Catalytic Potential-Guided Design of Multi-Enzymatic System for DHA Production from Glycerol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzyme Production and Purification
2.3. GlyDH Enzyme Activity Assay
2.4. NOX Enzyme Activity Assay
2.5. Protein Content Determination
2.6. Synthesis of Ag-Ni Supports
2.7. Immobilization of Enzymes
2.8. Stability of Biocatalyst and Determination of Catalytic Potential
2.9. Production of DHA from Glycerol in a Basket Reactor
3. Results and Discussions
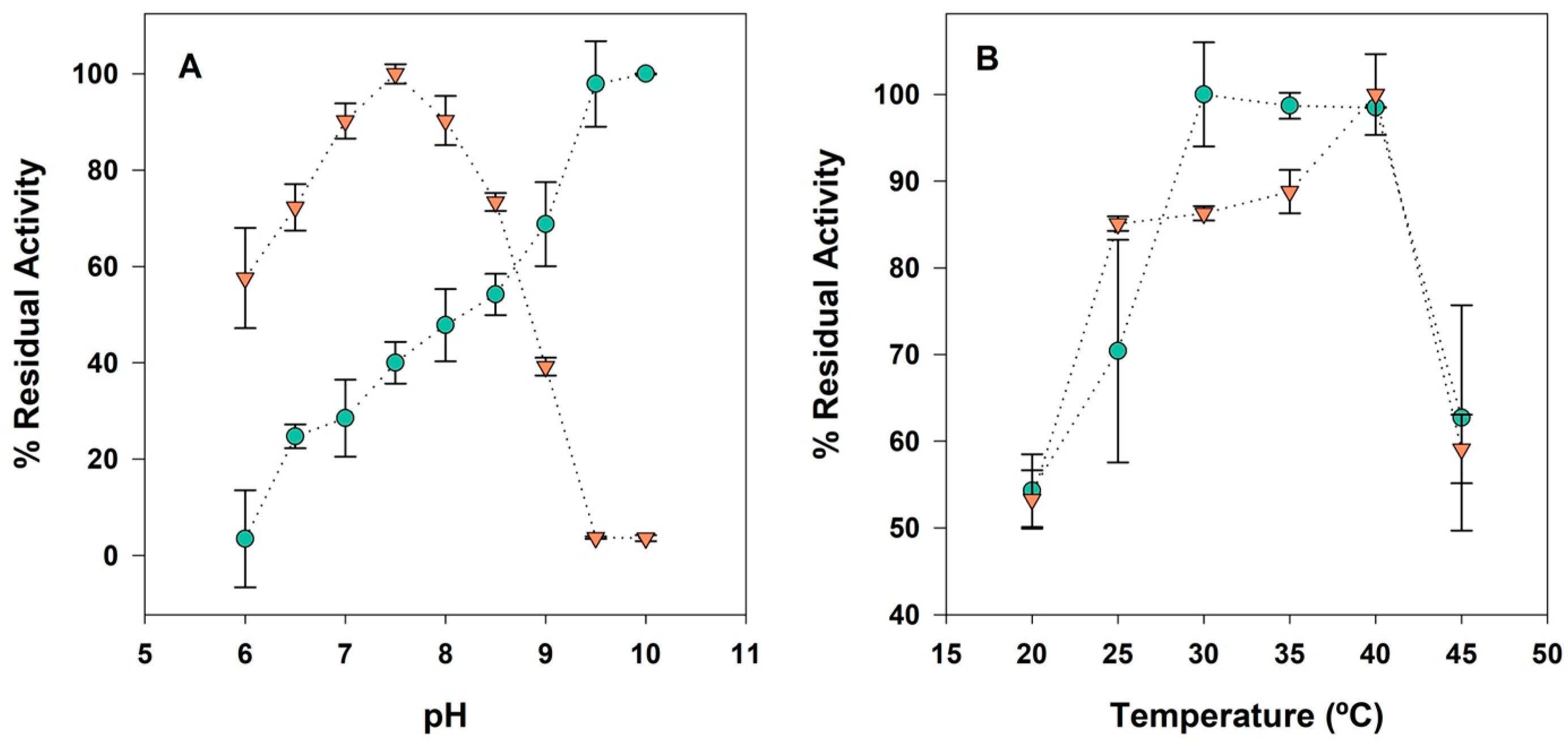
3.1. Characterization of Soluble Enzymes
3.2. Immobilization of Enzymes
3.3. Post-Immobilization Modifications for GlyDH and NOX Catalysts
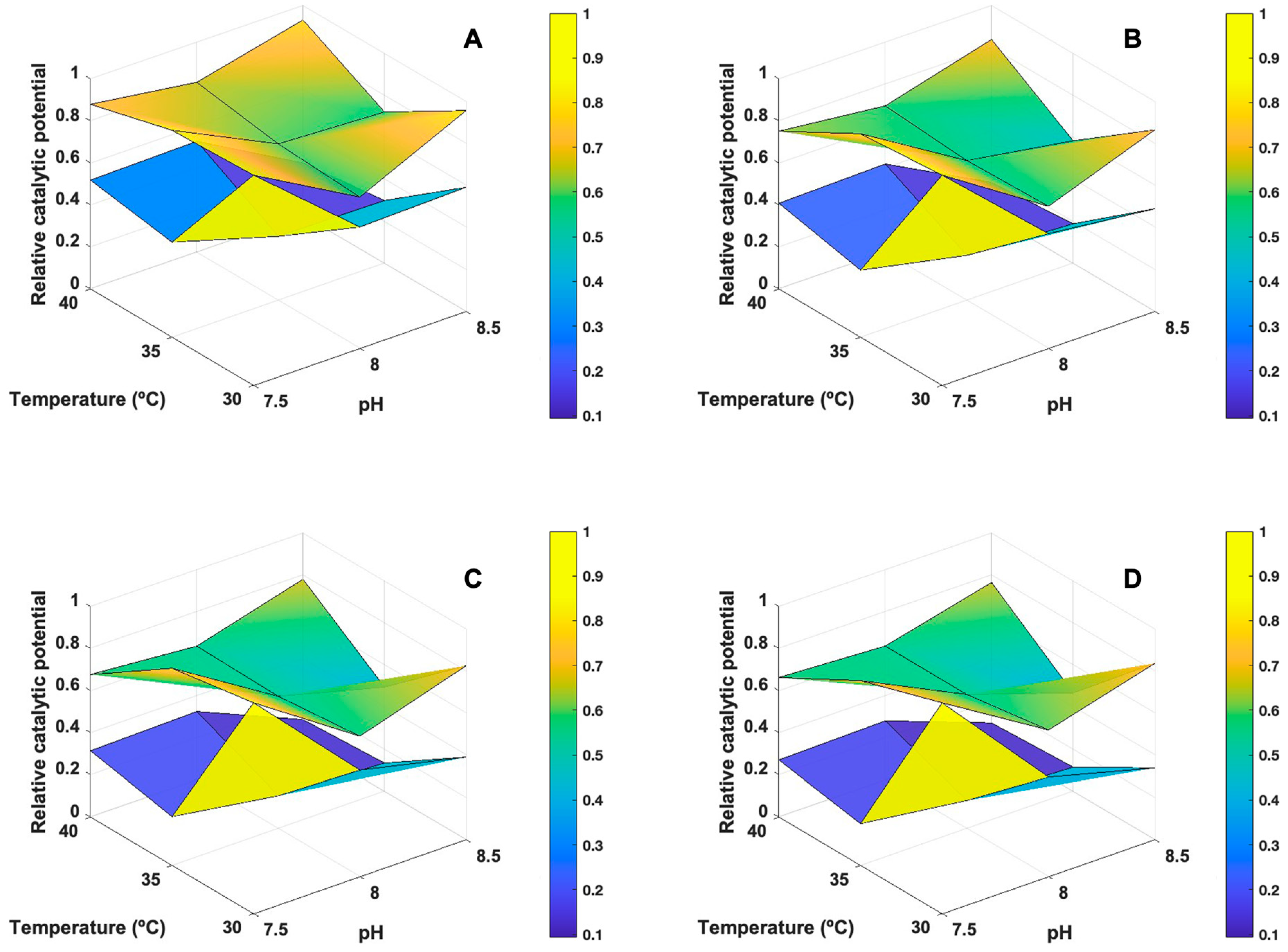
3.4. Determination of Operational Compromise Conditions
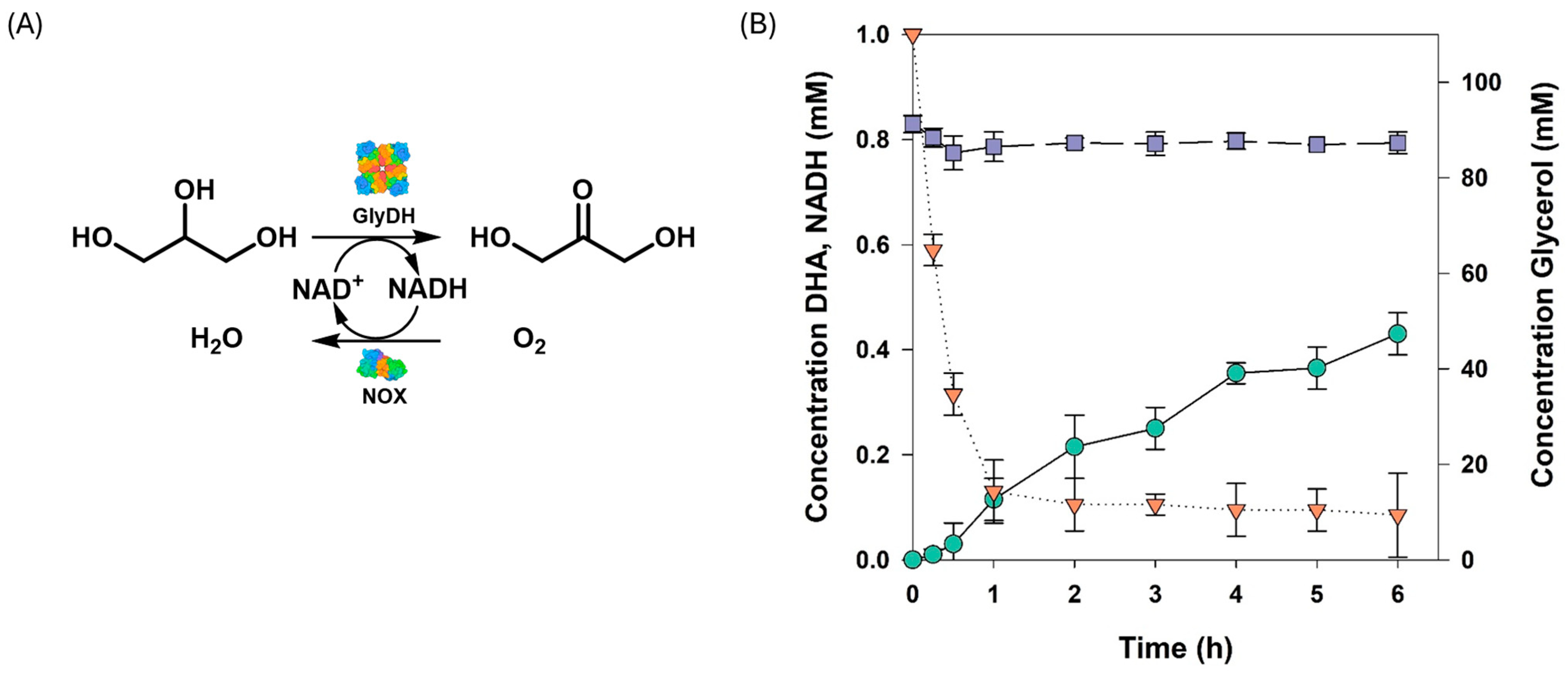
3.5. Production of Dihydroxyacetone from Glycerol Using the Development Multi-Enzymatic System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcántara, A.R.; Domínguez de María, P.; Littlechild, J.A.; Schürmann, M.; Sheldon, R.A.; Wohlgemuth, R. Biocatalysis as Key to Sustainable Industrial Chemistry. ChemSusChem 2022, 15, e202102709. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.M. New frontiers in biocatalysis for sustainable synthesis. Curr. Opin. Green. Sustain. Chem. 2020, 21, 22–26. [Google Scholar] [CrossRef]
- Ripoll, M.; Betancor, L. Opportunities for the valorization of industrial glycerol via biotransformations. Curr. Opin. Green Sustain. Chem. 2021, 28, 100430. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Dihydroxyacetone: An updated insight into an important bioproduct. Chemistryopen 2018, 7, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Wee, Y.; Lee, I.; Sun, H.J.; Zhao, X.; Xia, S.; Kim, S.; Lee, J.; Wang, P.; Kim, J. Stabilized glycerol dehydrogenase for the conversion of glycerol to dihydroxyacetone. J. Chem. Eng. 2015, 276, 283–288. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Acosta, A.; Berenguer, J.; Guisan, J.M.; Lopez-Gallego, F. Selective oxidation of glycerol to 1,3-dihydroxyacetone by covalently immobilized glycerol dehydrogenases with higher stability and lower product inhibition. Bioresour. Technol. 2014, 170, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, S.; Andexer, J.N. Round, round we go-strategies for enzymatic cofactor regeneration. Nat. Prod. Rep. 2020, 37, 1316–1333. [Google Scholar] [CrossRef]
- Gao, H.; Tiwari, M.K.; Kang, Y.C.; Lee, J.K. Characterization of H2O-forming NADH oxidase from Streptococcus pyogenes and its application in l-rare sugar production. Bioorg. Med. Chem. Lett. 2012, 22, 1931–1935. [Google Scholar] [CrossRef]
- Rehn, G.; Pedersen, A.T.; Woodley, J.M. Application of NAD(P)H oxidase for cofactor regeneration in dehydrogenase catalyzed oxidations. J. Mol. Catal. B Enzym. 2016, 134, 331–339. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Romero, O.; Ottone, C. Chapter 17—Future Perspectives in Enzyme Immobilization. In Biocatalyst Immobilization; Ferreira, M.L., Ed.; Academic Press: New York, NY, USA, 2023. [Google Scholar]
- Ottone, C.; Romero, O.; Urrutia, P.; Bernal, C.; Illanes, A.; Wilson, L. Enzyme Biocatalysis and Sustainability. In Nanostructured Catalysts for Environmental Applications; Piumetti, M., Bensaid, S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 383–413. [Google Scholar]
- Ardao, I.; Benaiges, M.D.; Caminal, G.; Alvaro, G. One step purification-immobilization of fuculose-1-phosphate aldolase, a class II DHAP dependent aldolase, by using metal-chelate supports. Enzyme Microb. Technol. 2006, 39, 22–27. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Illanes, A.; Wilson, L. Parameters for the Evaluation of Immobilized Enzymes Under Process Conditions. In Immobilization of Enzymes and Cells: Methods and Protocols; Guisan, J.M., Bolivar, J.M., López-Gallego, F., Rocha-Martín, J., Eds.; Springer: New York, NY, USA, 2020; pp. 65–81. [Google Scholar]
- Silva, P.; Illanes, A.; Wilson, L.; Conejeros, R. One-pot Heterogeneous Biocatalysis under Thermal Decay for Fructose Production from Lactose using Co-Immobilized Enzymes: Modeling and Simulation. ChemCatChem 2024, 16, e202301240. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Ottone, C.; Romero, O. Co-immobilized carrier-free enzymes for lactose upgrading. Curr. Opin. Green Sustain. Chem. 2022, 33, 100553. [Google Scholar] [CrossRef]
- Urrutia, P.; Mateo, C.; Guisan, J.M.; Wilson, L.; Illanes, A. Immobilization of Bacillus circulans β-galactosidase and its application in the synthesis of galacto-oligosaccharides under repeated-batch operation. Biochem. Eng. J. 2013, 77, 41–48. [Google Scholar] [CrossRef]
- Calleja, D.; Kavanagh, J.; De Mas, C.; Lopez-Sants, J. An engineering approach; Simulation and prediction of protein production in fed-batch E. coli cultures: An. engineering approach. Biotechnol. Bioeng. 2016, 113, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Bolivar, J.M.; Godoy, C.A.; Rocha-Martin, J.; Pessela, B.C.; Curiel, J.A.; Muñoz, R.; Guisan, J.M.; Fernández-Lorente, G. Improvement of enzyme properties with a two-step immobilizaton process on novel heterofunctional supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef]
- Henley, J.P.; Sadana, A. Deactivation Theory. Biotechnol. Bioeng. 1986, 28, 1277–1285. [Google Scholar] [CrossRef]
- Piattoni, C.V.; Figueroa, C.M.; Diez, M.D.; Parcerisa, I.L.; Antuña, S.; Comelli, R.A.; Guerrero, S.A.; Beccaria, A.J.; Iglesias, A.Á. Production and characterization of Escherichia coli glycerol dehydrogenase as a tool for glycerol recycling. Process Biochem. 2013, 48, 406–412. [Google Scholar] [CrossRef]
- Richter, N.; Neumann, M.; Liese, A.; Wohlgemuth, R.; Eggert, T.; Hummel, W. Characterisation of a recombinant NADP-dependent glycerol dehydrogenase from Gluconobacter oxydans and its application in the production of L-glyceraldehyde. ChemBioChem 2009, 10, 1888–1896. [Google Scholar] [CrossRef]
- Chauliac, D.; Wang, Q.; St John, F.J.; Jones, G.; Hurlbert, J.C.; Ingram, L.O.; Shanmugam, K.T. Kinetic characterization and structure analysis of an altered polyol dehydrogenase with d-lactate dehydrogenase activity. Protein Sci. 2020, 29, 2387–2397. [Google Scholar] [CrossRef]
- Ko, G.S.; Nguyen, Q.T.; Kim, D.H.; Yang, J.K. Biochemical and molecular characterization of glycerol dehydrogenase from klebsiella pneumoniae. J. Microbiol. Biotechnol. 2020, 30, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Shi, H.; Gu, H.; Zhang, Y.; Li, X.; Wang, F. Characterization of glycerol dehydrogenase from thermoanaerobacterium thermosaccharolyticum DSM 571 and GGG motif identification. J. Microbiol. Biotechnol. 2016, 26, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, Z.; Long, C.; He, B.; Hu, Z.; Jiang, C.; Zeng, B. Identification and functional characterization of glycerol dehydrogenase reveal the role in kojic acid synthesis in Aspergillus oryzae. World J. Microbiol. Biotechnol. 2020, 36, 136. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Shi, Y.; Zhang, J.X.; Gao, J.; Zhang, Y.W. Cloning, expression, characterization and homology modeling of a novel water-forming NADH oxidase from Streptococcus mutans ATCC 25175. Int. J. Biol. Macromol. 2018, 113, 1073–1079. [Google Scholar] [CrossRef]
- Zhang, J.D.; Cui, Z.M.; Fan, X.J.; Wu, H.L.; Chang, H.H. Cloning and characterization of two distinct water-forming NADH oxidases from Lactobacillus pentosus for the regeneration of NAD. Bioprocess. Biosyst. Eng. 2016, 39, 603–611. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Acosta, A.; Guisan, J.M.; López-Gallego, F. Immobilizing systems biocatalysis for the selective oxidation of glycerol coupled to in situ cofactor recycling and hydrogen peroxide elimination. ChemCatChem 2015, 7, 1939–1947. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazu, V.; López-Gallego, F.; Pessela, B.C.; Hidalgo, A.; Fernández-Lorente, G.; Fernández-Lafuente, R.; et al. Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Rivas, B.d.l.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. Rational Co-Immobilization of Bi-Enzyme Cascades on Porous Supports and Their Applications in Bio-Redox Reactions with In Situ Recycling of Soluble Cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- Diamanti, E.; Arana-Peña, S.; Ramos-Cabrer, P.; Comino, N.; Carballares, D.; Fernandez-Lafuente, R.; López-Gallego, F. Intraparticle Macromolecular Migration Alters the Structure and Function of Proteins Reversibly Immobilized on Porous Microbeads. Adv. Mater. Interfaces 2022, 9, 2200263. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Claiborne, A.L.; Miller, H.; Parsonage, D.; Ross, R.P. Protein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation. FASEB J. 1993, 7, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Villanueva, A.; Reyes-Vivas, H.; Oria-Hernández, J. Kinetic stability of the water-forming NADH oxidase from Giardia lamblia: Implications for biotechnological processes. Biotechnol. Biotechnol. Equip. 2021, 35, 1401–1408. [Google Scholar] [CrossRef]
- Spencer, P.; Atkinson, T.; Gore, M. The identification of a structurally important cysteine residue in the glycerol dehydrogenase from Bacillus stearothermophilus. Biochim. Biophys. Acta 1991, 1073, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Q.; Li, F.L.; Yu, W.Q.; Li, R.F.; Zhang, Y.W. Combined cross-linked enzyme aggregates of glycerol dehydrogenase and NADH oxidase for high efficiency in situ NAD+ regeneration. Int. J. Biol. Macromol. 2020, 144, 1013–1021. [Google Scholar] [CrossRef]
- Ashrafuddin Ahmed, S. The streptococcal flavoprotein nadh oxidase I. Evidence linking Nadh oxidase and Nadh peroxidase cysteinyl redox centers. J. Biol. Chem. 1989, 264, 19856–19863. [Google Scholar]
- Silva, P.; Arancibia, V.; Cid, D.; Romero, O.; Illanes, A.; Wilson, L. Catalyst replacement policy on multienzymatic systems: Theoretical study in the one-pot sequential batch production of lactofructose syrup. Catalysts 2021, 11, 1167. [Google Scholar] [CrossRef]
- Lye, G.J.; Woodley, J.M. Application of in situ product-removal techniques to biocatalytic processes. Trends Biotechnol. 1999, 17, 395–402. [Google Scholar] [CrossRef]

| Enzyme | Protein Loading (mg/g) | IYp (%) | IYact (%) | RA% | RCA% |
|---|---|---|---|---|---|
| GlyDH | 0.5 | 93.6 | 100.0 | 49.2 | 37.8 |
| GlyDH | 20 | 47.5 | 60.3 | 9.9 | 0.8 |
| NOX | 0.5 | 67.0 | 100.0 | 63.0 | 48.1 |
| NOX | 20 | 45.3 | 71.7 | 34.3 | 21.1 |
| Type of Support and Crosslinker | RCA (%) | Specific Activity (UI/g) | t1/2 (h) * |
|---|---|---|---|
| Soluble | - | 1.24 ** | 591 |
| Ag-Ni+2 | 21.41 | 0.94 | 497 |
| Ag-Ni PEI | 29.53 | 0.48 | 59 |
| Ag-Ni 0.0125% Glut | 16.14 | 0.80 | 973 |
| Ag-Ni 0.025% Glut | 15.09 | 0.79 | 1036 |
| Ag-Ni 0.05% Glut | 15.58 | 0.73 | 966 |
| Type of Support and Crosslinker | RCA (%) | Specific Activity (UI/g) | t1/2 (h) * |
|---|---|---|---|
| Soluble | - | 5.28 ** | 3.36 |
| Ag-Ni+2 | 13.77 | 5.27 | 6.74 |
| Ag-Ni PEI | 11.07 | 2.62 | 3.7 |
| Ag-Ni 0.0125% Glut | 12.36 | 4.42 | 3.19 |
| Ag-Ni 0.025% Glut | 11.09 | 4.27 | 3.01 |
| Ag-Ni 0.05% Glut | 7.02 | 2.7 | 3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pizarro, C.; Wilson, L.; Romero, O. Catalytic Potential-Guided Design of Multi-Enzymatic System for DHA Production from Glycerol. Processes 2024, 12, 2014. https://doi.org/10.3390/pr12092014
Fernández-Pizarro C, Wilson L, Romero O. Catalytic Potential-Guided Design of Multi-Enzymatic System for DHA Production from Glycerol. Processes. 2024; 12(9):2014. https://doi.org/10.3390/pr12092014
Chicago/Turabian StyleFernández-Pizarro, Carolina, Lorena Wilson, and Oscar Romero. 2024. "Catalytic Potential-Guided Design of Multi-Enzymatic System for DHA Production from Glycerol" Processes 12, no. 9: 2014. https://doi.org/10.3390/pr12092014
APA StyleFernández-Pizarro, C., Wilson, L., & Romero, O. (2024). Catalytic Potential-Guided Design of Multi-Enzymatic System for DHA Production from Glycerol. Processes, 12(9), 2014. https://doi.org/10.3390/pr12092014







