Qualitative and Quantitative Analysis of Banhasasim-Tang Using UHPLC-Q-Orbitrap-MS and UHPLC-TQ-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. BHSST Sample Preparation
2.3. Preparation of Standard Solutions and Control Samples
2.4. Analytical Conditions of UHPLC-Q-Orbitrap-MS and UHPLC-TQ-MS/MS
2.5. Validation of the Quantitative Method
3. Results
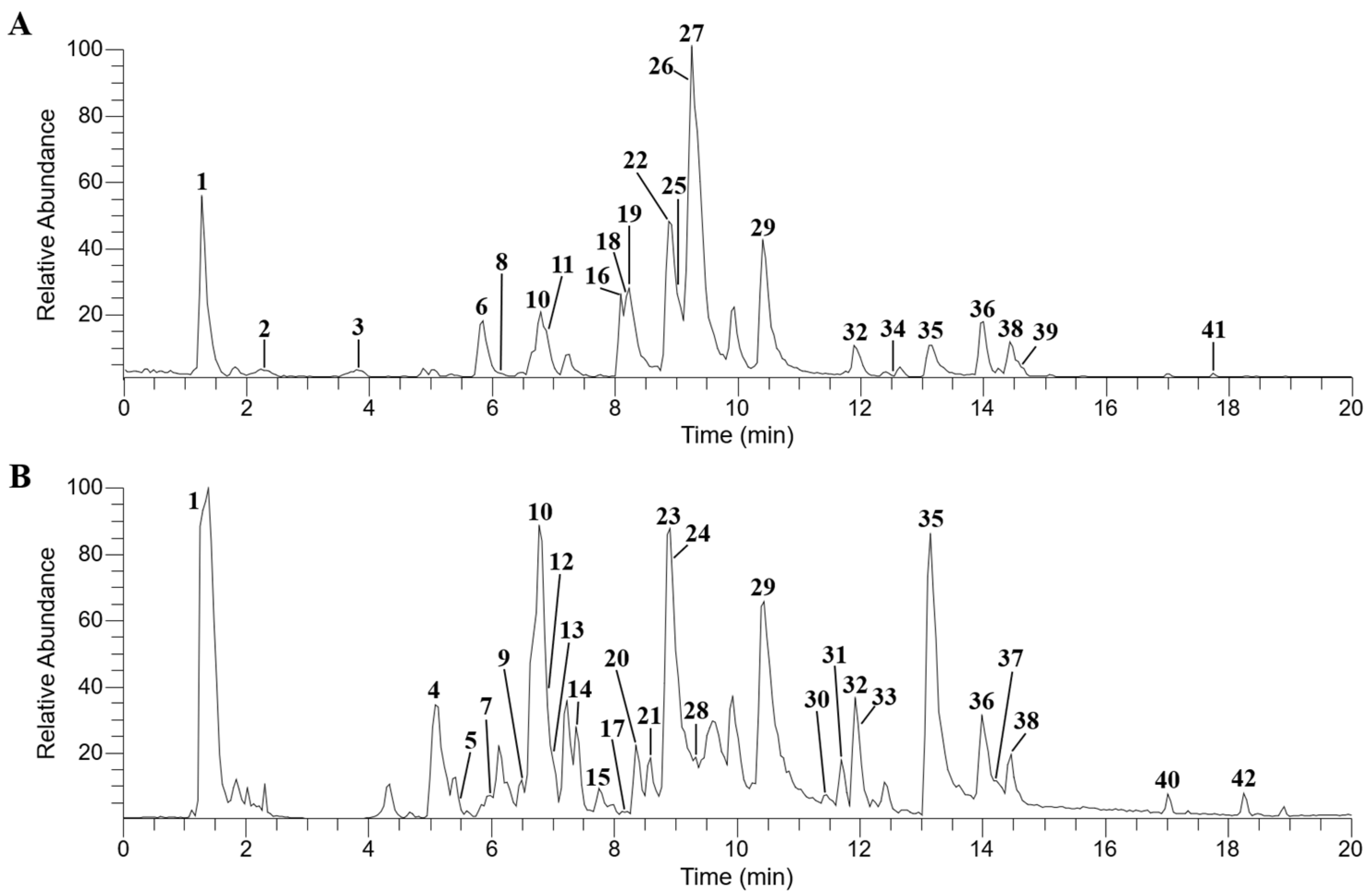
3.1. Characterization of Chemical Compounds in BHSST via UHPLC-Q-Orbitrap-MS
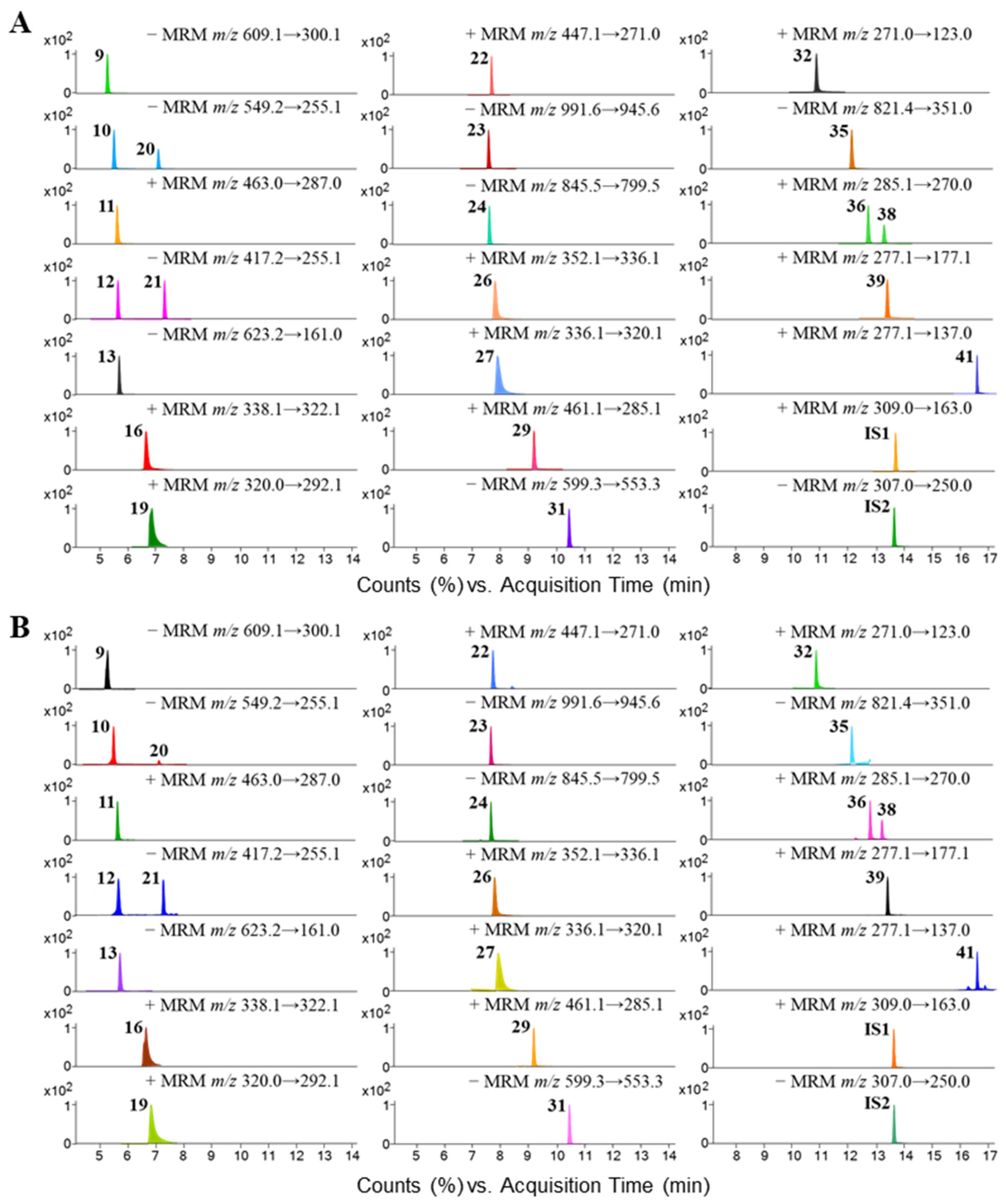
3.2. Quantitation of Chemical Compounds in BHSST via UHPLC-TQ-MS/MS
3.3. Method Validation
3.4. Quantitative Application to BHSST Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Yang, Y.; Liu, M.; Teng, Z.; Ye, J.; Xu, Y.; Cai, X.; Cheng, X.; Yang, J.; Hu, C.; et al. Banxia xiexin decoction protects against dextran sulfate sodium-induced chronic ulcerative colitis in mice. J. Ethnopharmacol. 2015, 166, 149–156. [Google Scholar] [CrossRef]
- Muluye, R.A.; Bian, Y.; Alemu, P.N. Anti-inflammatory and antimicrobial effects of heat-clearing Chinese herbs: A current review. J. Tradit. Complement. Med. 2014, 4, 93–98. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Li, X.; Wang, Z.; Yang, J.; Shen, Y.; Shi, M.; Chen, L.; Zhang, L.; Guo, Y.; et al. Exploration of the potential mechanism of Banxia Xiexin Decoction for the effects on TNBS-induced ulcerative colitis rats with the assistance of network pharmacology analysis. J. Ethnopharmacol. 2021, 277, 114197. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yang, Y.; Song, X.; Han, X.; Wang, W. Banxia Xiexin Decoction in the treatment of chronic atrophic gastritis: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e22110. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Fang, X.; Yin, X.; Li, Y. Investigation of molecular mechanism of Banxia xiexin decoction in colon cancer via network pharmacology and in vivo studies. Evid. Based Complement. Altern. Med. 2022, 2022, 4961407. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, W.; Li, X.; Li, C.; An, R.; Liang, K.; Wang, X. Comparative pharmacokinetics of four major flavonoids in normal and chronic gastritis rats after oral administration of different combinations of Banxia Xiexin decoction. Biomed. Chromatogr. 2022, 36, e5458. [Google Scholar] [CrossRef]
- Dai, X.; Yu, Y.; Zou, C.; Pan, B.; Wang, H.; Wang, S.; Wang, X.; Wang, C.; Liu, D.; Liu, Y. Traditional Banxia Xiexin decoction inhibits invasion, metastasis, and epithelial mesenchymal transition in gastric cancer by reducing lncRNA TUC338 expression. Heliyon 2023, 9, e21064. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, G.; Han, T.; Huang, H. Analysis of the pharmacological mechanism of Banxia Xiexin decoction in treating depression and ulcerative colitis based on a biological network module. BMC Complement. Med. Ther. 2020, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liu, W.; Huo, Y.; Hu, C.; Zhang, Y. Banxia Xiexin decoction ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis via inhibiting serine-threonine protein kinase (Akt)/mitogen-activated protein kinase (MAPK) signaling pathway. Biotechnol. Appl. Biochem. 2023, 70, 1530–1542. [Google Scholar] [CrossRef]
- Li, B.; Rui, J.; Ding, X.; Yang, X. Exploring the multicomponent synergy mechanism of Banxia Xiexin Decoction on irritable bowel syndrome by a systems pharmacology strategy. J. Ethnopharmacol. 2019, 233, 158–168. [Google Scholar] [CrossRef]
- Gong, B.-Y.; Xu, Z.; Wang, Y.; Feng, Z.-H.; Li, Z.-D.; Yu, G.-T.; Zhou, H.-F.; Bian, Y.-H. Efficacy and safety of Banxia Xiexin Decoction in the treatment of gastric ulcer: A systematic review and meta-analysis of twenty-seven randomized controlled trials. Gastroenterol. Hepatol. Res. 2023, 5, 9. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.Y.; Kwon, O.J.; Jung, S.Y.; Joung, J.Y.; Yang, C.S.; Lee, J.H.; Cho, J.H.; Son, C.G. Efficacy of a traditional herbal formula, Banha-Sasim-tang in functional dyspepsia classified as excess pattern. Front. Pharmacol. 2021, 12, 698887. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ke, J.; Wang, C.; Li, Y.; Wu, G.; Ding, Q.; Luo, Q.; Cai, R.; Lv, P.; Song, T.; et al. Efficacy and safety of Banxia XieXin decoction, a blended traditional Chinese medicine, as monotherapy for patients with advanced hepatocellular carcinoma. Integr. Cancer Ther. 2020, 19, 1534735420942587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Xiao, J.; Xu, R.; Wang, Q.; Wang, X. Simultaneous determination of baicalin, baicalein, wogonoside, wogonin, scutellarin, berberine, coptisine, ginsenoside Rb1 and ginsenoside Re of Banxia xiexin decoction in rat plasma by LC-MS/MS and its application to a pharmacokinetic study. Biomed. Chromatogr. 2018, 32, e4083. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, R.; Song, W.; Miao, W.J.; Liu, J.; Chen, H.B.; Guo, D.A.; Ye, M. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole Orbitrap mass spectrometry and key ion filtering. J. Chromatogr. A 2016, 1441, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Akram, W.; Luo, B.; Hu, S.; Faruque, M.O.; Ahmad, S.; Yasin, N.A.; Khan, W.U.; Ahmad, A.; Shikov, A.N.; et al. Metabolomic and pharmacologic insights of aerial and underground parts of Glycyrrhiza uralensis Fisch. ex DC. for maximum utilization of medicinal resources. Front. Pharmacol. 2021, 12, 658670. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Z.; Wu, C.; Dong, H.; Gan, C.; Fan, L.; Wang, W.; Yang, C. Ultrahigh-performance liquid chromatography with tandem mass spectrometry for the determination of 10 alkaloids in beagle plasma after the oral administration of the three Coptidis rhizoma extracts. J. Ethnopharmacol. 2019, 239, 111896. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, Y.; Tian, S.; Su, J.; Zhang, W. A novel and effective mode-switching triple quadrupole mass spectrometric approach for simultaneous quantification of fifteen ginsenosides in Panax ginseng. Phytomedicine 2018, 44, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; Zhu, Y.; Ma, G.F.; Liu, P.; Chen, L.L. Qualitative and quantitative analysis of major components of Renshen-Yangrong Pill by UPLC-LTQ/Orbitrap/MS and UPLC-MS/MS. J. Pharm. Biomed. Anal. 2023, 227, 115276. [Google Scholar] [CrossRef]
- Jin, S.E.; Kim, O.S.; Seo, C.S.; Shin, H.K.; Jeong, S.J. Comparative study on stability and efficacy of decoction depending on the preservation temperature and periods. J. Korean Med. 2016, 37, 21–33. [Google Scholar] [CrossRef]
- Seo, C.-S.; Hyeun-Kyoo, S. Quantitative determination of the thirteen marker components in Banhasasim-tang decoction using an ultraperformance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Korean J. Pharmacogn. 2016, 47, 62–72. [Google Scholar]
- Wang, Y.; Xu, R.; Xiao, J.; Zhang, J.; Wang, X.; An, R.; Ma, Y. Quantitative analysis of flavonoids, alkaloids and saponins of Banxia Xiexin decoction using ultra-high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 88, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Jia, Q.; Zhang, H.; Lin, C.; Zhao, X.; Zhang, M.; Wang, Y.; Hu, P. Chemical profiling and quality evaluation of Zhishi-Xiebai-Guizhi Decoction by UPLC-Q-TOF-MS and UPLC fingerprint. J. Pharm. Biomed. Anal. 2021, 194, 113771. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, D.Q.; Chen, Y.Y.; Fu, R.J.; Yue, S.J.; Yin, J.F.; Tang, Y.P. Qualitative and quantitative analysis of chemical components in Eupatorium lindleyanum DC. by ultra-performance liquid chromatography-mass spectrometry integrated with anti-inflammatory activity research. J. Sep. Sci. 2021, 44, 3174–3187. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, X.; Zhang, S.; Dai, X.; Liu, Y.; Tan, H.; Gao, L.; Xia, T. Quantification of flavonol glycosides in Camellia sinensis by MRM mode of UPLC-QQQ-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1017–1018, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.R.; Park, J.; Ko, S.J.; Kim, J.N.; Choi, W.; Park, J.W.; Kim, B.J. Prediction of the medicinal mechanisms of Pinellia ternata Breitenbach, a traditional medicine for gastrointestinal motility disorders, through network pharmacology. Plants 2022, 11, 1348. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Zhang, Q.Y.; Cao, J.; Cao, W.; Xu, J.J.; Peng, L.Q. Cyclodextrin-assisted liquid-solid extraction for determination of the composition of jujube fruit using ultrahigh performance liquid chromatography with electrochemical detection and quadrupole time-of-flight tandem mass spectrometry. Food Chem. 2016, 213, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Yudthavorasit, S.; Wongravee, K.; Leepipatpiboon, N. Characteristic fingerprint based on gingerol derivative analysis for discrimination of ginger (Zingiber officinale) according to geographical origin using HPLC-DAD combined with chemometrics. Food Chem. 2014, 158, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.W.; Li, Z.Z.; Wu, J.S.; Jin, W.Y.; Chang, X.Y.; Sun, H.; Dong, L.; Jiang, Z.P.; Shi, Y. Identification of the bioactive components of Banxia Xiexin Decoction that protect against CPT-11-induced intestinal toxicity via UPLC-based spectrum-effect relationship analyses. J. Ethnopharmacol. 2021, 266, 113421. [Google Scholar] [CrossRef]
- Wang, W.; Gu, W.; He, C.; Zhang, T.; Shen, Y.; Pu, Y. Bioactive components of Banxia Xiexin Decoction for the treatment of gastrointestinal diseases based on flavor-oriented analysis. J. Ethnopharmacol. 2022, 291, 115085. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Y.B.; Wei, X.; Song, C.H.; Qiao, M.Q.; Zhang, H.Y. Metabolic profiling of Shu-Yu capsule in rat serum based on metabolic fingerprinting analysis using HPLC-ESI-MSn. Mol. Med. Rep. 2016, 13, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, S.; Cheng, X.; Lu, Y.; Zou, Y.; Zhang, Q. UPLC-Q-TOF-MS/MS fingerprinting of Traditional Chinese Formula SiJunZiTang. J. Pharm. Biomed. Anal. 2013, 80, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Peng, C.; Ma, P.; Li, X. An integrated strategy of MS-network-based offline 2DLC-QTOF-MS/MS coupled with UHPLC-QTRAP®-MS/MS for the characterization and quantification of the non-polysaccharides in Sijunzi decoction. Anal. Bioanal. Chem. 2021, 413, 3511–3527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, X.; Yin, X.; Wang, M.; Zhao, J.; Ren, Y. A strategy integrating parent ions list-modified mass defect filtering-diagnostic product ions for rapid screening and systematic characterization of flavonoids in Scutellaria barbata using hybrid quadrupole-Orbitrap high-resolution mass spectrometry. J. Chromatogr. A 2022, 1674, 463149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, J.; Wang, Y.; Chen, L.; Liu, H.; Wang, Z.; Wang, B. Screening the Q-markers of TCMs from RA rat plasma using UHPLC-QTOF/MS technique for the comprehensive evaluation of Wu-Wei-Wen-Tong Capsule. J. Mass Spectrom. 2021, 56, e4711. [Google Scholar] [CrossRef]
- Li, Z.; Wen, R.; Du, Y.; Zhao, S.; Zhao, P.; Jiang, H.; Rong, R.; Lv, Q. Simultaneous quantification of fifteen compounds in rat plasma by LC-MS/MS and its application to a pharmacokinetic study of Chaihu-Guizhi decoction. J. Chromatogr. B 2019, 1105, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Guan, H.; Xu, R.; Luo, X.; Su, M.; Chang, X.; Tan, W.; Chen, J.; Shi, Y. Comparison of the chemical profiles and antioxidant activities of different parts of cultivated Cistanche deserticola using ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry and a 1,1-diphenyl-2-picrylhydrazyl-based assay. Molecules 2017, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.H.; Zhao, M.; Wang, D.G.; Yang, C.; Chen, G.T.; Zhao, X.; Pu, X.L.; Jiang, S. UPLC-Q-TOF/MS-based screening and identification of two major bioactive components and their metabolites in normal and CKD rat plasma, urine and feces after oral administration of Rehmannia glutinosa Libosch extract. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1001, 98–106. [Google Scholar] [CrossRef]
- Basera, I.A.; Girme, A.; Bhatt, V.P.; Saste, G.; Pawar, S.; Hingorani, L.; Shah, M.B. Development of validated UHPLC–PDA with ESI–MS-MS method for concurrent estimation of magnoflorine, berbamine, columbamine, jatrorrhizine, palmatine and berberine in Berberis aristata. Acta Chromatographica 2022, 34, 412–421. [Google Scholar] [CrossRef]
- Nakonieczna, S.; Grabarska, A.; Gawel, K.; Wróblewska-Łuczka, P.; Czerwonka, A.; Stepulak, A.; Kukula-Koch, W. Isoquinoline alkaloids from Coptis chinensis Franch: Focus on coptisine as a potential therapeutic candidate against gastric cancer cells. Int. J. Mol. Sci. 2022, 23, 10330. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gong, M.J.; Bai, J.Q.; Su, H.; Gong, L.; Huang, Z.H.; Qiu, X.H.; Xu, W.; Zhang, J. Differential metabolic profiles of ginsenosides in artificial gastric juice using ultra-high-pressure liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometry. Biomed. Chromatogr. 2022, 36, e5493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Cheng, M.; Huang, S.; Liu, D.; Zhao, Q.; Bai, Y.; Zhang, X. Various multicharged anions of ginsenosides in negative electrospray ionization with QTOF high-resolution mass spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Asamenew, G.; Kim, H.-W.; Lee, M.-K.; Lee, S.-H.; Kim, Y.J.; Cha, Y.-S.; Yoo, S.M.; Kim, J.-B. Characterization of phenolic compounds in normal ginger (Zingiber officinale Rosc.) and black ginger (Kaempferia parviflora Wall.) using UPLC–DAD–QToF–MS. Eur. Food Res. Technol. 2018, 245, 653–665. [Google Scholar] [CrossRef]
- Jo, S.K.; Kim, I.S.; Rehman, S.U.; Ha, S.K.; Park, H.-Y.; Park, Y.K.; Yoo, H.H. Characterization of metabolites produced from the biotransformation of 6-shogaol formed by Aspergillus niger. Eur. Food Res. Technol. 2016, 242, 137–142. [Google Scholar] [CrossRef]
| UHPLC | Q-Orbitrap-MS | TQ-MS/MS | ||||
|---|---|---|---|---|---|---|
| Parameter | Condition | Parameter | Condition | Parameter | Condition | |
| Column | Acquity BEH C18 column (100 × 2.1 mm, 1.7 µm) | Ion mode | Positive/ Negative | Ion mode | Positive/ Negative | |
| Column temp. | 40 °C | Ion source | ESI | Ion source | ESI | |
| Injection volume | 3.0 µL | Scan mode | Full MS-ddMS2 | Capillary voltage | 3500 V (pos) | |
| Sample temp. | 4 °C | Scan range | 100–1500 m/z | 3000 V (neg) | ||
| Mobile phase A | 0.1% Formic acid in water | Capillary temp. | 320 °C | Gas temp. | 130 °C | |
| Mobile phase B | Acetonitrile | Spray voltage | 3.8 kV | Nebulizer gas | 25 psi | |
| Flow rate | 0.25 mL/min | AUX gas | 10 au | Drying gas flow | 11 L/min | |
| Gradient program | Time (min) | B (%) | Sheath gas | 40 au | Nozzle voltage | 500 V (pos) |
| 0 | 3 | Collision energy | 25 eV | 1500 V (neg) | ||
| 1 | 3 | MS resolution | 70,000 | Software | MassHunter v.10.1 | |
| 2 | 15 | MS/MS resolution | 17,500 | |||
| 13 | 50 | Software | Xcalibur v.3.0 | |||
| 20 | 100 | Tracefinder v. 3.2 | ||||
| 23 | 100 | |||||
| 23.5 | 3 | |||||
| 27.5 | 3 | |||||
| No. | Compound Name | Rt (min) | Precursor Ion (m/z) | Product Ion (m/z) | Fragmentor (V) | Collision Energy (V) | Cell Accelerator (V) | Polarity |
|---|---|---|---|---|---|---|---|---|
| 9 | Rutin | 5.23 | 609.1 | 300.1 | 166 | 40 | 4 | Negative |
| 10 | Liquiritin apioside | 5.48 | 549.2 | 255.1 | 166 | 34 | 4 | Negative |
| 11 | Scutellarin | 5.58 | 463.0 | 287.0 | 166 | 22 | 4 | Positive |
| 12 | Liquiritin | 5.59 | 417.2 | 255.1 | 166 | 18 | 4 | Negative |
| 13 | Acteoside | 5.64 | 623.2 | 161.0 | 166 | 40 | 4 | Negative |
| 16 | Jatrorrhizine | 6.74 | 338.1 | 322.1 | 166 | 30 | 4 | Positive |
| 19 | Coptisine | 6.82 | 320.0 | 292.1 | 166 | 30 | 4 | Positive |
| 20 | Isoliquiritin apioside | 7.05 | 549.1 | 255.1 | 166 | 30 | 4 | Negative |
| 21 | Isoliquiritin | 7.37 | 417.0 | 255.1 | 166 | 18 | 4 | Negative |
| 22 | Baicalin | 7.58 | 447.1 | 271.0 | 166 | 22 | 4 | Positive |
| 23 | Ginsenoside Re | 7.52 | 991.6 | 945.6 | 166 | 26 | 4 | Negative |
| 24 | Ginsenoside Rg1 | 7.52 | 845.5 | 799.5 | 166 | 26 | 4 | Negative |
| 26 | Palmatine | 7.69 | 352.1 | 336.1 | 166 | 34 | 4 | Positive |
| 27 | Berberine | 7.81 | 336.1 | 320.1 | 166 | 34 | 4 | Positive |
| 29 | Wogonoside | 9.08 | 461.1 | 285.1 | 166 | 22 | 4 | Positive |
| 31 | Ginsenoside Rb1 | 10.32 | 599.3 | 553.3 | 166 | 14 | 4 | Negative |
| 32 | Baicalein | 10.60 | 271.0 | 123.0 | 166 | 34 | 4 | Positive |
| 35 | Glycyrrhizic acid | 11.86 | 821.4 | 351.0 | 166 | 40 | 4 | Negative |
| 36 | Wogonin | 12.67 | 285.1 | 270.0 | 166 | 26 | 4 | Positive |
| 38 | Oroxylin A | 13.22 | 285.0 | 270.0 | 166 | 26 | 4 | Positive |
| 39 | 6-Gingerol | 13.24 | 277.1 | 177.1 | 166 | 10 | 4 | Positive |
| 41 | 6-Shogaol | 16.50 | 277.1 | 137.0 | 166 | 22 | 4 | Positive |
| IS1 | Warfarin | 13.64 | 309.0 | 163.0 | 166 | 14 | 4 | Positive |
| IS2 | Warfarin | 13.64 | 307.0 | 250.0 | 166 | 22 | 4 | Negative |
| No. | Rt (min) | Identification | Formula | Adduct | Measured Mass (m/z) | Error (ppm) | MS2 Fragment (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | 1.26 | Maltose * | C12H22O11 | [M − H]− | 341.1088 | −0.3058 | 89.0228, 71.0122, 59.0122 |
| 2 | 2.24 | Leucine * | C6H13NO2 | [M + H]+ | 132.1021 | 1.1812 | 86.0972 |
| 3 | 3.80 | Phenylalanine | C9H11NO2 | [M + H]+ | 166.0863 | 0.3551 | 120.0811 |
| 4 | 5.09 | Darendoside A | C19H28O11 | [M − H]− | 431.1556 | −0.5575 | 125.0229, 101.0228, 57.0329 |
| 5 | 5.51 | Vicenin-2 * | C27H30O15 | [M − H]− | 593.1514 | 0.4016 | 473.1086, 353.0664 |
| 6 | 5.86 | Magnoflorine * | C20H24NO4 | [M]+ | 342.1698 | −0.4374 | 342.1700, 297.1122 |
| 7 | 5.98 | Schaftoside * | C26H28O14 | [M − H]− | 563.1406 | −0.0089 | 443.0982, 383.0774, 353.0665 |
| 8 | 6.09 | Daidzin * | C21H20O9 | [M + H]+ | 417.1177 | −0.6549 | 255.0659 |
| 9 | 6.54 | Rutin * | C27H30O16 | [M − H]− | 609.1462 | 0.2138 | 300.0272, 223.0607 |
| 10 | 6.77 | Liquiritin apioside * | C26H30O13 | [M − H]− | 549.1613 | −0.0803 | 255.0660 |
| 11 | 6.89 | Scutellarin * | C21H18O12 | [M + H]+ | 463.0869 | −0.4015 | 287.0553 |
| 12 | 6.91 | Liquiritin * | C21H22O9 | [M − H]− | 417.1191 | 0.0734 | 255.0660 |
| 13 | 7.00 | Acteoside * | C29H36O15 | [M − H]− | 623.1981 | −0.1298 | 161.0233 |
| 14 | 7.37 | Isoacteoside * | C29H36O15 | [M − H]− | 623.1981 | −0.0319 | 161.0233 |
| 15 | 7.70 | Acanthoside B * | C28H36O13 | [M − H]− | 579.2084 | 0.1105 | 417.1552, 181.0496 |
| 16 | 8.10 | Jatrorrhizine * | C20H20NO4 | [M]+ | 338.1384 | −0.9265 | 338.1385, 336.1230 |
| 17 | 8.17 | Scutellarin methylester * | C22H20O12 | [M − H]− | 475.0880 | −0.3300 | 299.0559, 113.0228 |
| 18 | 8.19 | Epiberberine * | C20H18NO4 | [M]+ | 336.1228 | −0.5853 | 336.1230 |
| 19 | 8.24 | Coptisine * | C19H14NO4 | [M]+ | 320.0916 | −0.3631 | 320.0917 |
| 20 | 8.36 | Isoliquiritin apioside | C26H30O13 | [M − H]− | 549.1613 | −0.0803 | 255.0660 |
| 21 | 8.68 | Isoliquiritin * | C21H22O9 | [M − H]− | 417.1190 | −0.2924 | 255.0659 |
| 22 | 8.89 | Baicalin * | C21H18O11 | [M + H]+ | 447.0919 | −0.6063 | 271.0600 |
| 23 | 8.91 | Ginsenoside Re * | C48H82O18 | [M + HCO2]− | 991.5478 | −0.534 | 945.5434, 637.4317, 113.0229, 101.0228 |
| 24 | 8.96 | Ginsenoside Rg1 * | C42H72O14 | [M + HCO2]− | 845.4902 | −0.2799 | 113.0229, 101.0228, 71.0121 |
| 25 | 9.02 | Ononin * | C22H22O9 | [M + H]+ | 431.1336 | −0.0546 | 269.0807 |
| 26 | 9.21 | Palmatine * | C21H22NO4 | [M]+ | 352.1541 | −0.7873 | 352.1543 |
| 27 | 9.26 | Berberine * | C20H18NO4 | [M]+ | 336.1229 | −0.4038 | 336.1230 |
| 28 | 9.32 | Liquiritigenin * | C15H12O4 | [M − H]− | 255.0662 | −0.2229 | 255.0652, 135.0073, 119.0487 |
| 29 | 10.41 | Wogonoside * | C22H20O11 | [M + H]+ | 461.1076 | −0.4435 | 285.0757 |
| 30 | 11.46 | Ginsenoside Rf * | C42H72O14 | [M + HCO2]− | 845.4905 | 0.0810 | 113.0229, 101.0228, 71.0121 |
| 31 | 11.69 | Ginsenoside Rb1 * | C54H92O23 | [M + HCO2]− | 1153.6008 | −0.2687 | 1107.5956, 179.0551, 101.0228 |
| 32 | 11.90 | Baicalein * | C15H10O5 | [M + H]+ | 271.0603 | 0.6353 | 271.0599 |
| 33 | 12.02 | Ginsenoside Rc * | C53H90O22 | [M + HCO2]− | 1123.5902 | −0.3293 | 1077.5838, 149.0442, 89.0227 |
| 34 | 12.56 | Formononetin * | C16H12O4 | [M + H]+ | 269.0809 | 0.3442 | 269.0808 |
| 35 | 13.15 | Glycyrrhizic acid * | C42H62O16 | [M − H]− | 821.3965 | 0.0431 | 351.0574, 193.0341 |
| 36 | 14.01 | Wogonin * | C16H12O5 | [M + H]+ | 285.0757 | −0.1259 | 285.0757 |
| 37 | 14.13 | Chrysin * | C15H10O4 | [M − H]− | 253.0505 | −0.4274 | 253.0504 |
| 38 | 14.53 | Oroxylin A * | C16H12O5 | [M + H]+ | 285.0757 | −0.2329 | 285.0757 |
| 39 | 14.62 | 6-Gingerol * | C17H26O4 | [M]+ | 294.1830 | 1.3355 | 150.0677, 137.0598 |
| 40 | 17.01 | Glabridin * | C20H20O4 | [M − H]− | 323.1290 | 0.2701 | 323.1287, 135.0438 |
| 41 | 17.75 | 6-Shogaol * | C17H24O3 | [M + H]+ | 277.1798 | −0.1477 | 137.0598 |
| 42 | 18.24 | Hederagenin * | C30H48O4 | [M − H]− | 471.3478 | −0.3631 | 471.3475 |
| No. | Compound Name | Regression Equation | R2 | Linear Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|---|
| 9 | Rutin | y = 0.0891x − 0.000393 | 0.9992 | 0.02–6.25 | 0.02 |
| 10 | Liquiritin apioside | y = 0.1956x − 0.007461 | 0.9994 | 0.20–50.00 | 0.20 |
| 11 | Scutellarin | y = 0.0107x + 0.000035 | 0.9993 | 0.02–6.25 | 0.02 |
| 12 | Liquiritin | y = 0.3732x − 0.006084 | 0.9994 | 0.10–25.00 | 0.10 |
| 13 | Acteoside | y = 0.1284x − 0.002549 | 0.9992 | 0.05–12.50 | 0.05 |
| 16 | Jatrorrhizine | y = 0.5496x − 0.004375 | 0.9993 | 0.02–6.25 | 0.02 |
| 19 | Coptisine | y = 0.2028x − 0.004268 | 0.9991 | 0.05–12.50 | 0.05 |
| 20 | Isoliquiritin apioside | y = 0.2073x − 0.001545 | 0.9994 | 0.05–12.50 | 0.05 |
| 21 | Isoliquiritin | y = 0.3112x − 0.001585 | 0.9994 | 0.02–6.25 | 0.02 |
| 22 | Baicalin | y = 0.0106x + 0.001108 | 0.9993 | 1.56–400.00 | 1.56 |
| 23 | Ginsenoside Re | y = 0.2309x − 0.002048 | 0.9992 | 0.05–12.50 | 0.05 |
| 24 | Ginsenoside Rg1 | y = 0.0690x − 0.001711 | 0.9992 | 0.10–25.00 | 0.10 |
| 26 | Palmatine | y = 0.5096x − 0.021645 | 0.9991 | 0.10–25.00 | 0.10 |
| 27 | Berberine | y = 0.3658x − 0.037572 | 0.9990 | 0.20–50.00 | 0.20 |
| 29 | Wogonoside | y = 0.0123x − 0.005180 | 0.9990 | 1.56–400.00 | 1.56 |
| 31 | Ginsenoside Rb1 | y = 0.2998x − 0.006713 | 0.9993 | 0.10–25.00 | 0.10 |
| 32 | Baicalein | y = 0.0120x − 0.000040 | 0.9990 | 0.05–12.50 | 0.05 |
| 35 | Glycyrrhizic acid | y = 0.0805x − 0.007355 | 0.9991 | 0.39–100.00 | 0.39 |
| 36 | Wogonin | y = 0.6316x − 0.001552 | 0.9992 | 0.02–6.25 | 0.02 |
| 38 | Oroxylin A | y = 0.1985x − 0.001333 | 0.9996 | 0.02–6.25 | 0.02 |
| 39 | 6-Gingerol | y = 0.0621x − 0.000717 | 0.9993 | 0.05–12.50 | 0.05 |
| 41 | 6-Shogaol | y = 0.1483x − 0.000504 | 0.9992 | 0.02–6.25 | 0.02 |
| No. | Compound Name | Recovery (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low Level | Medium Level | High Level | ||||||||
| Added (ng/mL) | Mean | RSD (%) | Added (ng/mL) | Mean | RSD (%) | Added (ng/mL) | Mean | RSD (%) | ||
| 9 | Rutin | 0.81 | 94.88 | 3.43 | 1.33 | 103.13 | 2.34 | 2.37 | 99.93 | 1.73 |
| 10 | Liquiritin apioside | 17.08 | 94.91 | 0.77 | 21.25 | 91.81 | 1.19 | 29.58 | 96.20 | 1.03 |
| 11 | Scutellarin | 1.01 | 93.39 | 2.80 | 1.53 | 106.49 | 1.07 | 2.57 | 102.51 | 3.40 |
| 12 | Liquiritin | 4.65 | 97.38 | 1.43 | 6.73 | 98.46 | 0.91 | 10.90 | 103.77 | 0.53 |
| 13 | Acteoside | 2.24 | 92.36 | 2.21 | 3.28 | 99.12 | 2.25 | 5.36 | 103.52 | 3.60 |
| 16 | Jatrorrhizine | 1.42 | 100.08 | 1.25 | 1.94 | 100.27 | 0.90 | 2.98 | 100.13 | 2.18 |
| 19 | Coptisine | 2.66 | 96.04 | 1.17 | 3.70 | 95.69 | 0.84 | 5.79 | 92.26 | 0.37 |
| 20 | Isoliquiritin apioside | 2.68 | 95.28 | 1.33 | 3.72 | 96.94 | 2.04 | 5.81 | 107.33 | 2.18 |
| 21 | Isoliquiritin | 0.96 | 96.12 | 1.28 | 1.48 | 104.95 | 2.30 | 2.52 | 106.23 | 1.59 |
| 22 | Baicalin | 97.13 | 94.94 | 1.00 | 130.46 | 102.59 | 0.80 | 197.13 | 101.22 | 0.72 |
| 23 | Ginsenoside Re | 2.66 | 95.33 | 1.94 | 3.70 | 100.32 | 2.05 | 5.78 | 103.62 | 0.74 |
| 24 | Ginsenoside Rg1 | 5.17 | 100.63 | 1.11 | 7.25 | 100.86 | 0.86 | 11.42 | 101.43 | 1.46 |
| 26 | Palmatine | 4.55 | 97.66 | 3.02 | 6.63 | 97.31 | 0.62 | 10.80 | 97.19 | 2.77 |
| 27 | Berberine | 14.56 | 94.83 | 1.13 | 18.72 | 97.63 | 0.93 | 27.06 | 105.44 | 0.95 |
| 29 | Wogonoside | 114.86 | 92.54 | 0.64 | 148.19 | 96.34 | 1.28 | 214.86 | 99.21 | 1.28 |
| 31 | Ginsenoside Rb1 | 4.99 | 98.38 | 1.40 | 7.07 | 104.24 | 1.27 | 11.24 | 110.54 | 0.65 |
| 32 | Baicalein | 2.50 | 97.19 | 3.56 | 3.55 | 108.86 | 0.44 | 5.63 | 102.40 | 1.43 |
| 35 | Glycyrrhizic acid | 23.07 | 93.11 | 1.46 | 31.41 | 99.03 | 1.89 | 48.07 | 101.14 | 0.52 |
| 36 | Wogonin | 1.02 | 92.33 | 0.96 | 1.54 | 101.54 | 1.56 | 2.58 | 105.80 | 0.78 |
| 38 | Oroxylin A | 1.12 | 93.05 | 1.70 | 1.64 | 95.02 | 1.10 | 2.68 | 91.32 | 1.00 |
| 39 | 6-Gingerol | 2.79 | 94.45 | 1.06 | 3.83 | 91.90 | 1.43 | 5.91 | 97.50 | 1.17 |
| 41 | 6-Shogaol | 0.82 | 94.58 | 1.38 | 1.34 | 100.26 | 2.12 | 2.38 | 99.37 | 0.62 |
| No. | Compound Name | Concentration (ng/mL) | Precision (RSD, %) | Accuracy (%) | ||
|---|---|---|---|---|---|---|
| Intraday | Interday | Intraday | Interday | |||
| 9 | Rutin | 4.17 | 1.80 | 5.53 | 110.26 | 104.14 |
| 1.04 | 1.50 | 2.64 | 102.93 | 99.99 | ||
| 0.26 | 1.20 | 2.71 | 94.01 | 94.63 | ||
| 10 | Liquiritin apioside | 33.33 | 1.58 | 5.97 | 110.77 | 103.67 |
| 8.33 | 0.82 | 4.24 | 105.55 | 100.93 | ||
| 2.08 | 1.29 | 1.07 | 102.83 | 103.91 | ||
| 11 | Scutellarin | 4.17 | 1.53 | 8.00 | 112.25 | 104.66 |
| 1.04 | 2.82 | 1.32 | 101.37 | 99.85 | ||
| 0.26 | 3.19 | 4.08 | 91.35 | 95.04 | ||
| 12 | Liquiritin | 16.67 | 1.12 | 5.27 | 109.75 | 103.53 |
| 4.17 | 0.50 | 3.64 | 104.67 | 100.47 | ||
| 1.04 | 1.39 | 3.73 | 100.93 | 103.91 | ||
| 13 | Acteoside | 8.33 | 1.07 | 2.51 | 108.43 | 107.36 |
| 2.08 | 1.04 | 1.45 | 103.12 | 104.74 | ||
| 0.52 | 1.04 | 8.08 | 93.47 | 101.33 | ||
| 16 | Jatrorrhizine | 4.17 | 0.82 | 3.59 | 102.85 | 99.29 |
| 1.04 | 2.13 | 4.77 | 102.28 | 96.94 | ||
| 0.26 | 1.84 | 5.11 | 98.13 | 94.13 | ||
| 19 | Coptisine | 8.33 | 0.42 | 1.15 | 99.75 | 98.73 |
| 2.08 | 1.42 | 2.04 | 97.36 | 95.12 | ||
| 0.52 | 0.73 | 2.88 | 93.17 | 91.33 | ||
| 20 | Isoliquiritin apioside | 8.33 | 0.63 | 7.21 | 111.90 | 103.33 |
| 2.08 | 1.13 | 5.26 | 108.42 | 102.21 | ||
| 0.52 | 1.88 | 1.39 | 100.09 | 101.48 | ||
| 21 | Isoliquiritin | 4.17 | 0.75 | 5.47 | 110.60 | 104.10 |
| 1.04 | 1.96 | 2.06 | 103.08 | 100.77 | ||
| 0.26 | 6.45 | 4.83 | 99.19 | 105.02 | ||
| 22 | Baicalin | 266.67 | 0.60 | 8.90 | 110.47 | 100.78 |
| 66.67 | 0.35 | 5.35 | 104.33 | 99.05 | ||
| 16.67 | 0.75 | 3.54 | 99.13 | 97.18 | ||
| 23 | Ginsenoside Re | 8.33 | 0.98 | 5.06 | 110.27 | 104.22 |
| 2.08 | 2.72 | 4.11 | 107.39 | 102.54 | ||
| 0.52 | 4.97 | 2.77 | 109.41 | 106.12 | ||
| 24 | Ginsenoside Rg1 | 16.67 | 1.06 | 5.75 | 111.14 | 104.22 |
| 4.17 | 1.74 | 2.66 | 104.24 | 101.20 | ||
| 1.04 | 3.48 | 1.67 | 100.17 | 101.18 | ||
| 26 | Palmatine | 16.67 | 1.11 | 5.02 | 105.18 | 100.01 |
| 4.17 | 0.86 | 1.07 | 95.02 | 94.77 | ||
| 1.04 | 0.61 | 1.75 | 95.26 | 93.52 | ||
| 27 | Berberine | 33.33 | 0.50 | 6.84 | 106.78 | 98.97 |
| 8.33 | 0.44 | 1.12 | 94.20 | 93.79 | ||
| 2.08 | 1.37 | 3.44 | 97.08 | 93.64 | ||
| 29 | Wogonoside | 266.67 | 0.64 | 4.22 | 102.30 | 97.56 |
| 66.67 | 1.98 | 3.52 | 99.72 | 95.83 | ||
| 16.67 | 1.38 | 3.42 | 92.78 | 94.75 | ||
| 31 | Ginsenoside Rb1 | 16.67 | 0.89 | 3.35 | 110.83 | 106.99 |
| 4.17 | 1.19 | 2.82 | 106.66 | 103.42 | ||
| 1.04 | 2.12 | 2.76 | 103.24 | 105.03 | ||
| 32 | Baicalein | 8.33 | 0.42 | 8.38 | 109.11 | 101.09 |
| 2.08 | 1.44 | 4.58 | 101.74 | 96.64 | ||
| 0.52 | 0.90 | 3.16 | 102.34 | 105.00 | ||
| 35 | Glycyrrhizic acid | 66.67 | 1.24 | 6.10 | 111.73 | 104.39 |
| 16.67 | 0.74 | 5.97 | 108.92 | 102.22 | ||
| 4.17 | 3.15 | 7.98 | 93.12 | 102.56 | ||
| 36 | Wogonin | 4.17 | 2.11 | 6.70 | 108.71 | 100.95 |
| 1.04 | 2.16 | 2.67 | 98.57 | 95.64 | ||
| 0.26 | 2.07 | 2.43 | 90.74 | 92.42 | ||
| 38 | Oroxylin A | 4.17 | 0.74 | 7.92 | 106.92 | 98.68 |
| 1.04 | 1.08 | 1.85 | 98.16 | 97.18 | ||
| 0.26 | 1.36 | 2.82 | 93.98 | 95.55 | ||
| 39 | 6-Gingerol | 8.33 | 0.66 | 6.13 | 104.57 | 97.87 |
| 2.08 | 0.50 | 1.11 | 92.29 | 93.43 | ||
| 0.52 | 1.24 | 2.33 | 97.30 | 94.78 | ||
| 41 | 6-Shogaol | 4.17 | 0.72 | 4.52 | 107.11 | 102.34 |
| 1.04 | 2.37 | 0.46 | 98.98 | 98.71 | ||
| 0.26 | 2.02 | 4.36 | 98.58 | 100.19 | ||
| No. | Compound | BHSST-1 | BHSST-2 | BHSST-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (mg/g) | SD | CV (%) | Mean (mg/g) | SD | CV (%) | Mean (mg/g) | SD | CV (%) | ||
| 9 | Rutin | 0.125 | 0.005 | 3.60 | 0.127 | 0.007 | 5.26 | 0.126 | 0.005 | 3.93 |
| 10 | Liquiritin apioside | 5.306 | 0.033 | 0.61 | 5.300 | 0.044 | 0.84 | 5.257 | 0.051 | 0.96 |
| 11 | Scutellarin | 0.256 | 0.012 | 4.57 | 0.263 | 0.011 | 4.31 | 0.263 | 0.004 | 1.58 |
| 12 | Liquiritin | 1.002 | 0.004 | 0.41 | 1.003 | 0.010 | 0.99 | 0.999 | 0.011 | 1.15 |
| 13 | Acteoside | 0.595 | 0.013 | 2.10 | 0.601 | 0.011 | 1.89 | 0.596 | 0.015 | 2.55 |
| 16 | Jatrorrhizine | 0.459 | 0.012 | 2.65 | 0.432 | 0.006 | 1.45 | 0.437 | 0.005 | 1.21 |
| 19 | Coptisine | 0.583 | 0.015 | 2.65 | 0.568 | 0.007 | 1.19 | 0.571 | 0.006 | 1.01 |
| 20 | Isoliquiritin apioside | 0.655 | 0.011 | 1.62 | 0.624 | 0.013 | 2.07 | 0.613 | 0.008 | 1.25 |
| 21 | Isoliquiritin | 0.171 | 0.004 | 2.45 | 0.170 | 0.006 | 3.60 | 0.167 | 0.005 | 2.99 |
| 22 | Baicalin | 35.157 | 0.521 | 1.48 | 34.848 | 0.388 | 1.11 | 34.661 | 0.401 | 1.16 |
| 23 | Ginsenoside Re | 0.558 | 0.008 | 1.52 | 0.554 | 0.006 | 1.16 | 0.551 | 0.004 | 0.69 |
| 24 | Ginsenoside Rg1 | 1.080 | 0.006 | 0.58 | 1.132 | 0.006 | 0.56 | 1.110 | 0.006 | 0.54 |
| 26 | Palmatine | 0.831 | 0.007 | 0.84 | 0.814 | 0.008 | 1.03 | 0.813 | 0.010 | 1.21 |
| 27 | Berberine | 2.749 | 0.065 | 2.35 | 2.654 | 0.041 | 1.55 | 2.678 | 0.042 | 1.58 |
| 29 | Wogonoside | 28.919 | 0.657 | 2.27 | 28.818 | 0.598 | 2.07 | 28.893 | 0.510 | 1.76 |
| 31 | Ginsenoside Rb1 | 1.120 | 0.008 | 0.75 | 1.112 | 0.008 | 0.68 | 1.091 | 0.007 | 0.61 |
| 32 | Baicalein | 0.626 | 0.011 | 1.80 | 0.630 | 0.007 | 1.10 | 0.617 | 0.012 | 1.97 |
| 35 | Glycyrrhizic acid | 4.869 | 0.059 | 1.20 | 4.898 | 0.058 | 1.19 | 4.894 | 0.050 | 1.01 |
| 36 | Wogonin | 0.186 | 0.004 | 2.14 | 0.183 | 0.001 | 0.81 | 0.183 | 0.001 | 0.78 |
| 38 | Oroxylin A | 0.204 | 0.012 | 5.77 | 0.202 | 0.009 | 4.40 | 0.205 | 0.006 | 2.69 |
| 39 | 6-Gingerol | 0.624 | 0.009 | 1.50 | 0.610 | 0.007 | 1.15 | 0.606 | 0.004 | 0.64 |
| 41 | 6-Shogaol | 0.101 | 0.001 | 0.82 | 0.098 | 0.001 | 1.47 | 0.100 | 0.001 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Hwang, Y.-H. Qualitative and Quantitative Analysis of Banhasasim-Tang Using UHPLC-Q-Orbitrap-MS and UHPLC-TQ-MS/MS. Processes 2024, 12, 1563. https://doi.org/10.3390/pr12081563
Jang S, Hwang Y-H. Qualitative and Quantitative Analysis of Banhasasim-Tang Using UHPLC-Q-Orbitrap-MS and UHPLC-TQ-MS/MS. Processes. 2024; 12(8):1563. https://doi.org/10.3390/pr12081563
Chicago/Turabian StyleJang, Seol, and Youn-Hwan Hwang. 2024. "Qualitative and Quantitative Analysis of Banhasasim-Tang Using UHPLC-Q-Orbitrap-MS and UHPLC-TQ-MS/MS" Processes 12, no. 8: 1563. https://doi.org/10.3390/pr12081563
APA StyleJang, S., & Hwang, Y.-H. (2024). Qualitative and Quantitative Analysis of Banhasasim-Tang Using UHPLC-Q-Orbitrap-MS and UHPLC-TQ-MS/MS. Processes, 12(8), 1563. https://doi.org/10.3390/pr12081563





