Ahmadabadi, A. Protective effects of Allium hirtifolium Boiss extract on cadmium-induced renal failure in rats. Environ. Sci. Pollut. R. 2019, 26, 18886–18892. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Benya, A.; Hota, S.; Kumar, M.S.; Singh, S. Eco-toxicity of hexavalent chromium and its adverse impact on environment and human health in Sukinda Valley of India: A review on pollution and prevention strategies. Environ. Chem. Ecotoxicol. 2023, 5, 46–54. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, W.M.; Niu, Y.J.; Guo, H.C.; Liu, X.H.; Hou, Y.C.; Zhao, L.J. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Environ. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef]
- Varoni, M.V.; Pasciu, V.; Gadau, S.D.; Baralla, E.; Serra, E.; Palomba, D.; Demontis, M.P. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 2946–2955. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Okonkwo, P.K.; Faboya, O.A.; Onikanni, S.A.; Fadaka, A.; Olayide, I.; Akinyemi, E.O.; Oboh, G. Curcumin improves episodic memory in cadmium induced memory impairment through inhibition of acetylcholinesterase and adenosine deaminase activities in a rat model. Metab. Brain Dis. 2017, 32, 87–95. [Google Scholar] [CrossRef]
- Mizuno, M.; Minato, K.I. Anti-inflammatory and immunomodulatory properties of polysaccharides in mushrooms. Curr. Opin. Biotechnol. 2024, 86, 103076. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Z.; Song, K.; Li, L.; Chen, M. Medicinal value of edible mushroom polysaccharides: A review. J. Future Foods 2023, 3, 16–23. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Zhao, Q.; Patrick Manzi, H.; Su, L.; Liu, D.; Huang, X.; Long, D.; Tang, Z.; Zhang, Y. The benefits of edible mushroom polysaccharides for health and their influence on gut microbiota: A review. Front. Nutr. 2023, 10, 1213010. [Google Scholar] [CrossRef] [PubMed]
- Muhaxi, M.; Liu, F.; Ng, T.B. Structural characterization and in vitro hepatoprotective activity of a novel antioxidant polysaccharide from fruiting bodies of the mushroom Pleurotus ferulae. Int. J. Biol. Macromol. 2023, 243, 125124. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Yen, Y.H.; Yang, J.P. Finding of polysaccharide-peptide complexes in Cordyceps militaris and evaluation of its acetylcholinesterase inhibition activity. J. Food Drug Anal. 2015, 23, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, P.; Lu, Y.; Zhang, Z.; Yao, C.; Tian, G.; Liu, Q. A Novel Polysaccharide From Heimioporus retisporus Displays Hypoglycemic Activity in a Diabetic Mouse Model. Front. Nutr. 2022, 9, 964948. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Hu, D.; Mo, J.; Ni, J. Black mulberry (Morus nigra L.) polysaccharide ameliorates palmitate-induced lipotoxicity in hepatocytes by activating Nrf2 signaling pathway. Int. J. Biol. Macromol. 2021, 172, 394–407. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Do, T.T.; Nguyen, T.D.; Pham, L.D.; Nguyen, V.D. Isolation and characteristics of polysaccharide from Amorphophallus corrugatus in Vietnam. Carbohyd. Polym. 2011, 84, 64–68. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Refregiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and amino acid composition analysis of proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yue, C.; Sun, Q.; Wang, Y.; Gong, Z.; Zhang, K.; Da, J.; Zou, H.; Zhu, J.; Zhao, H.; et al. Cadmium exposure exacerbates kidney damage by inhibiting autophagy in diabetic rats. Ecotoxicol. Environ. Saf. 2023, 186, 144544. [Google Scholar] [CrossRef]
- Guo, J.; Low, J.H.; Rajagopal Iyer, V.; Wong, P.; Ong, C.B.; Loh, W.L.; Yeow, C.H. A Preliminary Study on Grip-Induced Nerve Damage Caused by a Soft Pneumatic Elastomeric Gripper. Polymers 2022, 14, 4272. [Google Scholar] [CrossRef]
- Oiu, J.; Shi, W.; Miao, J.; Hu, H.; Gao, Y. Extraction, Isolation, Screening, and Preliminary Characterization of Polysaccharides with Anti-Oxidant Activities from Oudemansiella raphanipies. Polymers 2023, 15, 2917. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Bao, X.; Gao, L.; Tao, Y. Extraction of polysaccharides from black mulberry fruit and their effect on enhancing antioxidant activity. Int. J. Biol. Macromol. 2018, 120, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.; Wang, H.; Cui, Z.; Cheng, F.; Wang, K.P. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2016, 147, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-F.; Fan, Y.-M.; Xu, F.; Sun, R.-C. Structure and thermal stability of polysaccharide fractions extracted from the ultrasonic irradiated and cold alkali pretreated bamboo. J. Appl. Polym. Sci. 2011, 121, 176–185. [Google Scholar] [CrossRef]
- Choi, J.W.; Synytsya, A.; Capek, P.; Bleha, R.; Pohl, R.; Park, Y.I. Structural analysis and anti-obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydr. Polym. 2016, 146, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Kondo, N.; Hirabayashi, K.; Ogata, M.; Totani, K.; Ikematsu, S.; Osada, M. NMR spectroscopic structural characterization of a water-soluble beta-(1→3, 1→6)-glucan from Aureobasidium pullulans. Carbohydr. Polym. 2017, 174, 876–886. [Google Scholar] [CrossRef]
- Liu, X.; Liu, D.; Chen, Y.; Zhong, R.; Gao, L.; Yang, C.; Ai, C.; El-Seedi, H.R.; Zhao, C. Physicochemical characterization of a polysaccharide from Agrocybe aegirita and its anti-ageing activity. Carbohydr. Polym. 2020, 236, 116056. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, H.; Xu, Z.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Ding, C. Antioxidant and anti-aging activities and structural elucidation of polysaccharides from Panax notoginseng root. Process Bioch. 2019, 78, 189–199. [Google Scholar] [CrossRef]
- Ying, X.; Pan, Y.; Lan, J.; Fang, Y.; Ding, Y.; Gu, Z. Preparation of pectin from fingered citron peel and its protective effect on cadmium-induced liver and kidney damage in mice. Food Biosci. 2023, 56, 103359. [Google Scholar] [CrossRef]
- Han, P.; Tian, X.; Wang, H.; Ju, Y.; Sheng, M.; Wang, Y.; Cheng, D. Purslane (Portulacae oleracea L.) polysaccharide relieves cadmium-induced colonic impairments by restricting Cd accumulation and inhibiting inflammatory responses. Int. J. Biol. Macromol. 2024, 257, 128500. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ding, Y.; Kong, Y.; Wang, G.; Wang, S.; Cheng, D. Purslane (Portulacae oleracea L.) attenuates cadmium-induced hepatorenal and colonic damage in mice: Role of chelation, antioxidant and intestinal microecological regulation. Phytomedicine 2021, 92, 153716. [Google Scholar] [CrossRef] [PubMed]
- Musarurwa, H.; Tawanda, T.N. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef] [PubMed]




| Amino Acid | Content (mg/g) |
|---|---|
| Asp | 0.709 |
| Thr | 0.855 |
| Ser | 0.560 |
| Glu | 1.182 |
| Gly | 0.757 |
| Ala | 0.383 |
| Cys | 4.898 |
| Val | 0.926 |
| Met | 0.279 |
| Lys | 0.488 |
| Arg | 0.710 |
| Group | 7 d | 14 d | 21 d |
|---|---|---|---|
| CK | 1.63 ± 0.29 C | 1.18 ± 0.14 C | 1.39 ± 0.08 C |
| NG | 25.43 ± 1.76 A | 25.26 ± 4.54 A | 44.26 ± 3.28 A |
| DG-25 | 8.13 ± 1.74 B | 7.86 ± 0.70 B | 10.74 ± 0.21 B |
| DG-50 | 3.48 ± 0.58 C | 2.24 ± 0.24 C | 10.49 ± 0.05 B |
| DG-100 | 2.28 ± 0.06 C | 1.96 ± 0.03 C | 9.47 ± 0.49 B |
| Group | 7 d | 14 d | 21 d |
|---|---|---|---|
| CK | 10.3 ± 1.25 D | 47.18 ± 5.98 D | 44.58 ± 3.51 F |
| NG | 313.83 ± 21.83 A | 400.17 ± 29.16 A | 370.7 ± 5.25 A |
| DG-25 | 95.91 ± 3.9 C | 150.4 ± 6.6 B | 190.9 ± 11.39 B |
| DG-50 | 35.9 ± 4.68 C | 113.24 ± 5.35 C | 168.17 ± 1.1 C |
| DG-100 | 5.6 ± 0.35 D | 70.83 ± 3.55 D | 91.5 ± 2.66 E |
| Group | 7 d | 14 d | 21 d |
|---|---|---|---|
| CK | 3.89 ± 0.39 D | 1.98 ± 0.39 E | 10.83 ± 0.60 CD |
| NG | 47.71 ± 2.70 A | 37.49 ± 3.9 A | 44.30 ± 4.34 A |
| DG-25 | 23.67 ± 2.31 B | 19.67 ± 1.26 | 22.53 ± 1.06 B |
| DG-50 | 16.4 ± 2.65 C | 14.13 ± 3.53 BC | 15.5 ± 1.57 C |
| DG-100 | 5.6 ± 0.35 D | 5.83 ± 0.47 DE | 7.98 ± 0.11 D |
| Group | 7 d | 14 d | 21 d |
|---|---|---|---|
| CK | 137.87 ± 1.56 E | 134.80 ± 5.01 E | 31.36 ± 12.29 E |
| NG | 469.58 ± 12.12 A | 413.47 ± 11.40 A | 399.29 ± 6.46 A |
| DG-25 | 331.95 ± 8.90 B | 276.91 ± 4.28 B | 252.94 ± 5.03 B |
| DG-50 | 261.83 ± 3.60 C | 209.76 ± 3.23 C | 200.18 ± 4.38 C |
| DG-100 | 211.57 ± 2.98 D | 177.6 ± 2.76 D | 171.39 ± 3.69 D |
| Group | 7 d | 14 d | 21 d |
|---|---|---|---|
| CK | 29.38 ± 3.5 E | 28.85 ± 3.58 E | 142.90 ± 7.17 B |
| NG | 286.19 ± 8.05 A | 216.45 ± 9.29 A | 178.89 ± 9.29 A |
| DG-50 | 133.29 ± 8.05 C | 119.88 ± 4.65 C | 93.06 ± 8.05 D |
| DG-25 | 189.62 ± 8.05 B | 162.8 ± 12.29 B | 141.34 ± 8.05 C |
| DG-100 | 109.15 ± 8.04 D | 93.06 ± 8.05 D | 60.87 ± 8.05 E |
| Group | HEART | KIDNEY | LIVER | SERUM |
|---|---|---|---|---|
| CK | 0.0044 ± 0.0023 D | 0.03 ± 0.0054 C | 0.02 ± 0.0012 D | 0.00425 ± 0.0014 E |
| NG | 2.83 ± 0.12 A | 44.43 ± 4.54 A | 91.28 ± 10.64 A | 0.443 ± 0.058 A |
| DG-100 | 0.72 ± 0.040 C | 13.27 ± 0.32 B | 45.79 ± 1.43 B | 0.129 ± 0.0053 D |
| DG-50 | 0.92 ± 0.022 B | 15.23 ± 0.64 B | 40.60 ± 1.21 BC | 0.144 ± 0.015 CD |
| DG-25 | 0.95 ± 0.076 B | 16.97 ± 0.50 B | 31.24 ± 2.49 C | 0.1925 ± 0.013 BC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalimaimaiti, N.; Dong, Y.; Jia, P.; Feng, X.; Luo, Y.; Hao, J.; Jia, W.; Chen, H.; Zhu, Q.; Liang, Z.; et al. A Glycopeptide from Agaricus balchaschensis Mitigates Cadmium Damage in Mice. Processes 2025, 13, 168. https://doi.org/10.3390/pr13010168
Yalimaimaiti N, Dong Y, Jia P, Feng X, Luo Y, Hao J, Jia W, Chen H, Zhu Q, Liang Z, et al. A Glycopeptide from Agaricus balchaschensis Mitigates Cadmium Damage in Mice. Processes. 2025; 13(1):168. https://doi.org/10.3390/pr13010168
Chicago/Turabian StyleYalimaimaiti, Nuerziya, Yongqiang Dong, Peisong Jia, Xiaobin Feng, Ying Luo, Jingzhe Hao, Wenjie Jia, Haoyu Chen, Qi Zhu, Zhihao Liang, and et al. 2025. "A Glycopeptide from Agaricus balchaschensis Mitigates Cadmium Damage in Mice" Processes 13, no. 1: 168. https://doi.org/10.3390/pr13010168
APA StyleYalimaimaiti, N., Dong, Y., Jia, P., Feng, X., Luo, Y., Hao, J., Jia, W., Chen, H., Zhu, Q., Liang, Z., & Luo, C. (2025). A Glycopeptide from Agaricus balchaschensis Mitigates Cadmium Damage in Mice. Processes, 13(1), 168. https://doi.org/10.3390/pr13010168





