Computational Fluid Dynamics Modelling of a Laboratory Spray Dry Scrubber for SO2 Removal in Flue Gas Desulphurisation—Effect of Drying Models
Abstract
1. Introduction
2. Materials and Methods
3. Numerical Model
3.1. Hydrodynamic Modelling
3.1.1. Continuous Phase
3.1.2. Dispersed Phase
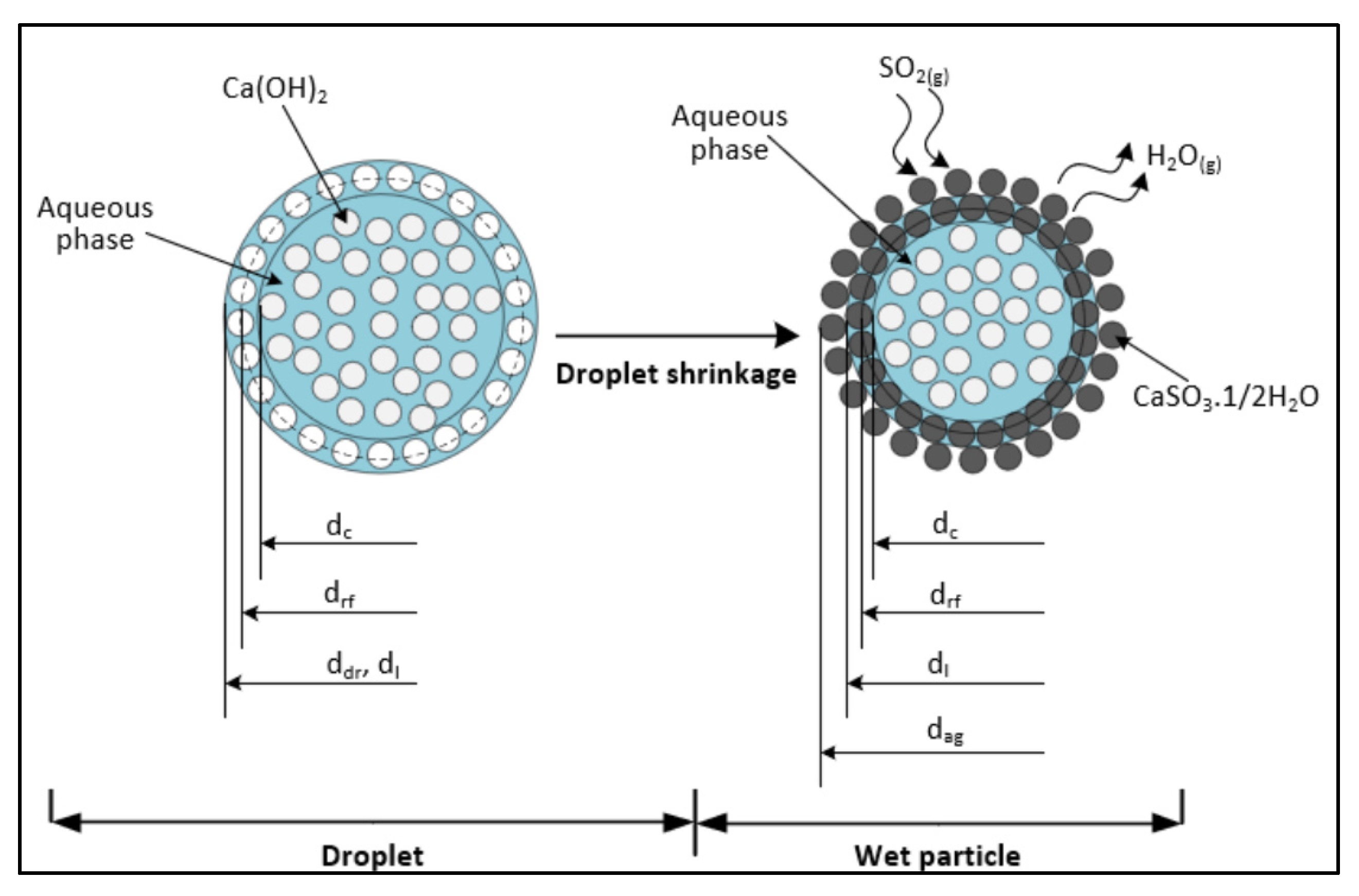
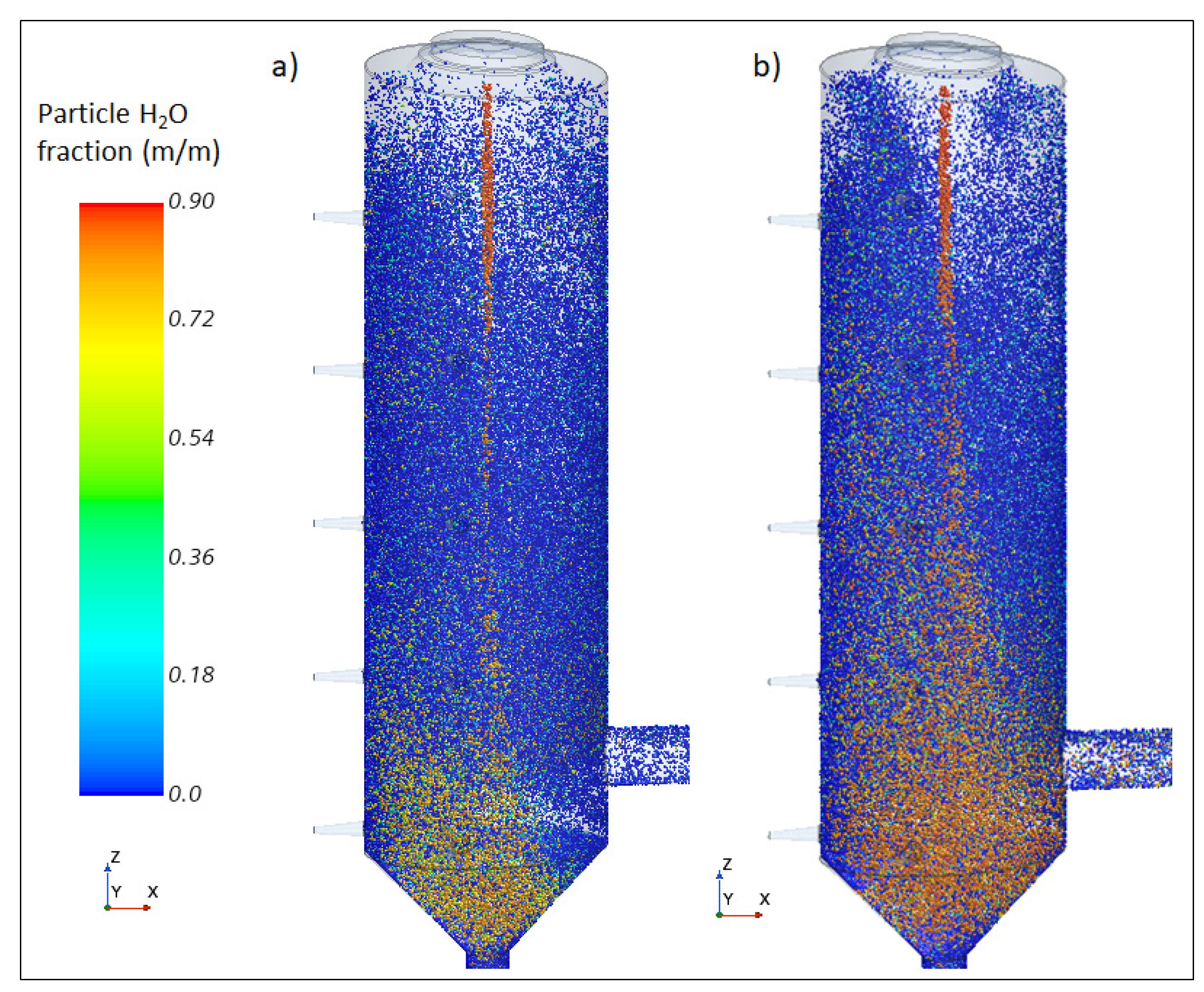
3.2. Droplet Drying Modelling
3.3. SO2 Absorption Modelling
3.4. Effect of pH on the Reaction Chemistry
4. Numerical Solution Approach
5. Results and Discussion
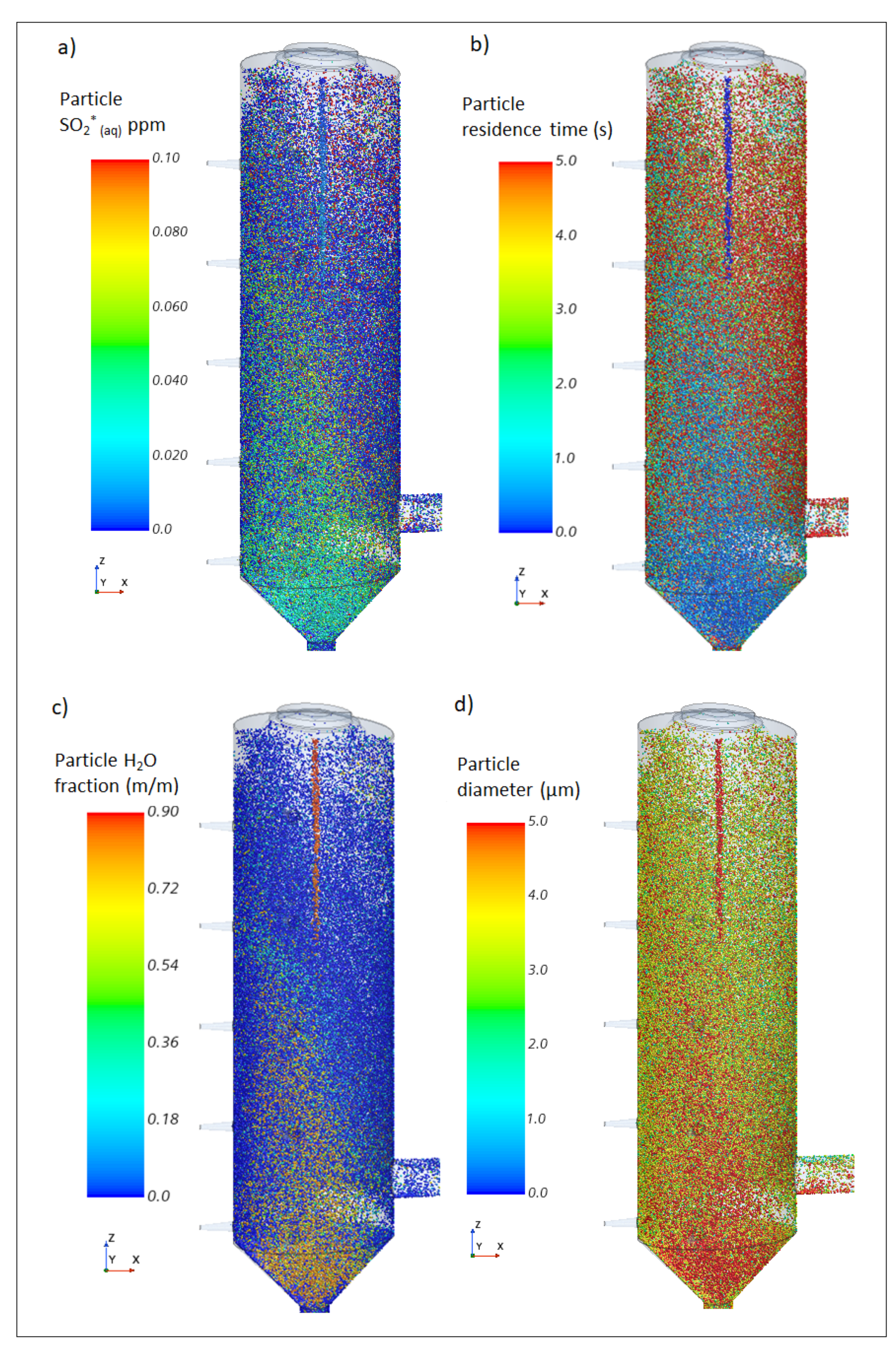
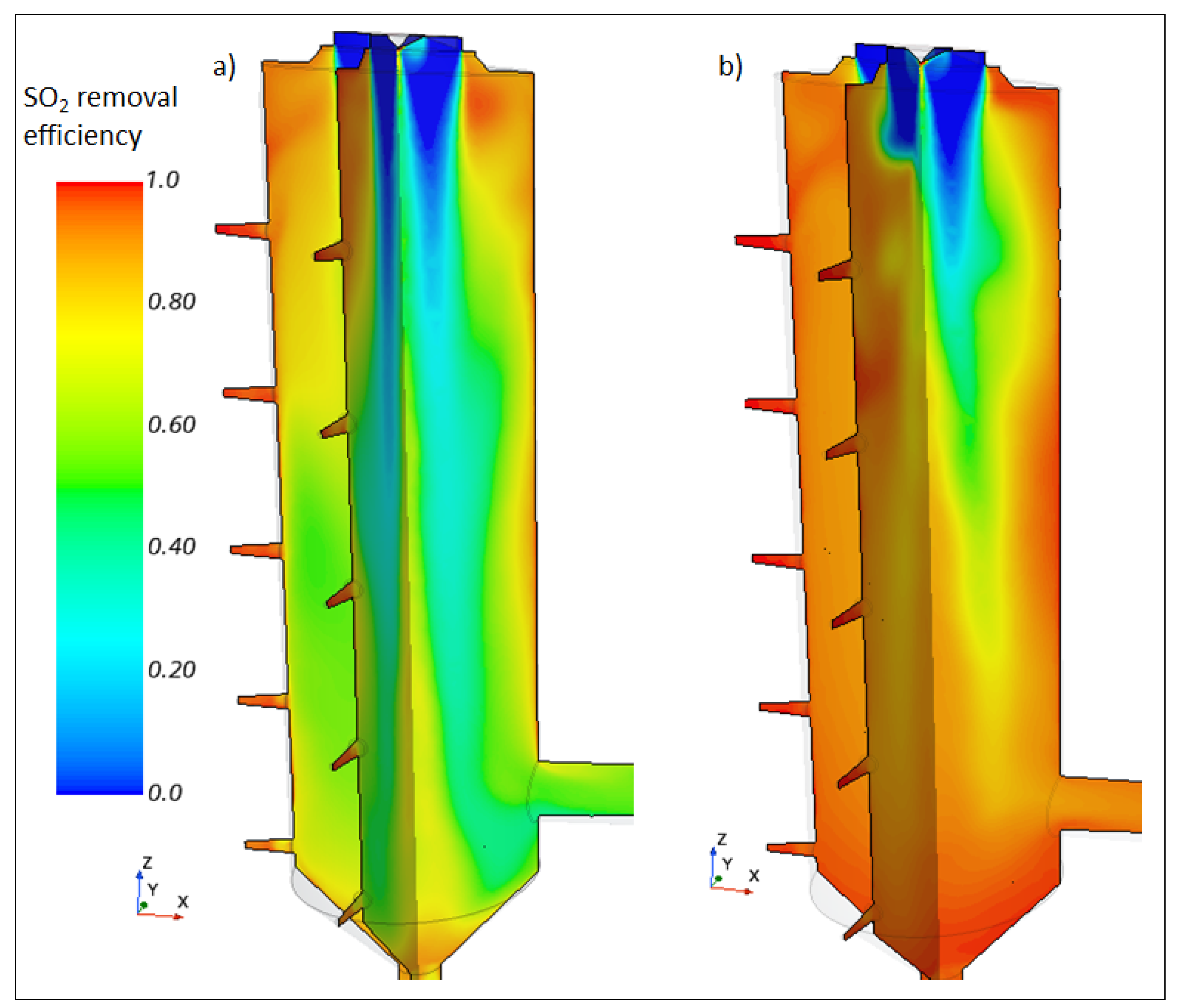
5.1. SO2 Removal and Associated Variables
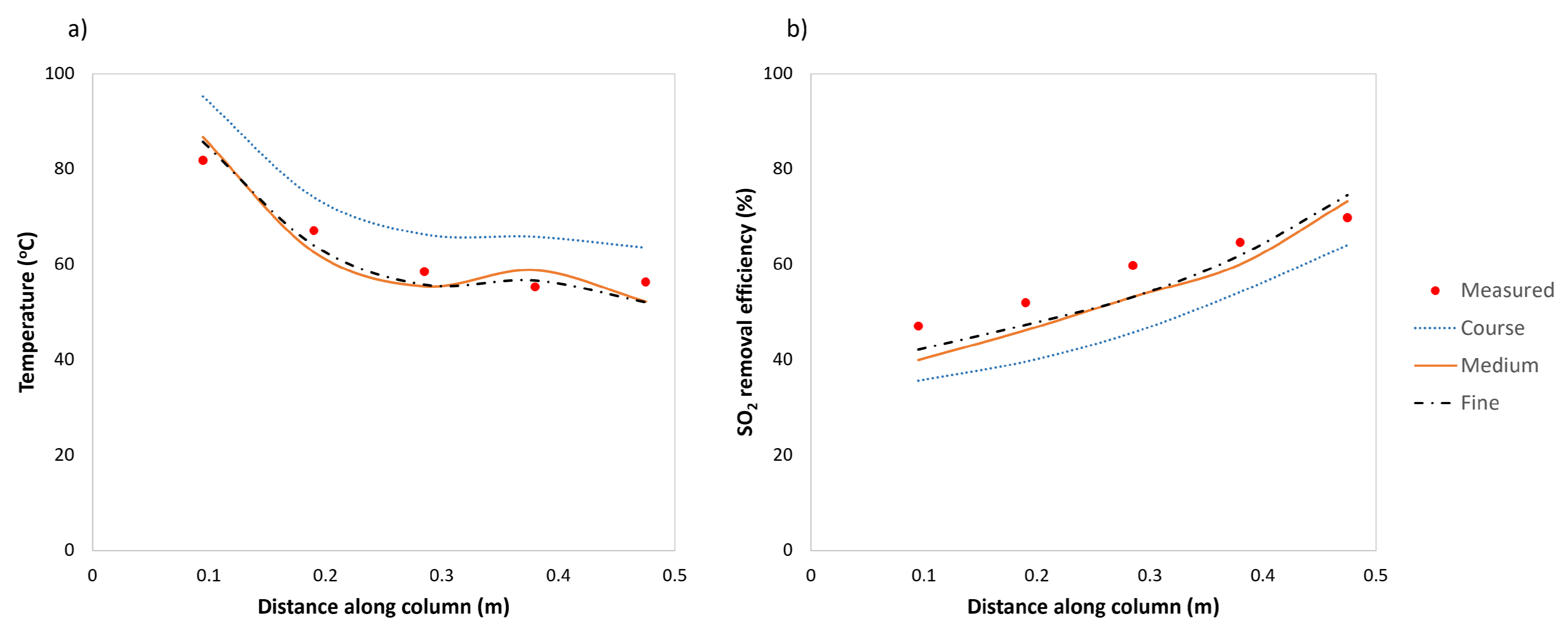
5.2. Validation of the Drying Model
5.3. Sensitivity Analysis
5.3.1. Effect of Inlet Temperature on SO2-Removal Efficiency
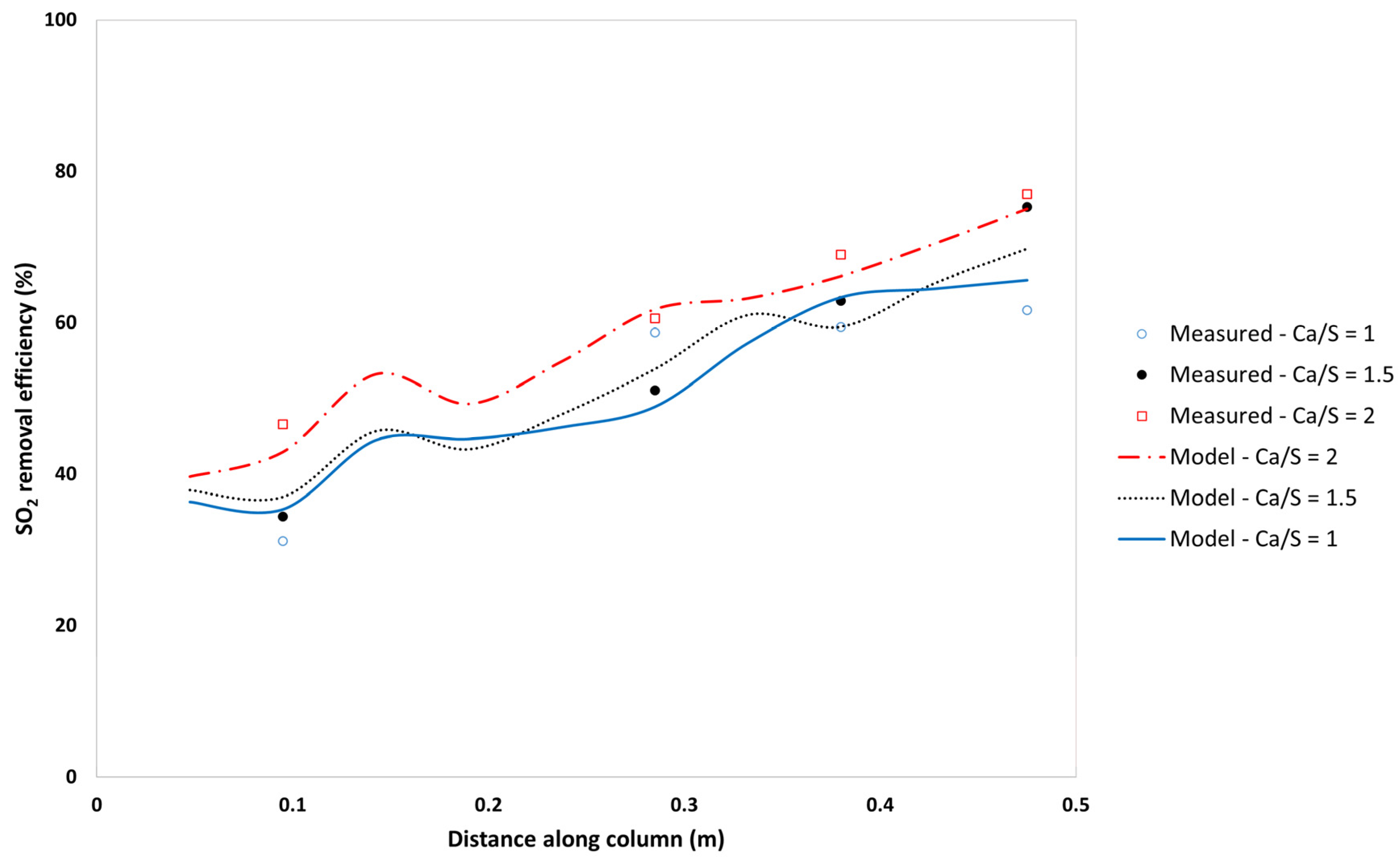
5.3.2. Effect of the Ca/S on SO2-Removal Efficiency and Sorbent Utilisation
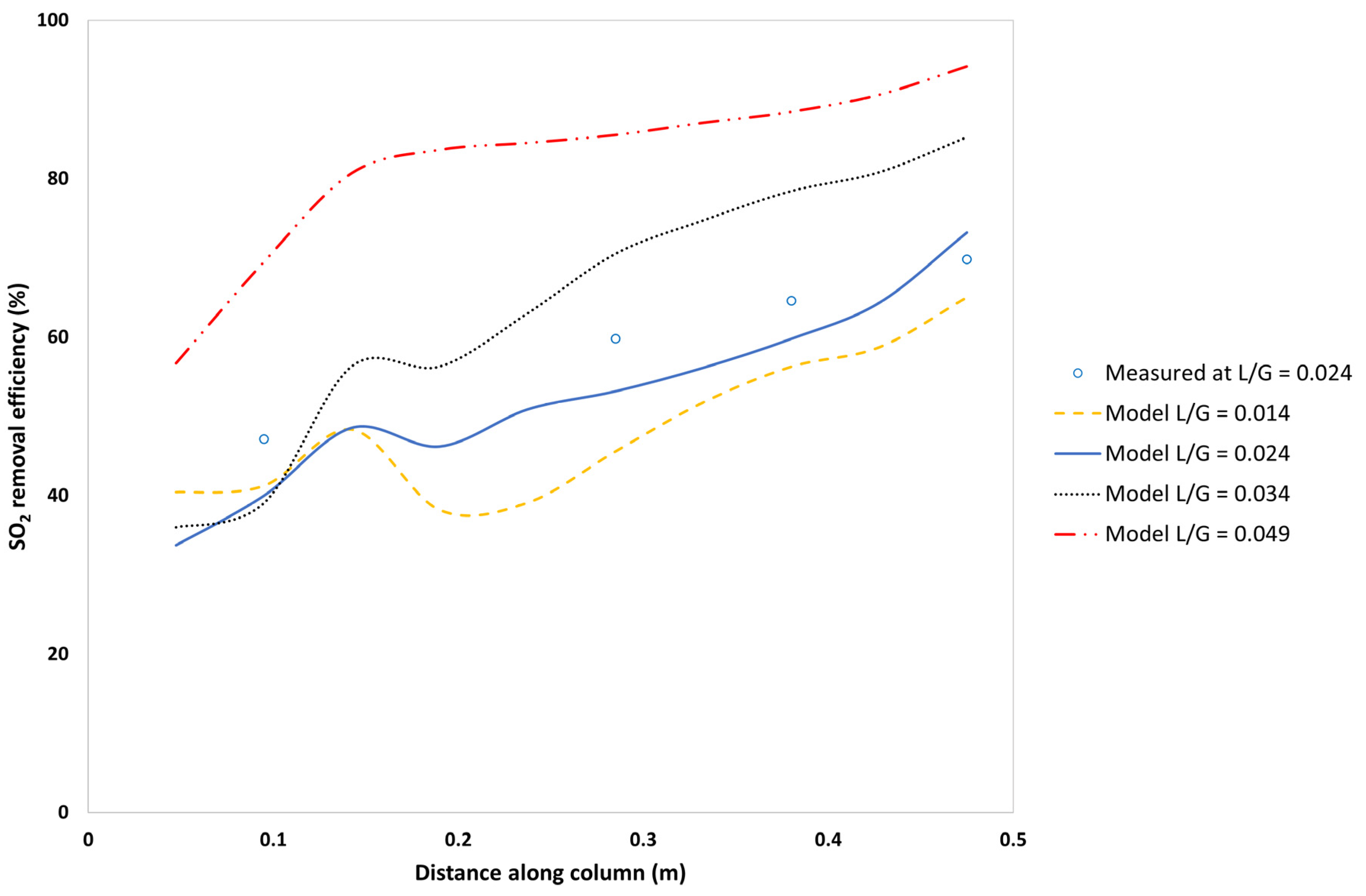
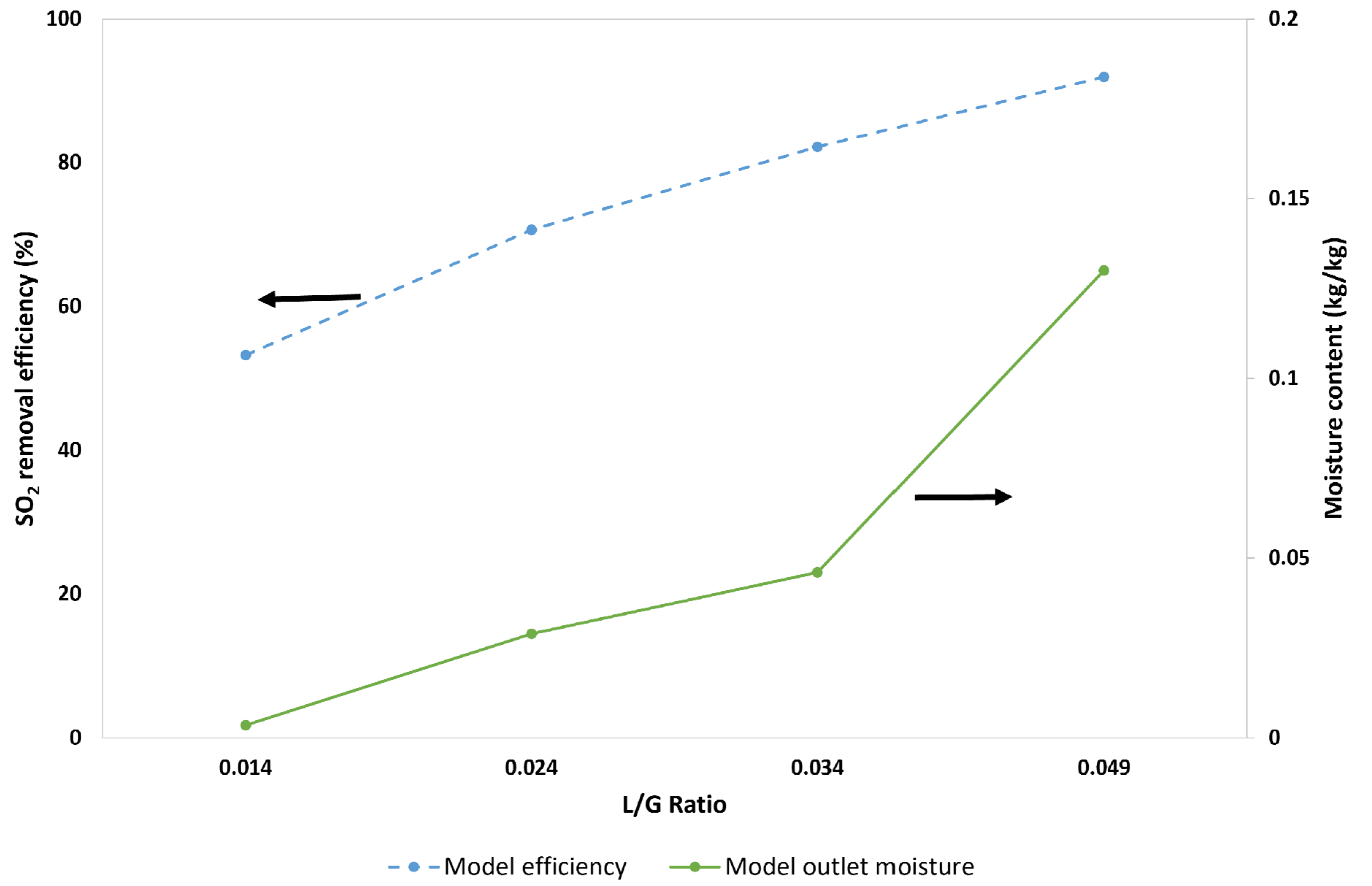
5.3.3. Effect of the L/G Ratio on the SO2-Removal Efficiency
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| Density (kg m–3) | Viscosity (Pa s) | ||
| Velocity (m s–1) | Eddy viscosity (Pa s) | ||
| Species mass fraction (-) | Gravitational term (m1 s–2) | ||
| Mass diffusivity (m–2 s–1) | Re | Reynolds number (–) | |
| Time (s–1) | Prandtl number (–) | ||
| Energy per unit mass (J kg–1) | Schmidt number (–) | ||
| Pressure (Pa) | Coefficient of drag (–) | ||
| Partial pressure (Pa) | Specific heat capacity (J K–1) | ||
| Thermal conductivity (W m–1 K–1) | Heat transfer coefficient (W m–2 K–1) | ||
| Stress tensor (Pa) | Area (m2) | ||
| Reynolds stress tensor (Pa) | Latent heat of vaporisation (J kg–1) | ||
| Temperature (K) | ki, g | Gas-phase mass transfer coefficient for component i (m s–1) | |
| Specific dissipation rate (s–1) | Ki,c | Overall mass transfer coefficient for component i (m s–1) | |
| Turbulent kinetic energy (m2 s–2) | Geometric parameter (m) | ||
| Position (m) | Agglomerate area (m2) | ||
| Diameter (m) | Tortuosity parameter (m) | ||
| Particle velocity (m s–1) | Molar flow rate (mol) | ||
| Subscripts | |||
| A | Species A | mom | Momentum |
| B | Species B | k | kth particle |
| d | Droplet | g | gas |
| p | Particle | l | liquid |
| m | mass | Bulk phase | |
| s | Droplet/particle surface | E | energy |
| Superscripts | |||
| ‾ | vector term | · | Rate |
References
- Sun, W.; Zhou, Y.; Lv, J.; Wu, J. Assessment of multi-air emissions: Case of particulate matter (dust), SO2, NO and CO2 from iron and steel industry of China. J. Clean. Prod. 2019, 232, 350–358. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Xu, Y.; Chen, L.; Wang, Q.; Ma, Q.; Yuan, X. A multi-criteria group decision making framework for sustainability evaluation of sintering flue gas treatment technologies in the iron and steel industry. J. Clean. Prod. 2023, 389, 136048. [Google Scholar] [CrossRef]
- Bešenić, T.; Baleta, J.; Pachler, K.; Vujanović, M. Numerical modelling of sulfur dioxide absorption for spray scrubbing. Energy Convers. Manag. 2020, 217, 112762. [Google Scholar] [CrossRef]
- Lerotholi, L.; Everson, R.C.; Koech, L.; Neomagus, H.W.J.P.; Rutto, H.L.; Branken, D.; Hattingh, B.B.; Sukdeo, P. Semi-dry flue gas desulphurization in spray towers: A critical review of applicable models for computational fluid dynamics analysis. Clean Technol. Environ. Policy 2022, 24, 2011–2060. [Google Scholar] [CrossRef]
- Córdoba, P. Status of flue gas desulphurisation (FGD) systems from coal-fired power plants: Overview of the physic-chemical control processes of wet limestone FGDs. Fuel 2015, 144, 274–286. [Google Scholar] [CrossRef]
- Kumar, L.; Jana, S.K. Advances in absorbents and techniques used in wet and dry FGD: A critical review. Rev. Chem. Eng. 2021, 38, 843–880. [Google Scholar] [CrossRef]
- Ladwig, K.J.; Blythe, G.M. Flue-gas desulfurization products and other air emissions controls. In Coal Combustion Products; Robl, T., Overlink, A., Jones, R., Eds.; Elsevier: Cambridge, UK, 2017; pp. 67–95. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Jozewicz, W. Flue gas desulfurization: The state of the art. J. Air Waste Manag. 2001, 51, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Koech, L.; Rutto, H.; Lerotholi, L.; Everson, R.; Neomagus, H.; Branken, D.; Moganelwa, A. Spray drying absorption for desulphurization: A review of recent developments. Clean Technol. Environ. Policy 2021, 23, 1665–1686. [Google Scholar] [CrossRef]
- Carpenter, A.M. Low Water FGD Technologies; IEA Clean Coal Centre: London, UK, 2012. [Google Scholar]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef]
- Meuleman, E.; Cottrell, A.; Ghayur, A. Treatment of flue-gas impurities for liquid absorbent-based post-combustion CO2 capture processes. In Absorption-Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Elsevier: Cambridge, UK, 2016; pp. 519–551. [Google Scholar] [CrossRef]
- Scala, F.; D’Ascenzo, M.; Lancia, A. Modeling flue gas desulfurization by spray-dry absorption. Sep. Purif. Technol. 2004, 34, 143–153. [Google Scholar] [CrossRef]
- Hill, F.F.; Zank, J. Flue gas desulphurization by spray dry absorption. Chem. Eng. Process. 2000, 39, 45–52. [Google Scholar] [CrossRef]
- Katolicky, J.; Jicha, M. Influence of lime slurry droplet spectrum on the efficiency of semi-dry flue gas desulfurization. Chem. Eng. Technol. 2012, 36, 156–166. [Google Scholar] [CrossRef]
- Marocco, L.; Inzoli, F. Multiphase Euler–Lagrange CFD simulation applied to wet flue gas desulphurisation technology. Int. J. Multiph. Flow 2009, 35, 185–194. [Google Scholar] [CrossRef]
- Qu, J.; Qi, N.; Li, Z.; Zhang, K.; Wang, P.; Li, L. Mass transfer process intensification for SO2 absorption in a commercial-scale wet flue gas desulfurization scrubber. Chem. Eng. Process.—Process Intensif. 2021, 166, 108478. [Google Scholar] [CrossRef]
- Hong, M.; Xu, G.; Lu, P.; Huang, Z.; Miao, H.; Zhang, Y.; Ma, C.; Zhang, Q. CFD analysis of the spray droplet parameters effect on gas-droplet flow and evaporation characteristics in circulating fluidized bed desulfurization tower. Chem. Eng. Res. Design 2023, 196, 184–203. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhu, T.; Jing, P.; Wang, J. Simulation of the heterogeneous semi-dry flue gas desulfurization in a pilot CFB riser using the two-fluid model. Chem. Eng. J. 2015, 264, 479–486. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Feng, M.; Cui, J. Research on the characteristics of flue gas purification reaction in spouted bed. Chem. Eng. Sci. 2023, 282, 119329. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, R.; Yuan, Z.; Shao, B.; Fan, J. Numerical simulation of semi-dry desulfurization spouted bed using the discrete element method (DEM). Powder Technol. 2021, 378, 191–201. [Google Scholar] [CrossRef]
- Hrdlička, J.; Dlouhý, T. Full-scale evaluation of SO2 capture increase for semi-dry FGD technology. J. Energy Inst. 2018, 92, 1399–1405. [Google Scholar] [CrossRef]
- Koech, L.; Everson, R.C.; Hattingh, B.; Rutto, H.; Lerotholi, L.; Neomagus, H.W. Comparative study of sorbents for spray dry scrubbing of SO2 from flue gases. ACS Omega 2023, 8, 23401–23411. [Google Scholar] [CrossRef]
- Liu, P.; Yang, S.; Hu, J.; Wang, H. Numerical analysis of SO2 removal characteristics in industrial flue gas desulfurization reactor by spray drying adsorption. Sep. Purif. Technol. 2023, 323, 124475. [Google Scholar] [CrossRef]
- Katolicky, J.; Jicha, M. Optimization of Flue Gas Desulphurization Absorber by Means of Computational Fluid Dynamics Analysis. In Proceedings of the 12th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Costa de Sol, Spain, 11–13 July 2016. [Google Scholar]
- Mei, D.; Shi, J.; Zhu, Y.; Xu, X.; Xing, F.; Shi, L. Optimization of the operation parameters of SDA desulfurization tower by coupling chemical reaction model. Polish J. Chem. Technol. 2020, 22, 35–45. [Google Scholar] [CrossRef]
- Grosshans, H. Evaporation of a Droplet. Project Report 2012MVK160; Heat and Mass Transport: Lund, Sweden, 2012. [Google Scholar]
- Sirignano, W.A. Fluid Dynamics and Transport of Droplets and Sprays, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Patel, K.; Chen, X.D.; Jeantet, R.; Schuck, P. One-dimensional simulation of co-current, dairy spray drying systems—Pros and cons. Dairy Sci. Technol. 2010, 90, 181–210. [Google Scholar] [CrossRef]
- Ochowiak, M.; Bielecki, Z.; Bielecki, M.; Włodarczak, S.; Krupińska, A.; Matuszak, M.; Choiński, D.; Lewtak, R.; Pavlenko, I. The D2-law of droplet evaporation when calculating the droplet evaporation process of liquid containing solid state catalyst particles. Energies 2022, 15, 7642. [Google Scholar] [CrossRef]
- Dalla Barba, F.; Wang, J.; Picano, F. Revisiting D2-law for the evaporation of dilute droplets. Phys. Fluids 2021, 33, 051701. [Google Scholar] [CrossRef]
- BÜCHI Labortechnik. B-290 Mini Spray Dryer Operation Manual. 2018. Available online: https://www.labotec.co.za/wp-content/uploads/2016/07/B-290-Mini-Spray-Dryer-Data-Sheet.pdf (accessed on 30 August 2024).
- Kievet, F.G. Modelling Quality in Spray Drying. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 1997. [Google Scholar]
- Gabites, J.R.; Abrahamson, J.; Winchester, J.A. Air flow patterns in an industrial milk powder spray dryer. Chem. Eng. Res. Des. 2010, 88, 899–910. [Google Scholar] [CrossRef][Green Version]
- Langrish, T.A.G.; Williams, J.; Fletcher, D.F. Simulation of the effects of inlet swirl on gas flow patterns in a pilot-scale spray dryer. Chem. Eng. Res. Des. 2004, 82, 821–833. [Google Scholar] [CrossRef]
- Partridge, G.P.; Davis, W.T.; Counce, R.M.; Reed, G.D. A mechanistically based mathematical model of sulfur dioxide absorption into calcium hydroxide slurry in a spray dryer. Chem. Eng. Commun. 1990, 96, 97–112. [Google Scholar] [CrossRef]
- Mchabe, D.; Everson, R.C.; Ramachandran, P.A.; Neomagus, H.W.J.P.; Branken, D.J. Development of an integrated model for absorption of sulphur dioxide in limestone slurry. Chem. Eng. Sci. 2020, 229, 116050. [Google Scholar] [CrossRef]
- Neveux, T.; Le Moullec, Y. Wet industrial flue gas desulfurization unit: Model development and validation on industrial data. Ind. Eng. Chem. Res. 2011, 50, 7579–7592. [Google Scholar] [CrossRef]
- Hanus, M.J.; Langrish, T.A.G. Re-entrainment of wall deposits from a laboratory-scale spray dryer. Asia-Pac. J. Chem. Eng. 2007, 2, 90–107. [Google Scholar] [CrossRef]
- Welty, J.R. Engineering Heat Transfer—SI Version; John Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Woo, M.W.; Daud, W.R.W.; Mujumdar, A.S.; Wu, Z.; Meor Talib, M.Z.; Tasirin, S.M. CFD evaluation of droplet drying models in a spray dryer fitted with a rotary atomizer. Dry. Technol. 2008, 26, 1180–1198. [Google Scholar] [CrossRef]
- Poozesh, S.; Grib, S.W.; Renfro, M.W.; Marsac, P.J. Near-field dynamics of high-speed spray dryer coannular two fluid nozzle: Effects of operational conditions and formulations. Powder Technol. 2018, 333, 439–448. [Google Scholar] [CrossRef]
- Neathery, J.K. Model for flue-gas desulfurization in a circulating dry scrubber. AIChE J. 1996, 42, 259–268. [Google Scholar] [CrossRef]







| Process Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Tin (°C) | 108 | 130 | 142 | 108 | 130 | 142 | 142 |
| Ca/S ratio (mol/mol) | 1 | 1 | 1 | 1.5 | 1.5 | 1.5 | 2 |
| Regime | I | II | III |
|---|---|---|---|
| pH | pH ≥ 6.83 at 323.15 K | 6.83 > pH > 3 at 323.15 K | pH ≤ 6.83 at 323.15 K |
| Gas-side resistance | Highly significant | Significant | Insignificant |
| Liquid-side resistance | Insignificant | Significant | Highly significant |
| Dissociation of aqueous SO2 | Highly favoured | Favoured | Not favoured |
| concentration | Insignificant | Significant | Highly significant |
| concentration | Highly significant | Significant | Insignificant |
| Parameter | Units | Value |
|---|---|---|
| Continuous phase—Flue gas | ||
| Inlet boundary—velocity inlet | ||
| Inlet temperature | °C | 108; 130; 142 |
| Inlet L/G ratio | - | 0.014; 0.024; 0.034; 0.049 |
| Inlet Ca/S ratio | - | 1; 1.5; 2 |
| Outlet boundary—pressure outlet | Exit gas | |
| Pressure (cyclone exit) | kPa | 84.6 |
| Wall | ||
| Convective heat transfer coefficient | W/m2·K | 5 |
| Thermal conductivity | W/m·K | 0.74 |
| Free stream temperature | °C | 20 |
| Dispersed phase—Lime slurry | ||
| Inlet boundary—velocity inlet | Atomisation air | |
| Mass flow rate | kg/h | 0.473 |
| Dispersed phase—Injection properties | ||
| Mass flow rate | kg/h | 0.9 |
| Temperature | °C | 20 |
| Parcel number | - | 80 |
| Mean diameter | µm | 10.5 |
| Measured Variable | Axial Distance from the Top of the Column | RMSE | ||||
|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | ||
| Model SO2-removal efficiency | 40.0 | 46.2 | 53.2 | 60.0 | 73.2 | 5.7 |
| Measured SO2-removal efficiency | 47.1 | 52.0 | 59.8 | 64.6 | 69.8 | |
| Absolute error (Efficiency) | 7.14 | 5.82 | 6.63 | 4.83 | 3.35 | |
| Model T (°C) | 86.7 | 62.6 | 55.4 | 58.8 | 52.2 | 4.1 |
| Measured T (°C) | 81.8 | 67.1 | 58.5 | 55.3 | 56.3 | |
| Absolute error (Temperature) | 4.9 | 4.5 | 3.0 | 3.5 | 4.1 | |
| Process Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| RMSE of the efficiency | 4.7 | 5.7 | 6.0 | 5.5 | 5.6 | 3.8 | 2.6 |
| Coefficient of determination (R2) | 0.92 | 0.90 | 0.77 | 0.85 | 0.89 | 0.94 | 0.95 |
| Process Variable | Case 3 | Case 6 | Case 7 |
|---|---|---|---|
| Ca/S ratio | 1 | 1.5 | 2 |
| Model outlet efficiency (%) | 70.7 | 73.9 | 78.6 |
| Model calculated outlet utilisation (%) | 70.7 | 49.3 | 39.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerotholi, L.; Everson, R.C.; Hattingh, B.B.; Koech, L.; Le Roux, I.; Neomagus, H.W.J.P.; Rutto, H.L. Computational Fluid Dynamics Modelling of a Laboratory Spray Dry Scrubber for SO2 Removal in Flue Gas Desulphurisation—Effect of Drying Models. Processes 2024, 12, 1862. https://doi.org/10.3390/pr12091862
Lerotholi L, Everson RC, Hattingh BB, Koech L, Le Roux I, Neomagus HWJP, Rutto HL. Computational Fluid Dynamics Modelling of a Laboratory Spray Dry Scrubber for SO2 Removal in Flue Gas Desulphurisation—Effect of Drying Models. Processes. 2024; 12(9):1862. https://doi.org/10.3390/pr12091862
Chicago/Turabian StyleLerotholi, Letsabisa, Raymond C. Everson, Burgert B. Hattingh, Lawrence Koech, Ignus Le Roux, Hein W. J. P. Neomagus, and Hilary L. Rutto. 2024. "Computational Fluid Dynamics Modelling of a Laboratory Spray Dry Scrubber for SO2 Removal in Flue Gas Desulphurisation—Effect of Drying Models" Processes 12, no. 9: 1862. https://doi.org/10.3390/pr12091862
APA StyleLerotholi, L., Everson, R. C., Hattingh, B. B., Koech, L., Le Roux, I., Neomagus, H. W. J. P., & Rutto, H. L. (2024). Computational Fluid Dynamics Modelling of a Laboratory Spray Dry Scrubber for SO2 Removal in Flue Gas Desulphurisation—Effect of Drying Models. Processes, 12(9), 1862. https://doi.org/10.3390/pr12091862








