Abstract
Pain, a prevalent clinical symptom, significantly demands attention in the current public health system due to its profound impact on patients’ quality of life, daily activities, and economic circumstances. Despite being a pervasive issue, many forms of pain remain ineffectively addressed, hence posing an enormous burden on patients. Pharmaceutical treatments, the first-line approach for various forms of pain, continue to face considerable challenges due to their limited efficacy, lack of long-lasting effects, and adverse side effects. In recent years, the rapid advancements in science and technology, especially the incorporation of micro and nano technologies across various domains, have accelerated the development of novel therapeutics. This review underscores the merits and drawbacks of different pharmacological strategies for pain management. It focuses on the research progress and applications of poly (lactic-co-glycolic acid)(PLGA) as drug delivery carriers, elucidating their potential therapeutic influence over pain management. The review concludes with a thorough summary of current research outcomes and limitations, a discussion of potential clinical transformations, and projections for future pain management research and effective care strategies.
1. Introduction
Pain, the body’s primary warning signal indicating damage or disease, is a widely experienced clinical symptom. As per the World Health Organization (WHO) statistics, over 520 million people worldwide experience varying degrees of pain, with a significant 75% enduring moderate-to-severe discomfort. The International Association for the Study of Pain (IASP) characterizes pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage [1]. Pain perception correlates with the activation of nociceptors within primary afferent fibers subjected to noxious stimuli (thermal, chemical, or mechanical, etc.). These primary afferent fibers comprise unmyelinated C-fibers and myelinated Aδ-fibers [2], with both the peripheral and central nervous systems playing crucial roles in pain perception [3]. Moreover, the spinal and supraspinal components of the central nervous system (CNS) significantly contribute to this process.
Pain can be classified into acute and chronic categories based on the disease duration. Acute pain refers to sudden, transient pain that usually lasts for minutes to hours, or even days. It is usually caused by trauma, surgery, infection, and other transient irritation, such as fracture, sprain, toothache, and so on. Acute pain is characterized by a high degree of pain, but it usually does not last long. Chronic pain refers to pain that recurs over a long period of time, usually lasting from weeks to months, or even years. Chronic pain is usually due to a long-term illness, injury, or inflammatory response, such as arthritis, muscle strain, neuropathy, etc. Chronic pain is characterized by a low level of pain, but its duration may be long and may have a serious impact on the patient’s quality of life.
Managing pain has been a longstanding clinical challenge. Pharmacological interventions have always been the primary approach for pain management, but each drug brings with it its own advantages and disadvantages. Consequently, healthcare professionals must tailor their treatment and management strategies to individual patients’ specific conditions. It is incumbent upon researchers to strive for an optimized drug and its administration methods, considering the existing challenges. PLGA is a kind of biodegradable polymer widely used in the biomedical field. Its mechanism of action includes controlled release, biocompatibility, improved drug stability, targeted delivery, and enhanced solubility. By adjusting the ratio of lactic acid to glycolic acid [4], PLGA can achieve the sustained and controlled release of drug molecules, reduce the frequency of administration, improve patient compliance, and reduce the side effects of drugs. PLGA is broken down in the body into lactic acid and glycolic acid, two small molecules that can be eliminated through metabolic pathways in the body, usually without harmful side effects. PLGA can also be used to prepare microcapsules and nanoparticles, which can protect drugs from the environment in vivo and help to pass through the cell barrier, improving the efficiency of the intracellular delivery of drugs. In the design and use of PLGA as a drug-delivery system, the nature of the drug, the expected effect of action, and the pharmacokinetic properties in vivo need to be considered in order to ensure optimal efficacy and safety. PLGA nanoparticles containing baclofen and lamotrigine were prepared by using polymers and surfactants, as well as a high-speed homogenizer and sonicator, as shown in the patent of Kuldeep Nigam et al. [5], and these nanoparticles were able to effectively co-deliver these two therapeutic agents for the treatment of chronic neuropathic pain and can be administered via a variety of routes, such as orally, intranasally, and intravenously, to achieve better therapeutic efficacy and brain distribution. Some PLGA drug-loaded drugs have been approved by FDA for clinical application and have entered the frontier of medicine [6]. This paper reviews the present clinical status of pain drug therapy, highlighting the role of PLGA as carriers for drug optimization for different forms of pain.
2. Materials and Methods
In the database of PubMed and Web of science, the keywords PLGA, nano, and pain were used to search, and 116 articles were screened out for writing this review.
3. Pharmacological Interventions
Non-steroidal anti-inflammatory drugs (NSAIDs), opioids, and local anesthetics (LAs) represent the three main therapeutic categories for pain management, and other drugs that are more etiologically specific are also used, such as muscle relaxants, represented by chlorzoxazone, fluoxetine, and tizanidine [7].
3.1. Nonsteroidal Anti-Inflammatory Drug (NSAID) Pharmacological Interventions
NSAIDs, also known as cyclooxygenase (COX) inhibitors, are the first-line medications for pain management. They inhibit the catalytic activity of two isoforms of cyclooxygenase: COX-1 and COX-2 [8,9]. This process blocks the conversion of arachidonic acid into prostaglandins. Prostaglandins and thromboxane A2 produced through COX-1 catalysis regulate gastrointestinal mucosal barriers, the kidney balance, platelet aggregation, and other physiological functions. In contrast, COX-2 is associated with inflammation, pain, and fever, making it an ideal target for pain treatment [10]. However, these inhibitors may cause significant side effects, such as gastrointestinal issues, ulcers, bleeding, strokes, and heart attacks [11,12]. Prolonged use could precipitate severe complications, including gastric perforation, hemorrhagic ulcers, cardiovascular diseases, and kidney issues [13,14].
3.2. Opioids
Considered highly effective for pain management, opioids are often deemed the most potent treatment for pain. They are suitable for managing benign-to-severe acute pain and cancer pain [15]. Opioids are categorized based on their source (natural, synthetic, or semi-synthetic), potency (moderate to high), and the duration of action (slow and fast) [16]. They function both presynaptically and postsynaptically, producing an analgesic effect. Undesirable side effects include cardiovascular and respiratory diseases, endocrine system suppression, depression, nausea, and itching. Long-term opioid use can lead to risks such as tolerance, hyperalgesia, and allodynia [17].
3.3. Local Anesthetics (LAs)
LAs, such as lidocaine and propivacaine, are commonly used to minimize neuropathic and inflammatory pain by blocking the sodium channel in the neuron cell membrane. They are regularly used during surgical procedures to manage postoperative pain [18]. Despite advancements in clinical practice, LAs can still cause systemic toxicity. Side effects include skin tingling, allergic reactions, hypotension, headaches, muscle tremors, convulsions, or paralysis, among others [19].
3.4. Capsaicin and Cannabinoids
Capsaicin, a natural compound found in chili peppers, is incorporated into diets across numerous countries. The significant mechanism of capsaicin in pain treatment involves influencing the TRPV1 channels of nociceptive sensory neurons [20]. Capsaicin is effective in treating neuropathic pain linked to conditions like peripheral neuropathy, diabetic neuropathy, chronic peripheral polyneuropathy, and post-herpetic neuralgia. It can also alleviate pain caused by chronic illnesses, such as osteoarthritis, rheumatoid arthritis, and psoriasis.
Cannabinoids bring about various effects by activating G protein-coupled cannabinoid receptors present in the brain and peripheral tissues [21]. These receptors regulate a range of responses, including pain, mood, and memory. Tetrahydrocannabinol (THC) is the primary source of the pharmacological effects induced by marijuana consumption [22]. However, during clinical administration, controlling the dose of cannabinoids accurately often proves challenging [23,24]. Beyond the psychotropic threshold, marijuana intake usually promotes a sense of well-being and relaxation, enhancing the overall sensory experience. However, overdoses can trigger acute adverse reactions, such as anxiety, panic attacks, and changes in heart rate and blood pressure [25].
3.5. Tricyclic Antidepressants and Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs)
Tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors (SNRIs), including amitriptyline, imipramine, nortriptyline, and others, serve as antiepileptic drugs. They basically control and inhibit asynchronous neuronal discharges, which lead to seizures. This inhibition can alleviate pain and treat related symptoms. Gabapentin and pregabalin are also effectively used in managing the pain associated with fibromyalgia and neuropathic pain, with manageable risks [26]. The most common side effects encompass gastrointestinal damage, like nausea, vomiting, diarrhea, and constipation [27,28,29]. Other issues can impact the nervous system, primarily expressing as dizziness, headaches, tremors, extrapyramidal reactions, and skin ailments, like rashes, erythema, itching, hyperhidrosis, and alopecia [27,30].
The significant shortcomings of clinically available drugs have redirected drug development strategies towards enhancing drug targeting, minimizing side effects, and extending the release duration of active compounds [31]. As multidisciplinary advances and technological progress unfold, micron and nano-medical technology has increasingly come within our purview. The integration of pharmacology with micron and nanotechnology could possibly be a pivotal leap towards creating more effective and less harmful drugs for chronic pain [32]. Presently, many research findings related to pain treatment have been published. Among them, poly (lactic-co-glycolic Acid) (PLGA) as a carrier stands out due to its safety, non-toxic nature, simple preparation method, and excellent functionality.
3.6. Muscle Relaxants
Muscle relaxants help relieve pain caused by tight muscles by reducing muscle spasms and tension. Commonly used muscle relaxants include chlorzoxazone, fluoxetine, and tizanidine. Chlorzoxazone is a central muscle relaxant that acts on spinal alpha motor neurons to inhibit the transmission of nerve impulses and reduce muscle tone [33]. Fluoxetine is often used to treat muscle stiffness and spasms caused by stroke sequelae, multiple sclerosis, etc., and can indirectly relieve pain by reducing the muscle tension related to anxiety [34]. Tizanidine has the effect of relaxing muscles by increasing the activity of gamma-aminobutyric acid (GABA) [35] and is suitable for the treatment of muscle spasms caused by cerebral palsy and peripheral neuropathy. Doctors will choose the appropriate medication and dosage according to the patient’s specific symptoms and medical history.
4. PLGA
PLGA is a biocompatible and biodegradable polymer. It is synthesized via the copolymerization of lactic acid (LA) and glycolic acid (GA). Given its desirable mechanical properties [36], non-toxic nature, biocompatibility, and biodegradability, it is widely employed in various medical disciplines, including drug delivery, gene therapy, and the production of medical fiber materials [37].
The relative molecular mass of PLGA lies within the range of 38,000 and 54,000 g·mol−1. The preparation of PLGA requires the fermentation or chemical synthesis of two monomers of lactic acid and glycolic acid. Under the catalysis of tin octoate, octanol, and the like, the monomers are polymerized through a condensation reaction to form the PLGA in an anhydrous environment protected by a vacuum or inert gas. The polymerized PLGA is further purified by washing, drying, and grinding. In addition, the molecular weight and its distribution of PLGA can be controlled by adjusting the polymerization conditions, such as the reaction time, temperature, and monomer concentration. In order to facilitate the subsequent drug encapsulation and release, PLGA is usually made into films via solvent casting, melt extrusion, or calendering. The interplay between the ratio of LA and GA and the relative molecular mass of the polymer during the polymerization process impinges upon its hydrophobicity, crystallinity, mechanical properties, size, and biodegradation rate [38,39]. Therefore, altering the ratio of LA and GA enables the generation of diverse forms of PLGA [40].
Methods, including electrostatic adsorption, blending, and emulsification, allow for the encapsulation of drugs within these structures. Techniques for preparing PLGA nanoparticles encompass single emulsification, double emulsification, nanoprecipitation, coacervation, and spray drying [41,42]. A single emulsification method is a method for preparing a PLGA drug carrier and relates to the following steps (Figure 1): firstly, the drug is dissolved in a solvent, which is immiscible with an oil phase to form an internal water phase [43]. Next, PLGA is dissolved in an organic solvent to form a transparent external aqueous phase. The inner aqueous phase is then added to the outer aqueous phase and stirred at high speed to mix the two liquids [44]. In order to form a stable emulsion, it may be necessary to add a surfactant as an emulsifier. Due to the density difference between oil and water, the internal water phase will be dispersed into small droplets. The oil droplets are separated out by evaporating the organic solvent or adjusting the pH value, and the oil droplets are solidified to form solid microspheres or other structures. Finally, the unreacted monomers and other impurities are removed by washing with appropriate solvents, and the final product is obtained after drying. The double emulsification method is a method for preparing a PLGA drug carrier, which comprises the following steps: firstly preparing an aqueous-phase solution containing a drug as an inner aqueous phase [45], then mixing the inner aqueous phase with an outer aqueous phase, stirring at a high speed to form a primary emulsion, mixing the primary emulsion with the outer aqueous phase, mixing the obtained primary emulsion with an oil phase, stirring at a high speed for the second time to form a double emulsion, and solidifying oil droplets using the method; collecting the obtained microspheres or other structures, and finally carrying out subsequent treatment on the solidified product, including the steps of washing, drying, and the like, to obtain the final drug carrier.
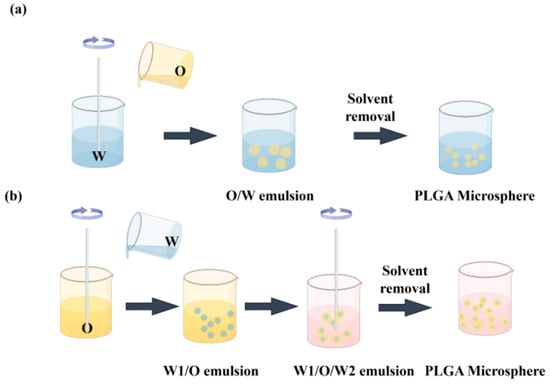
Figure 1.
Schematic representation of PLGA microspheres prepared through (a) single emulsion preparation and (b) the double emulsion method. “O” represents the organic solvent phase of the water-insoluble component. “W” represents an aqueous solution containing an emulsifier, which is the medium in which the PLGA solution is emulsified.
Different preparation methods and conditions will have different effects on the prepared products. The study by Xu X et al. [46] compared the morphologies of Lidocaine @ PLGA microcapsules prepared using the premixed membrane emulsification method, Figure 2A,B, with those prepared using the nonpremixed membrane emulsification method (C) at (a) 1000×, (b) 3000×, or (b) 5000×. And Figure 2D shows morphological changes in Lidocaine @ PLGA microcapsules after sonication. Figure 2E shows optical photographs of 3 mg/mL Lidocaine @ PLGA microcapsules with (right) and without (left) premixed membrane emulsification after standing in water for (a) 0 h, (b) 6 h, and (c) 12 h. In this SEM image, we visually see the difference between PLGA and lidocaine @ PLGA microcapsules formed under different preparation methods and conditions [46].
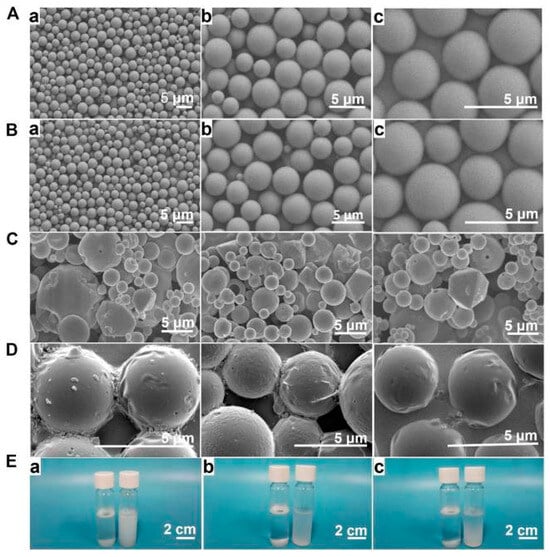
Figure 2.
(A) Preparing PLGA microcapsules using a premixed membrane emulsification method. Lidocaine @ PLGA microcapsules prepared with (B) and without (C) a premixed membrane emulsification method were observed via electron microscopy at (A) 1000×, (B) 3000×, or (C) 5000×. (D) Morphological changes in Lidocaine @ PLGA microcapsules after sonication. (E) Optical photographs of 3 mg/mL lidocaine @ PLGA microcapsules with (right) and without (left) premixed membrane emulsification after standing in water for (a) 0 h, (b) 6 h, and (c) 12 h. Reprinted from ref. [46] under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). https://doi.org/10.3389/fbioe.2022.1072205 (accessed on 24 November 2023).
In Table 1, we collected some of the drug-delivery systems studied with PLGA as the carrier. Long L et al. achieved sustained release of the drug in their experiment [47]. Experimental studies by Kao CW et al. showed that high doses of lidocaine and hEGF were released over 32 and 27 days, respectively, helping to prevent chronic neuropathic pain and sustained postoperative pain relief [48]. The drug-delivery system constructed by Lee FY et al. could continuously release effective doses of epinephrine and lidocaine for more than 3 weeks [49]. In addition, Nigam K et al. found that their intranasally administered PLGA NPs showed more than 100% drug-targeting efficiency [50]. The bufalin-PLGA microparticles constructed by Long L et al. can prolong the analgesic effect to 3 days and enhance the analgesic effect of bufalin under the condition of reducing the number of medications [47]. These features make PLGA an effective drug-delivery system, especially for pain control and wound healing.

Table 1.
The poly (lactic-co-glycolic acid)-based biodegradable drug delivery carriers for pain management.
PLGA also has better biodegradability compared to other polymers, such as PEG (polyethylene glycol), chitosan, or lipid-based carriers. Its degradation to LA and GA in vivo can be excreted from the body via metabolism to CO2 [69] without in vivo accumulation, and biodegradation occurs only through water-neutral reactions, independent of any enzymatic activity [70]. However, PEG degrades slowly in vivo, and long-term accumulation may lead to toxicity. Compared with PEG, PLGA can control the degradation rate by adjusting the ratio of lactic acid and glycolic acid, so that it can change over weeks to years, thus having modifiable degradation characteristics, enabling it to achieve sustained drug release in drug-delivery systems. For example, triamcinolone acetonide Loaospheres developed by Rudnik-Jansen I et al. [71] for the treatment of arthritis were released in vitro for 35 days (Figure 3A). Kao CW et al. [46] developed a PLGA nanofiber membrane loaded with lidocaine/human EGF (hEGF) for surgical wounds, which could release lidocaine and hEGF for more than 32 and 27 days, respectively. DED PLGA microlidocaine and hEGF were efficiently released for >2 weeks in vitro and in vivo (Figure 3B,C). Compared with chitosan and lipid nanoparticles, PLGA nanoparticles also have higher stability and drug loading. Although lipid carriers also have good biocompatibility, their stability is poor, and they easily aggregate or leak during storage; the comparison of paclitaxel in PLGA nanoparticles and liposomes also showed that PLGA nanoparticles had higher encapsulation efficiency and more stable release characteristics [72].
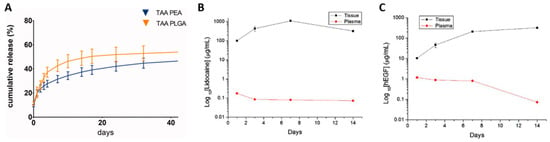
Figure 3.
(A) Cumulative triamcinolone (TAA) release from PLGA and PEA microspheres in PBS buffer. The release concentration of TAA in PBS medium was determined via HPLC over the entire 42-day period. For each polymer, three batches were used as technical replicates. Reprinted from ref. [71] under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). https://doi.org/10.3390/pharmaceutics11020070 (accessed on 5 October 2023). (B) In vivo release of lidocaine from the nanofibrous films and (C) in vivo release of hEGF from the nanofibrous films. Reprinted from ref. [42] under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by-nc/3.0/). https://doi.org/10.3390/pharmaceutics13040500 (accessed on 5 October 2023).
In terms of biological safety, Chen H et al. [73] experimentally evaluated the safety of PLGA, CS-, and Bio-CS-PLGA NPs using cell co-culture. The results showed that the cells had good viability after 24 h and 48 h of culture (Figure 4A,B). The preimplantation embryo is one of the most sensitive systems for assessing toxicity in biological systems. Exposure to factors above threshold levels during embryonic and fetal life may result in a variety of developmental abnormalities in animals and their offspring. Therefore, clarifying the effects of PLGA on embryonic development can more accurately evaluate the biological safety. Kim YS et al. [74] was used to visualize NP delivery into mouse gametes and preimplantation embryos by using TRITC-conjugated PLGA-NP (PLGA-NP), referred to as TRITC-labeled nanotracer (TnT). In this experiment, fluorescence activated cell sorting (FACS) analysis and neonatal mouse fibroblast karyotype analysis were carried out, and the results showed that there was no fluorescence residue of PLGA in the fibroblasts of all groups of live offspring (Figure 4D,E), and the chromosomes were normal (Figure 4F), which proved the reproductive safety of PLGANP (and TnT) for the first time. It does not interfere with the developmental programming of early mouse embryos and their next generations.
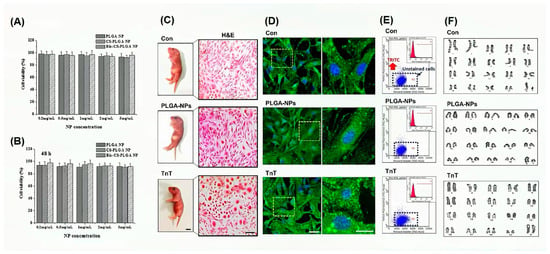
Figure 4.
Biological safety of PLGA. In the NP concentration range of 0.2–5 mg/mL, all blank NPs, including PLGA NP, CS-, and Bio-CS-PLGA NPs, had no significant effect on the proliferation of MCF-7 cells at 24 h (A) and 48 h (B). Reprinted with permission from ref. [73]. https://doi.org/10.1016/j.colsurfb.2015.11.033 (accessed on 20 June 2024). Copyright 2023 Elsevier, under the license number 5811291008791. The toxicity of PLGA embryo exposure was studied by tracing (TnT) with TRITC, and pups from each group were randomly selected for karyotyping and fluorescence-activated cell sorting (FACS) analysis. Fibroblasts were isolated from live pups born in each group and cultured (C). The lipophilic tracer did not detect residual TRITC groups in cultured fibroblasts of any of the three cells (D), and FACS analysis confirmed that the fibroblasts did not contain TRITC (E). In addition, karyotyping of the fibroblasts showed that all chromosomes were normal in the pups undergoing TnT (F). Reprinted with permission from ref. [74]. Copyright 2023 Elsevier, under the license number 5811290554390. https://doi.org/10.1016/j.biomaterials.2018.08.042 (accessed on 20 June 2024).
5. Results
PLGA as a drug delivery carrier has achieved remarkable results in prolonging the drug release time, enhancing targeting, and reducing side effects of treatment. Advanced technologies for the surface modification and functionalization of PLGA nanoparticles have been developed to enhance targeting specificity and improve therapeutic efficacy. Attachment by ligand involves the conjugation of a targeting moiety, such as an antibody, peptide, or aptamer, to the nanoparticle surface to facilitate targeted delivery to a particular cell or tissue. For example, Reddy GA et al. formulated NPs with a size of ≈170 nm, PDI ≈ 0.25, zeta potential ≈ −4.0 mV, drug loading ≈ 6.8%, and capture efficiency of 82%, and the transferrin-modified NPs exhibited tailored release in nearly 12 h and in vitro antibacterial activity in 14 h. Cellular uptake studies were performed on the RAW264.7 cell line to better determine transferrin uptake by manufactured NPs, and the results suggest that PLGA nanoparticles modified with transferrin improve the brain delivery of encapsulated drugs for the treatment of neurological diseases [75]; PEGylation, the process of attaching polyethylene glycol chains to the nanoparticle surface, can prolong the circulating half-life and reduce immune recognition. In addition, the incorporation of targeted moieties, such as folic acid, can enhance the uptake of nanoparticles by cancer cells overexpressing folate receptors [76], thereby improving the efficacy of encapsulated chemotherapeutic drugs. These advanced technologies hold significant promise for optimizing the therapeutic potential of PLGA nanoparticles for various clinical applications, including pain management. After continuous research and development, the clinical pain treatment involving PLGA may usher in a bright future. The following is the research progress of PLGA as a drug carrier in different pain treatments.
5.1. Neuropathic Pain
Neuropathic pain is a chronic pain condition that is triggered by an injury or pathology affecting the somatosensory system [77,78]. A multitude of potential mechanisms, including mitochondrial dysfunction, increased levels of reactive oxygen species (ROS), and autophagy, are believed to contribute to its emergence and subsequent progression. PLGA nanoparticles can directly interact with mitochondria to activate or inhibit the intracellular PI3K/Akt signaling pathway, thereby regulating the mitochondrial membrane potential, promoting mitochondrial fusion and fission, and further affecting the mitochondrial function of the neuropathic pain model. In a neuropathic pain model, PLGA nanoparticles can activate Nrf2/ARE and other signaling pathways, thereby reducing ROS levels and alleviating oxidative stress damage to nerve cells. At the same time, PLGA nanoparticles can also affect the level of autophagy in neuropathic pain models by directly affecting the expression of autophagy-related proteins, changing the intracellular environment to activate autophagy, or participating in the regulation of Beclin-1, LC3, and other signaling pathways. In this context, a compelling body of research has sought to delineate these complex underpinnings and harness novel, innovative therapy strategies.
Shin N et al. [79] conducted a remarkable study wherein they subdurally injected PLGA nanoparticles entrapping p66Shc siRNA (p66Shc siRNA-PLGA NPs) to attenuate the abnormal autophagy, mitochondrial phagocytosis, and neuroinflammation associated with spinal nerve ligation. In some related studies, p62 levels were increased in the spinal cord 7 days after spinal nerve ligation (SNL) in mice. It is suggested that the accumulation of p62 represents the disruption of the autophagic process and autophagic flux [80], and the inhibition of autophagy can reduce the miR-195-induced increase in neuroinflammation and neuropathic pain after SNL [81,82]. PINK1 expression in spinal dorsal horn neurons is increased in SNL-induced neuropathic pain. In contrast, neuropathic hypersensitivity is reduced in PINK1-knockout mice [83], suggesting that PINK1 expression plays a role in the development of neuropathic pain. P66shc has been shown to be associated with mitochondrial dysfunction [84]. In the study by Shin N et al., changes in cleaved p66Shc, caspase-3 (apoptotic marker), p62 (autophagy marker), and PINK1 (mitotic marker) levels in the L5 spinal cord after SNL were assessed. Compared with the SNL group, the cleaved p-p66shc level in the p66shc siRNA group was significantly lower than that in the SNL group (Figure 5A), and the caspase-3 level was reduced by 19.1 ± 0.24%, and the p62 and PINK1 levels were also reduced by 38.3 ± 0.03% and 74.2 ± 0.09%, respectively. Activated microglia can mediate neuroinflammatory processes in the spinal cord, leading to the development and maintenance of neuropathic pain. Therefore, in this study, they also examined the activation of microglia in the ipsilateral dorsal horn of the L5 spinal cord after SNL. In the SNL group, the number of IBA-1-immunoreactive microglia in the ipsilateral dorsal horn of the L5 spinal cord increased significantly after seven days of SNL. On the other hand, the number of Iba-1 immunoreactive microglia was significantly reduced in the p66Shc siRNA group compared to the SNL group (Figure 5B). At the same time, the increase of p66shc, caspase-3, p62, and PINK1 protein levels in the group treated with p66Shc siRNA was significantly inhibited in HT22 cells stimulated by H2O2 (Figure 5C), and the expression level of pro-inflammatory mediator mRNA was significantly reduced. TNF-α, IL-1β, IL-6, COX-2, and iNOS decreased by 38.5 ± 0.05%, 61.3 ± 0.05%, 44.0 ± 0.07%, 30.9 ± 0.18%, and 28.5 ± 0.15%, respectively (Figure 5D).
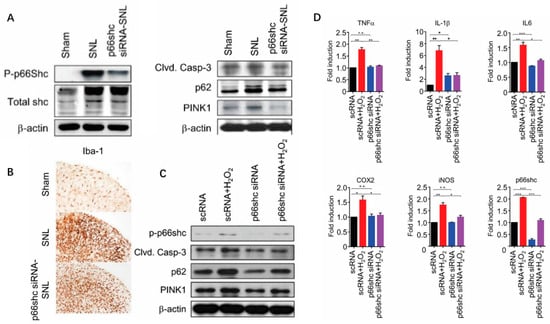
Figure 5.
(A) Phosphorylation of p66shc was decreased after treatment with p66shc siRNA-PLGA NPs and SNL-induced increases in cleaved caspase-3, p62, and PINK1 protein levels were significantly attenuated. (B) Microglial activation was reduced in the p66shc siRNA-PLGA NP group compared with the SNL group. Scale bar = 100 μm. (C) Induction of oxidative stress by H2O2 after p66shc knockdown in the HT22 neuronal cell line reduced the protein levels of phosphorylated p66shc, cleaved caspase-3, p62, and PINK1. (D) P66shc knockdown resulted in decreased mRNA levels of proinflammatory mediators in H2O2-induced HT22 cells. Reprinted from ref. [79] under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). https://doi.org/10.3390/polym12051014 (accessed on 18 June 2024).
Furthermore, Xu X et al. [46] have ingeniously designed lidocaine-polylactic-glycolic acid (Lidocaine@PLGA) microcapsules that effectively respond to ultrasound to alleviate sciatic neuropathic pain. These lidocaine @ PLGA microcapsules are marked by their uniform size, excellent stability, optimal injectability, excellent biocompatibility (Figure 2A), and their protracted in vivo and in vitro release kinetics. Notably, the incorporation of the ultrasonic response feature allows a tunable “on-off” drug release mechanism. In essence, using ultrasound as a trigger switch can catalyze the rapid release of lidocaine from these microcapsules, effectively providing both sustained and short-term ultrasound-triggered release.
Microglial activation, influential in the onset and maturation of neuropathic pain, was extensively studied by Lee S et al. [85]. They explored whether the delivery of IKKβ siRNA (IKBKB small interference RNA) encapsulated in PLGA nanoparticles could mollify microglial activity and ameliorate neuropathic pain in a rat model. After the intrathecal injection of these nano-formulations, the IKBKB expression level was markedly mitigated. This interference in IKBKB expression led to a welcome reduction in hypersensitivity and inflammatory responses facilitated by NF-κB.
Adding another dimension to this rich tapestry of research, Kim SI et al. [86] examined the beneficial potential of PLGA nanoparticles in enhancing the analgesic effect of Duloxetine (DLX), a selective serotonin and norepinephrine reuptake inhibitor with a marked incidence of side effects (10–20%). These DLX NPs show promise in enhancing the microglial drug targeting to offer prolonged analgesic effects while limiting side effects and the potential risk of drug abuse and overdose.
5.2. Inflammatory Pain
Inflammatory pain is the other most important pain besides neuropathic pain. Common inflammatory pain conditions encompass both acute and chronic arthritis, rheumatic diseases, skeletal muscle inflammation, prostatitis, and pelvic inflammation, among others.
Triamcinolone acetonide extended-release tablet 32 mg (Zilretta ®) is approved in the United States for the treatment of knee osteoarthritis pain, administered as a single 5 mL intra-articular injection [87], representing a key therapeutic category. (Zilretta ®) is manufactured by Flexion Therapeutics, Burlington, USA. Free steroids usually show transient efficacy, and the novel sustained-release microsphere formulation of triamcinolone acetonide injection (Zilretta ®) can overcome the limitations of the efficacy of traditional intra-articular steroid injections and the systemic adverse effects associated with corticosteroids. These microspheres can stably release triamcinolone acetonide into the synovium and persist in the joint after dripping into the joint cavity. This avoids frequent intra-articular injections that can lead to systemic exposure, reduces the risk of infection, and mitigates corticosteroid-related adverse events such as elevated blood glucose. Nonetheless, to assess the adapted arthritis model’s applicability for long-term drug-delivery system testing, Rudnik-JansenI et al. [56] compared triamcinolone acetonide released from the novel self-regulating polyesteramide (PEA) microsphere platform with that from poly (lactic-glycolic acid) (PLGA) microspheres. The study demonstrated that TAA-PEA microspheres seemed to be more efficient in alleviating joint swelling and pain behavior compared to TAA-PLGA microspheres.
The exact etiology and pathogenesis of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) remain elusive, and existing treatments often fall short of adequate symptom control. In response to this, Cheng Y et al. [88] devised a novel PLGA-coupled autoantigen peptide (PLGA-T2) therapy to enhance the management of CP/CPPS. Results from the study’s mouse model revealed that mice treated with PLGA-T2 exhibited elevated pain thresholds, reduced urinary frequency, and less severe edema and prostate tissue inflammation compared to control groups. Further, the PLGA-T2 treatment group showed a significant decrease in TNF-α and CRP levels, while IL-10 was markedly increased, compared to the other groups. These results suggest the PLGA-T2-coupled nanoparticles could ameliorate or potentially even cure CP/CPPS in mice, providing a simplified, practical, and cost-effective strategy to better manage the clinical presentations of CP/CPPS and inspire improved future clinical treatments.
5.3. Traumatic Pain
Among the various strategies employed for the comprehensive management of trauma analgesia, postoperative analgesia has been found to be the most feasible and utilized. Postoperative pain, as a result of its multifaceted physiological response to disease/ailment and subsequent tissue injury, can antagonize a number of physiological processes, effectively impacting the functions of the respiratory, circulatory, digestive, and endocrine systems [89]. In cases of failure of control and management, postoperative pain may even precipitate severe immune dysfunctions, as well as metabolic disorders [90]. Given the nature of the operation, as well as the potential risk to patients, a panoply of techniques and methodologies to alleviate postoperative pain—primarily comprising a wide range of analgesics—has found extensive clinical applications. However, it has been observed that the ongoing outcomes are still nascent and somewhat unsatisfactory due to the associated side effects of their usage. Consequently, the continuous intravenous administration of opioids is gaining traction as an alternative method of treatment [91]. However, opioids are known to have limited beneficial effects on pain related to physical activity. Additionally, they are likely to cause undesirable effects, including nausea, vomiting, drowsiness, and respiratory depression.
An innovative initiative was undertaken by Suzuki T et al. [92] where they prepared and used a controlled-release lidocaine consolidated with PLGA in the form of a tablet. They administered this new formulation to patients who underwent tooth extraction surgeries, and they would receive the administration of these lidocaine controlled-release tablets for postoperative pain management. The results revealed that the differential in the positive impacts of analgesic effects between the group receiving the lidocaine sustained-release tablets and other groups was statistically insignificant. However, what was noteworthy about this study was that the use of PLGA seemed to extend the duration of the administration of SRLS, and it demonstrated significant clinical potential for treating post tooth-extraction discomfort (Figure 6).
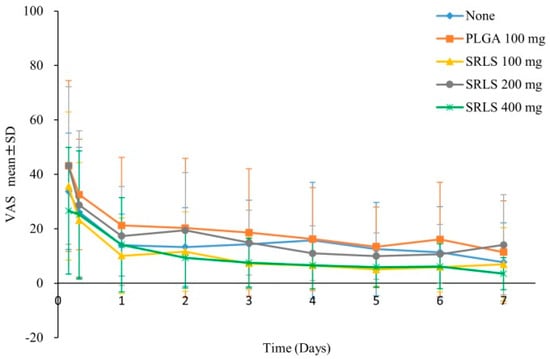
Figure 6.
VAS score of postoperative pain. The efficacy or dose response of the sustained-release lidocaine sheet (SRLS) was evaluated by comparing the VAS scores of the unadministered group, the PLGA control group, and the 100 mg, 200 mg, and 400 mg groups of SRLS. Reprinted from ref. [92] under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). https://doi.org/10.1371/journal.pone.0200059 (accessed on 18 August 2023).
The research of Fu X et al. [93] also analyzed the analgesic effects of a single administration of ropivacaine loaded onto a PLGA-PEG-PLGA thermosensitive gel at the incision site in rat models. The benefits of the pain-relieving effects lasted for up to 48 h, a period significantly lengthened relative to the usage of ropivacaine alone.
In another experiment by Pek YS et al. [94], they utilized a distinct approach where they adopted core-shell polymer microspheres consisting of a PLGA core and a poly (l-lactide) shell. The aim was to ensure the continuous release of bupivacaine to alleviate the discomfort of knee pain after surgery. The gathered data indicated that, at least in goat models post-surgery, the aforementioned microsphere was able to linearly release bupivacaine for a fortnight. Concurrently, Yu YH and colleagues [95] made significant strides in developing a biodegradable PLGA/lidocaine nanofiber membrane to manage the discomfort associated with rib fractures during the course of operation. On application, the release effect of PLGA/lidocaine was slightly better than the other groups. Compared with the same dose of lidocaine alone, the effect of PLGA was significantly improved. Lidocaine sustained-release tablets after PLGA improvement can maintain no differential treatment effect on the basis of prolonged release and alleviating side effects, which we believe can reduce the frequency of administration and improve treatment compliance. The application of the PLGA/lidocaine nanofiber membrane, therefore, could potentially be a viable technique for enabling extended relief from pain.
5.4. Cancerous Pain
Discerning the category of pain a patient encounters is central to effective pain management, given that treatments fluctuate depending on the type of pain. Tumor-related pain can be segregated into three types: neuropathic pain resultant from local compression and subsequent neuropathy, somatic pain due to tumor invasion and destruction, and visceral pain [96].
In the treatment of cancer pain, PLGA can be used to encapsulate various analgesic drugs, such as non-opioid analgesics, opioid analgesics, and other adjuvant drugs. It is slowly released in the body in the form of PLGA nanoparticles or microspheres, which provides continuous pain relief, reduces the dependence on single doses, and reduces the risk of side effects. PLGA nanoparticles can also be modified via surface modification and functionalization technology to enhance targeting specificity and improve the therapeutic effect.
The first-line treatment for tumors, comprised of surgical resection along with radiotherapy and chemotherapy, often inflicts grave harm upon the human body whilst targeting the tumors. Consequently, the primary focus of cancer pain alleviation rests on the treatment of the cancer itself [97,98].
In a study, Ma Y et al. [99] devised PLGA nanoparticles infused with doxorubicin (DOX), which were layered onto planar (1D) microneedles. This method was aimed at addressing recurrent oral cancer. The results from the tests on a 1D tissue model and microscopic examination of pig buccal tissue demonstrated that DOX could permeate both horizontally and vertically into the tissue, thereby causing cytotoxicity. The microneedle array ensured the even distribution of DOX in the cadaver’s buccal tissue, reaching depths greater than 3 mm. Through PLGA nanoparticles, the effect of DOX was significantly enhanced, and the sustained release effect was increased. This miniaturized device delivering drugs consistently to localized oral cancer sites and could potentially be transformative for oral cancer treatment regimens. Furthermore, an evaluation conducted by Ma Y et al. analyzed the sustained release effect of DOX within a 24 h period. The evaluations revealed that the nanoparticles released roughly 30% of the total DOX over the first three hours, subsequently slowing down to a release of 34% over the next 21 h. Such continuous release of DOX—along with the subsequent diffusion of the remaining 66% of drugs contained within the nanoparticles—could enhance the cytotoxicity against tumor cells, offering a favorable condition for chemotherapy.
Hepatocellular carcinoma dominates as one of the most commonplace cancers in East Asian countries and is the third leading cause of cancer-related deaths on a global scale [100]. Transcatheter arterial chemoembolization (TACE) is a beneficial technique in which embolic agents and chemotherapeutic drugs are injected into the hepatic artery to incapsulate the tumor, resulting in necrosis [101]. Owing to its efficacy, TACE is often the primary choice of treatment for advanced, non-resectable liver cancers [102]. Bio-degradable PLGA employed as a drug-delivery system for TACE during liver tumor treatments has attained FDA approval for specific clinical interventions [103]. Li X [104] used a liquid solvent diffusion method to formulate sorafenib (SOR)- and catalase (CAT)-coated PLGA microspheres (SOR-CAT-PLGAMS), forming a SOR-CAT-PLGA microsphere sustained-release system to treat VX2 liver tumors in rabbits. The continuous-release effect of PLGA was clearly demonstrated in this system. Sorafenib inhibits tumor angiogenesis, and catalase catalyzes the breakdown of hydrogen peroxide (H2O2), producing oxygen in the tumor. The SOR-CAT-PLGAMS demonstrated significant on-site efficacy, both in vitro and in vivo, in ameliorating the efficacy of hepatic artery embolization while treating the VX2 liver tumor in rabbits (Figure 7). The system proved to modulate tumor hypoxia and the immunosuppressive microenvironment, achieving near-complete, rapid necrosis of the liver tumor. Salerno et al. developed alendronate (ALN) -conjugated PLGA nanoparticles loaded with doxorubicin (DOX) for the treatment of bone metastases from breast cancer [105], and the obtained ALN-PLGA-DOX nanoparticles had good biocompatibility and the ability to target tumor-induced osteolytic sites.
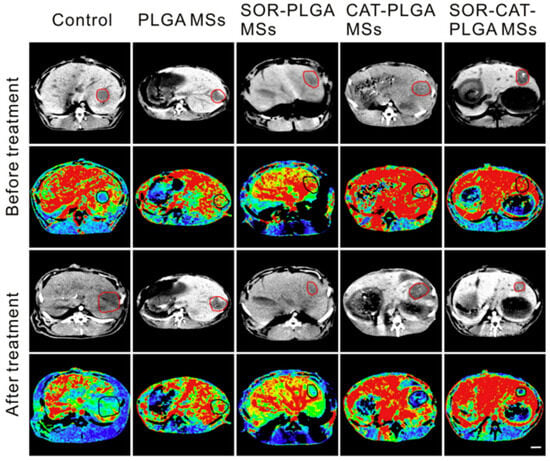
Figure 7.
Perfusion CT images of liver tumors in different groups before and after treatment. The red and black circles in the perfusion CT image indicate the liver tumor. The scale bar on the right is 1 cm in length. Reprinted from ref. [105] under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). https://doi.org/10.1016/j.biopha.2020.110512 (accessed on 25 August 2023).
PLGA is indicated for the relief of long-term or chronic pain and for the reduction of opioid dependence. For patients with cancer pain, PLGA nanoparticles or microspheres can be used to encapsulate non-opioid and opioid analgesics to achieve precise drug-release control, improve efficacy, and reduce side effects. PLGA can improve the specificity and effectiveness of treatment through advanced technologies, such as targeted drug delivery and combination therapy. The continuous monitoring and adjustment of treatment based on patient feedback is also a key step to ensure optimal pain management. The application of PLGA can provide an effective pain-management strategy for patients with cancer pain and help to improve patient compliance and quality of life.
5.5. Angina
Angina pectoris denotes a type of chest discomfort primarily triggered by inadequate blood supply to the coronary artery [106]. The sensation is generally described as oppressive or constrictive, localized behind the sternum, or at the chest’s center [107]. Typical manifestations of angina encompass chest pain and tightness, which may project to the left shoulder, arm, neck, lower jaw, or back. Accompanying symptoms may include breathlessness, perspiration, nausea, or vomiting [108]. The overarching cause of angina pectoris is usually coronary artery stenosis or obstruction, a derivative of coronary atherosclerosis. The primary therapeutic objectives for angina pectoris include symptom relief, myocardial infarction prevention, and enhancement of life quality [109].
To augment the bioavailability of felodipine and address its oral administration challenges through the gastrointestinal barrier, felodipine-loaded PLGA nanoparticles were devised via the nano-precipitation technique by Shah U et al. [110]. These nanoparticles are designed to target M cells within Peyer’s patches. Pharmacodynamic investigations in rats demonstrated that felodipine-loaded PLGA nanoparticles are capable of normalizing both blood pressure and electrocardiogram alterations (specifically ST-segment elevation) within a three-day period when compared to conventional drug suspensions. By augmenting bioavailability, these felodipine-loaded PLGA nanoparticles present a viable alternative medicine for managing hypertension and angina pectoris. Zhang X et al. [111] developed an acidic nanoparticle-delivery system using PLGA that can concentrate in macrophage lysosomes and maintain the acidic pH necessary for lysosomal function, provide functional benefits to macrophages in the presence of atherogenic lipids, and improve the complexity of atherosclerotic plaques, including a reduction in cytotoxic protein aggregation, apoptosis, and necrotic core formation. This work has important implications for the future treatment of angina.
In summary, PLGA-based drug-delivery systems have been extensively explored in pain management trials, focusing on safety, extended release time, enhanced therapeutic efficacy, and a reduced dosing frequency and side effects. For example, Zhang W et al. studied the safety and pharmacokinetics of subcutaneous bupivacaine PLGA microspheres for postoperative pain control [61]. The subcutaneous injection of tramadol hydrochloride using PLGA nanoparticles has been shown to enhance efficacy while reducing systemic side effects by Lalani J et al. [112]. The continuous monitoring and adjustment of treatment based on patient feedback will be a critical step to ensure optimal pain management, and the application of PLGA provides an effective pain-management strategy that can help improve patient compliance and quality of life. Combining analgesics with immunotherapy is also an effective mode of addressing underlying inflammation or lesions in pain management. PLGA nanoparticles can efficiently deliver proteins, DNA, or other biologics to endothelial cells and induce favorable cellular responses for vascular graft healing and remodeling. For example, it was reported by Davda et al. [113] that PLGA NPs with bovine serum albumin as a model protein and 6-coumarin as a fluorescent label were successfully prepared. This study shows that nanoparticles can be used to implant therapeutic agents or genes into endothelial cells. Nanoparticles located in endothelial cells can prolong the efficacy of drugs because of their sustained-release characteristics and also protect the encapsulated drugs from enzymatic degradation. D J Smith et al. [114] determined the effect of mucosal delivery of Streptococcus sobrinus glucosyltransferase (GTF) in bioadhesive PLGA microparticles on the induction of salivary IgA and serum IgG antibody responses in SD rats. The results indicate that the intranasal delivery of GTF-containing PLGA bioadhesive microparticles induced the highest and longest duration of salivary immune responses with any mucosal or systemic route or vehicle tested, promising a useful approach for inducing mucosal immunity.
In the clinical trial, Elisabeth Kjær Jensen et al. performed human experiments with a novel microparticle extended-release local anesthetic containing bupivacaine/PLGA (PLGA LIQ865A) [115]. Serial comparisons were made between the assay and plain bupivacaine (LIQ865B). Dose safety/tolerability and pharmacodynamics were examined. The randomized subcutaneous injections of LIQ865A (n = 16) or LIQ865B (n = 12) and diluent, contralaterally, were administered in a dose-ascending manner (150 to 600 mg bupivacaine). Subjects were admitted 24 h post-injection and followed for 30 days post-injection. The risk ratio (RR; 95% CI) of erythematous reactions for LIQ865A versus diluent was 9.00 (1.81–52.23; p = 0.006), and for LIQ865B versus diluent, it was 2.50 (0.69–9.94; p = 0.37). The RRs for the development of hematomas (LIQ865A versus diluent) were 3.25 (1.52–8.16; p = 0.004) and 4.00 (0.72–24.89; p = 0.32) (LIQ865B versus diluent). Subcutaneous indurations persisting for 4–13 weeks were seen in 6/16 subjects receiving LIQ865A. One subject receiving LIQ865A (600 mg bupivacaine) developed intermittent central nervous system (CNS) symptoms of local anesthetic systemic toxicity (85 min to 51 h post-injection) coinciding with plasma peak bupivacaine concentrations (490–533 ng/mL). Both LIQ865 formulations demonstrated dose-dependent hypoesthesia and hypoalgesia. The duration of analgesia ranged between 37 and 86 h. The overall number of local adverse events, however, prohibits clinical application without further pharmacological modifications. In the clinical use of PLGA drug-delivery systems, continuous monitoring and adjustment of treatment based on patient feedback may be a key step to ensure optimal pain management. In the future, the application of PLGA may provide effective pain-management strategies, improve patient compliance, and achieve a better quality of life.
6. Discussion
Pain, as one of the most common clinical symptoms, inflicts significant negative impacts on many patients’ lives [116]. Tasked with the prospect of efficacious pain management, clinicians face immense challenges while simultaneously harboring enormous potential for future developments. This article provides an exhaustive review concerning the efficacy and side-effects related to first-line pain-management pharmacological treatments, in conjunction with investigating the application of PLGA as a drug-delivery medium.
When compared with other polymers, PLGA triumphs due to its optimal biocompatibility, customizability pertaining to release rates, and potential to alter characteristics to achieve superior delivery efficacy. Importantly, the Food and Drug Administration (FDA) has accorded its approval for the clinical usage of PLGA in humans, and numerous PLGA-loaded therapeutics have pervaded the frontline of clinical medicine, thereby setting a promising precedent for future clinical applications involving PLGA.
While numerous validations support the biocompatibility of PLGA, currently, the sector faces a glaring deficiency in longitudinal follow-up post-clinical applications. Numerous challenges remain in translating clinical trials fully into clinical practice, alongside ensuring that all safety requirements mandated for clinical approval are comprehensively met. Presently, most insights into efficacy and safety predominantly originate from animal studies, primarily involving rodents, such as mice and rats.
Current research predominantly concentrates on PLGA nanoparticles and microparticles, encapsulating various RNAs and therapeutics. Already, numerous analgesics have been successfully incorporated within PLGA nanoparticles. These advancements are not confined to encapsulation alone, with some innovations taking forms, such as nano-films and other structures, or integrating with established practices, like costal periosteum and nanoparticle microneedle coatings, as referenced in this text. Common administration routes include localized administration, intrathecal injection, oral administration, and embolization.
Beyond biocompatibility, drug-loaded PLGA also holds advantageous potential in amplifying analgesic effects, enhancing continuous-release capabilities, and prolonging therapeutic action. These benefits are paving the way toward reduced the frequency and dosage for patients. Through the application of surface modifications and functionalization, selected types of PLGA may achieve targeted delivery, accurately navigating therapeutics to pain-sensation organs or inflammation regions. Certain degrees of attenuation or circumvention of the original side-effects become feasible when drugs are encapsulated. While PLGA drug loading maintains therapeutic stability, it also serves an indispensable role for unstable or easily degradable therapeutics. This technique extends their in vivo retention period and beneficial effects, dramatically improving pain management and enhancing patients’ quality of life.
An increasing amount of research is converging on the intersection of PLGA and pain-management therapeutics, paving the way for numerous potentialities in further clinical translation. Particularly, in neuroanalgesia, PLGA nanoparticles demonstrate their strengths through enhanced targeting and continuous drug release to spinal microglia. The triumphant applications of gene therapy or drug-encapsulated nanoparticles in neuropathic pain management attest to the broad potential of this innovative delivery system. In the near future, with the accumulation of valuable animal studies, it will become feasible to identify optimal therapeutics tailored to distinct pain-management requirements.
However, challenges and opportunities reside hand in hand. Although PLGA can govern therapeutic release rate timings, its release behavior remains subject to variables, such as size, molecular weight, and drug solubility. Additionally, the influence of PLGA integration on therapeutic activity, the limitations of PLGA-synthesis methods, quality control in manufacturing scale-up, technical transferability capabilities, and cost-effectiveness pose obstacles to its clinical implementation. Therefore, moving forward necessitates further research to optimize PLGA microsphere and nanoparticle design parameters, monitor in vivo performance and safety, and refine synthesis technology.
With the resolution of these challenges, the production of PLGA will become simplified, controllable, and scalable, ensuring production efficiency and quality consistency. Consequently, the production and commercialization of PLGA at scale will transform into a plausible reality. In the foreseeable future clinical translations, designing an appropriate PLGA-drug-delivery system according to the types of pain, individual patient characteristics, and treatment requirements may facilitate personalized pain management. As the clinical translation of PLGA analgesics make strides, it becomes imperative to rigorously conduct preclinical and early clinical safety and efficacy trials. Through these extended investigations and clinical verifications, we may discover superior PLGA-related analgesics, providing innovative pain-management solutions, sparking new breakthroughs and progress in the field of pain management.
Author Contributions
T.L. wrote the original draft. J.G. was responsible for revising the article and preparing Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7. R.F. drew Table 1. Y.Z. and K.T. collected information, X.X. and J.C. guided and revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Natural Science Foundation of Zhejiang Province (LQ21H300008), Zhejiang students’ technology and innovation program, and XinMiao program (2023R420027) and Scientific research project of Zhejiang Rehabilitation Medical Center, China (Zk2001), (ZKJC2203).
Conflicts of Interest
The author declares no conflicts of interest, financial or otherwise.
References
- Gottschalk, A.; Smith, D.S. New concepts in acute pain therapy: Preemptive analgesia. Am. Fam. Physician 2001, 63, 1979–1984. [Google Scholar] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Dahl, J.B. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth. Analg. 1993, 77, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Todaro, B.; Moscardini, A.; Luin, S. Pioglitazone-Loaded PLGA Nanoparticles: Towards the Most Reliable Synthesis Method. Int. J. Mol. Sci. 2022, 23, 2522. [Google Scholar] [CrossRef] [PubMed]
- Kuldeep, N. Co-Delivery of Baclofen & Lamotrigine via Plga Nanoparticles, India. IN202011001014A, 9 January 2020. [Google Scholar]
- Yoo, J.; Won, Y.Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Peterson, K.; Helfand, M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: A systematic review. J. Pain Symptom Manag. 2004, 28, 140–175. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47 (Suppl. S2), S78–S87. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.J.; Fudin, J. Nonsteroidal Antiinflammatory Drugs for Acute and Chronic Pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef]
- James, D.S. The multisystem adverse effects of NSAID therapy. J. Am. Osteopath. Assoc. 1999, 99 (Suppl. S11), S1–S7. [Google Scholar] [CrossRef]
- Arfè, A.; Scotti, L.; Varas-Lorenzo, C.; Nicotra, F.; Zambon, A.; Kollhorst, B.; Schink, T.; Garbe, E.; Herings, R.; Straatman, H.; et al. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. BMJ 2016, 354, i4857. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Dreischulte, T.; Morales, D.R.; Bell, S.; Guthrie, B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015, 88, 396–403. [Google Scholar] [CrossRef]
- Bach-Rojecky, L.; Vađunec, D.; Žunić, K.; Kurija, J.; Šipicki, S.; Gregg, R.; Mikula, I.; Primorac, D. Continuing war on pain: A personalized approach to the therapy with nonsteroidal anti-inflammatory drugs and opioids. Per. Med. 2019, 16, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.; Wallace, M. Opioids, Analgesia, and Pain Management. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef]
- Wadlund, D.L. Local Anesthetic Systemic Toxicity. AORN J. 2017, 106, 367–377. [Google Scholar] [CrossRef]
- Macfarlane, A.J.R.; Gitman, M.; Bornstein, K.J.; El-Boghdadly, K.; Weinberg, G. Updates in our understanding of local anaesthetic systemic toxicity: A narrative review. Anaesthesia 2021, 76 (Suppl. S1), 27–39. [Google Scholar] [CrossRef]
- Pasierski, M.; Szulczyk, B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules 2022, 27, 2484. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Acevedo, A.P.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Hladun, O.; Siles, A.; Torrens, M.; Busardo, F.P.; Farré, M. Oral Administration of Cannabis and Δ-9-tetrahydrocannabinol (THC) Preparations: A Systematic Review. Medicina 2020, 56, 309. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Mathieson, S.; Lin, C.C.; Underwood, M.; Eldabe, S. Pregabalin and gabapentin for pain. BMJ. 2020, 369, m1315. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Rex, J.; Eith, T.; Heilmaier, C. Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. Ger. Med. Sci. 2014, 12, Doc15. [Google Scholar]
- Browning, K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 2015, 9, 413. [Google Scholar] [CrossRef]
- Qin, J.; Zhuang, L.; Yao, X.; Guo, D. Analysis of 233 cases of adverse drug reactions / events reported by SSRIs and SNRIs drugs. Drug Appl. Monit. China 2020, 17, 249–252. [Google Scholar]
- Gao, Y.J.; Ji, R.R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010, 7, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom [data storage]. J. Microelectromech. Syst. 1992, 1, 60–66. [Google Scholar] [CrossRef]
- Nielsen, R.V.; Fomsgaard, J.S.; Siegel, H.; Martusevicius, R.; Mathiesen, O.; Dahl, J.B. The effect of chlorzoxazone on acute pain after spine surgery. A randomized, blinded trial. Acta Anaesthesiol. Scand. 2016, 60, 1152–1160. [Google Scholar] [CrossRef]
- Barakat, A.; Hamdy, M.M.; Elbadr, M.M. Uses of fluoxetine in nociceptive pain management: A literature overview. Eur. J. Pharmacol. 2018, 829, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Ghanavatian, S.; Derian, A. Tizanidine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vázquez, N.; Sánchez-Arévalo, F.; Maciel-Cerda, A.; Garnica-Palafox, I.; Ontiveros-Tlachi, R.; Chaires-Rosas, C.; Piñón-Zarate, G.; Herrera-Enríquez, M.; Hautefeuille, M.; Vera-Graziano, R.; et al. Influence of the PLGA/gelatin ratio on the physical, chemical and biological properties of electrospun scaffolds for wound dressings. Biomed. Mater. 2019, 14, 045006. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGANanoparticle-Based Formulations to Cross the Blood-Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.-L.; Hamid, Z.A.A.; Mariatti, M.; Yahaya, B.H.; Lim, K.; Bee, S.-T.; Sin, L.T. Approaches to Improve Therapeutic Efficacy of Biodegradable PLA/PLGA Microspheres: A Review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Xu, X.; Chang, S.; Zhang, X.; Hou, T.; Yao, H.; Zhang, S.; Zhu, Y.; Cui, X.; Wang, X. Fabrication of a controlled-release delivery system for relieving sciatica nerve pain using an ultrasound-responsive microcapsule. Front. Bioeng. Biotechnol. 2022, 10, 1072205. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhong, W.; Guo, L.; Ji, J.; Nie, H. Effect of Bufalin-PLGA Microspheres in the Alleviation of Neuropathic Pain via the CCI Model. Front. Pharmacol. 2022, 13, 910885. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.W.; Tseng, Y.Y.; Liu, K.S.; Liu, Y.W.; Chen, J.C.; He, H.L.; Kau, Y.C.; Liu, S.J. Anesthetics and human epidermal growth factor incorporated into anti-adhesive nanofibers provide sustained pain relief and promote healing of surgical wounds. Int. J. Nanomed. 2019, 14, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Lee, D.; Lee, T.C.; Chen, J.K.; Wu, R.C.; Liu, K.C.; Liu, S.J. Fabrication of Multi-Layered Lidocaine and Epinephrine-Eluting PLGA/Collagen Nanofibers: In Vitro and In Vivo Study. Polymers 2017, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Nigam, K.; Kaur, A.; Tyagi, A.; Nematullah, M.; Khan, F.; Gabrani, R.; Dang, S. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Shen, S.J.; Hsu, Y.H.; Chou, Y.C.; Yu, P.C.; Liu, S.J. Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair. Polymers 2022, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Berrocoso, E.; Rey-Brea, R.; Fernández-Arévalo, M.; Micó, J.A.; Martín-Banderas, L. Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomedicine 2017, 13, 2623–2632. [Google Scholar] [CrossRef]
- Rani, S.; Gothwal, A.; Pandey, P.K.; Chauhan, D.S.; Pachouri, P.K.; Gupta, U.D.; Gupta, U. HPMA-PLGA Based Nanoparticles for Effective In Vitro Delivery of Rifampicin. Pharm. Res. 2018, 36, 19. [Google Scholar] [CrossRef]
- Liu, K.S.; Chen, W.H.; Lee, C.H.; Su, Y.F.; Liu, S.J. Extended pain relief achieved by analgesic-eluting biodegradable nanofibers in the Nuss procedure: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 8355–8364. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Cheng, Y.S.; Hsu, Y.H.; Yu, Y.H.; Liu, S.J. Biodegradable nanofiber-membrane for sustainable release of lidocaine at the femoral fracture site as a periosteal block: In vitro and in vivo studies in a rabbit model. Colloids Surf. B Biointerfaces 2016, 140, 332–341. [Google Scholar] [CrossRef]
- Nigam, K.; Kaur, A.; Tyagi, A.; Manda, K.; Gabrani, R.; Dang, S. Baclofen-Loaded Poly (D,L-Lactide-Co-Glycolic Acid) Nanoparticles for Neuropathic Pain Management: In Vitro and In Vivo Evaluation. Rejuvenation Res. 2019, 22, 235–245. [Google Scholar] [CrossRef]
- Kim, S.R.; Ho, M.J.; Kim, S.H.; Cho, H.R.; Kim, H.S.; Choi, Y.S.; Choi, Y.W.; Kang, M.J. Increased localized delivery of piroxicam by cationic nanoparticles after intra-articular injection. Drug Des. Dev. Ther. 2016, 10, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, M.; Baskaran, P.; Arulsamy, N.; Thyagarajan, B. Preparation and Evaluation of PLGA-Coated Capsaicin Magnetic Nanoparticles. Pharm. Res. 2017, 34, 1255–1263. [Google Scholar] [CrossRef]
- Zhu, M.; Whittaker, A.K.; Smith, M.T.; Han, F.Y. Bioerodable Ketamine-Loaded Microparticles Fabricated Using Dissolvable Hydrogel Template Technology. J. Pharm. Sci. 2019, 108, 1220–1226. [Google Scholar] [CrossRef]
- Kandilli, B.; Ugur Kaplan, A.B.; Cetin, M.; Taspinar, N.; Ertugrul, M.S.; Aydin, I.C.; Hacimuftuoglu, A. Carbamazepine and levetiracetam-loaded PLGA nanoparticles prepared by nanoprecipitation method: In vitro and in vivo studies. Drug Dev. Ind. Pharm. 2020, 46, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ning, C.; Xu, W.; Hu, H.; Li, M.; Zhao, G.; Ding, J.; Chen, X. Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere. Theranostics 2018, 8, 3331–3347. [Google Scholar] [CrossRef]
- Wang, T.; Hurwitz, O.; Shimada, S.G.; Tian, D.; Dai, F.; Zhou, J.; Ma, C.; LaMotte, R.H. Anti-nociceptive effects of bupivacaine-encapsulated PLGA nanoparticles applied to the compressed dorsal root ganglion in mice. Neurosci. Lett. 2018, 668, 154–158. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, H.; Du, S.; Zhang, X.Q.; Lin, F.; Ji, F.; Tsou, Y.H.; Li, Z.; Feng, Y.; Ticehurst, K.; et al. Injectable PLGA-Coated Ropivacaine Produces A Long-Lasting Analgesic Effect on Incisional Pain and Neuropathic Pain. J. Pain. 2021, 22, 180–195. [Google Scholar] [CrossRef]
- Han, F.Y.; Whittaker, A.; Howdle, S.M.; Naylor, A.; Shabir-Ahmed, A.; Smith, M.T. Sustained-Release Hydromorphone Microparticles Produced by Supercritical Fluid Polymer Encapsulation. J. Pharm. Sci. 2019, 108, 811–814. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Schrijver, K.; Woike, N.; Tellegen, A.; Versteeg, S.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Intra-articular injection of triamcinolone acetonide releasing biomaterial microspheres inhibits pain and inflammation in an acute arthritis model. Drug Deliv. 2019, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef]
- Shin, J.; Yin, Y.; Park, H.; Park, S.; Triantafillu, U.L.; Kim, Y.; Kim, S.R.; Lee, S.Y.; Kim, D.K.; Hong, J.; et al. p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation. Nanomedicine 2018, 13, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Malewicz, N.M.; Rattray, Z.; Oeck, S.; Jung, S.; Escamilla-Rivera, V.; Chen, Z.; Tang, X.; Zhou, J.; LaMotte, R.H. Topical Capsaicin in Poly(lactic-co-glycolic)acid (PLGA) Nanoparticles Decreases Acute Itch and Heat Pain. Int. J. Mol. Sci. 2022, 23, 5275. [Google Scholar] [CrossRef]
- Cavalier, M.; Benoit, J.P.; Thies, C. The formation and characterization of hydrocortisone-loaded poly((+/–)-lactide) microspheres. J. Pharm. Pharmacol. 1986, 38, 249–253. [Google Scholar] [CrossRef]
- Wu, X.S. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers: Part III. Drug delivery application. Artif. Cells Blood Substit. Immobil. Biotechnol. 2004, 32, 575–591. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Woike, N.; de Jong, S.; Versteeg, S.; Kik, M.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Applicability of a Modified Rat Model of Acute Arthritis for Long-Term Testing of Drug Delivery Systems. Pharmaceutics 2019, 11, 70. [Google Scholar] [CrossRef]
- Pramual, S.; Lirdprapamongkol, K.; Atjanasuppat, K.; Chaisuriya, P.; Niamsiri, N.; Svasti, J. PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells. Molecules 2022, 27, 8295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, J.S.; Park, M.; Ko, M.Y.; Yi, S.W.; Yoon, J.A.; Yang, S.; Shim, S.H.; Park, K.H.; Song, H. PLGA nanoparticles with multiple modes are a biologically safe nanocarrier for mammalian development and their offspring. Biomaterials 2018, 183, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.A.; Handa, M.; Garabadu, D.; Kumar, R.; Kushawaha, P.K.; Shukla, R. Transferrin decorated PLGA encumbered moxifloxacin nanoparticles and in vitro cellular studies. Drug Dev. Ind. Pharm. 2023, 49, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xie, Y.; Li, X.; Yao, X.; Wang, X.; Wang, M.; Li, Z.; Cao, F. Folic Acid/Peptides Modified PLGA-PEI-PEG Polymeric Vectors as Efficient Gene Delivery Vehicles: Synthesis, Characterization and Their Biological Performance. Mol. Biotechnol. 2021, 63, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Szok, D.; Tajti, J.; Nyári, A.; Vécsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Shin, H.J.; Yi, Y.; Beom, J.; Lee, W.; Lee, C.H.; Kim, D.W. p66shc siRNA-Encapsulated PLGA Nanoparticles Ameliorate Neuropathic Pain Following Spinal Nerve Ligation. Polymers 2020, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Berliocchi, L.; Russo, R.; Maiarù, M.; Levato, A.; Bagetta, G.; Corasaniti, M.T. Autophagy impairment in a mouse model of neuropathic pain. Mol. Pain. 2011, 7, 83. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z. Upregulated TLR3 promotes neuropathic pain by regulating autophagy in rat with l5 spinal nerve ligation model. Neurochem. Res. 2017, 42, 634–643. [Google Scholar] [CrossRef]
- Yi, M.H.; Shin, J.; Shin, N.; Yin, Y.; Lee, S.Y.; Kim, C.S.; Kim, S.R.; Zhang, E.; Kim, D.W. PINK1 mediates spinal cord mitophagy in neuropathic pain. J. Pain. Res. 2019, 12, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Shi, J.; Liu, K.; Liu, N.; Wang, Y.; Fu, Z.; Ding, J.; Jia, L.; Yuan, W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 2013, 61, 504–512. [Google Scholar] [CrossRef]
- Bhat, S.S.; Anand, D.; Khanday, F.A. p66Shc as a switch in bringing about contrasting responses in cell growth: Implications on cell proliferation and apoptosis. Mol. Cancer 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, H.J.; Noh, C.; Kim, S.I.; Ko, Y.K.; Lee, S.Y.; Lim, C.; Hong, B.; Yang, S.Y.; Kim, D.W.; et al. IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. Int. J. Mol. Sci. 2021, 22, 5657. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Shin, J.; Tran, Q.; Park, H.; Kwon, H.H.; Shin, N.; Hwang, J.A.; Shin, H.J.; Lee, J.; Lee, W.H.; et al. Application of PLGA nanoparticles to enhance the action of duloxetine on microglia in neuropathic pain. Biomater. Sci. 2021, 9, 6295–6307. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Duggan, S.T.; Keam, S.J. Triamcinolone Acetonide Extended-Release: A Review in Osteoarthritis Pain of the Knee. Drugs 2019, 79, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cao, Y.; Ihsan, A.U.; Khan, F.U.; Li, X.; Xie, D.; Cui, X.; Wang, W.; Liu, Z.; Li, C.; et al. Novel Treatment of Experimental Autoimmune Prostatitis by Nanoparticle-Conjugated Autoantigen Peptide T2. Inflammation 2019, 42, 1071–1081. [Google Scholar] [CrossRef]
- Jirkof, P. Side effects of pain and analgesia in animal experimentation. Lab Anim. 2017, 46, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mattila, K.; Toivonen, J.; Janhunen, L.; Rosenberg, P.H.; Hynynen, M. Postdischarge symptoms after ambulatory surgery: First-week incidence, intensity, and risk factors. Anesth. Analg. 2005, 101, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; DeRoche, A.; Adams, L.; Slocum, K.V.; Clark, C.J.; Fino, N.F.; Shen, P. Effect of epidural compared to patient-controlled intravenous analgesia on outcomes for patients undergoing liver resection for neoplastic disease. J. Surg. Oncol. 2017, 115, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kosugi, K.; Suto, T.; Tobe, M.; Tabata, Y.; Yokoo, S.; Saito, S. Sustained-release lidocaine sheet for pain following tooth extraction: A randomized, single-blind, dose-response, controlled, clinical study of efficacy and safety. PLoS ONE 2018, 13, e0200059. [Google Scholar] [CrossRef]
- Fu, X.; Zeng, H.; Guo, J.; Liu, H.; Shi, Z.; Chen, H.; Li, D.; Xie, X.; Kuang, C. A PLGA-PEG-PLGA Thermosensitive Gel Enabling Sustained Delivery of Ropivacaine Hydrochloride for Postoperative Pain Relief. Chem. Pharm. Bull. 2017, 65, 229–235. [Google Scholar] [CrossRef]
- Pek, Y.S.; Pitukmanorom, P.; Ying, J.Y. Sustained release of bupivacaine for post-surgical pain relief using core-shell microspheres. J. Mater. Chem. B 2014, 2, 8194–8200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Hsu, Y.H.; Chou, Y.C.; Fan, C.L.; Ueng, S.W.; Kau, Y.C.; Liu, S.J. Sustained relief of pain from osteosynthesis surgery of rib fracture by using biodegradable lidocaine-eluting nanofibrous membranes. Nanomedicine 2016, 12, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Grossberg, G.T. Phantom limb pain: Mechanisms and treatment approaches. Pain. Res. Treat. 2011, 2011, 864605. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.B.; Krempl, G.A.; Canfield, V.; Bogardus, C.; Kojouri, K.; Kaneaster, S.K.; Medina, J.E. Treatment of base of tongue cancer with paclitaxel, ifosfamide, and cisplatinum induction chemotherapy followed by chemoradiotherapy. Laryngoscope 2008, 118, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Boese, S.E.; Luo, Z.; Nitin, N.; Gill, H.S. Drug coated microneedles for minimally-invasive treatment of oral carcinomas: Development and in vitro evaluation. Biomed. Microdevices 2015, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Dreher, M.R. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J. Control Release 2012, 161, 338–350. [Google Scholar] [CrossRef]
- Cammà, C.; Schepis, F.; Orlando, A.; Albanese, M.; Shahied, L.; Trevisani, F.; Andreone, P.; Craxì, A.; Cottone, M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: Meta-analysis of randomized controlled trials. Radiology 2002, 224, 47–54. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, J.H.; Baek, S.Y.; Kim, D.D.; Kim, H.C.; Cho, H.J. Doxorubicin-loaded poly(lactic-co-glycolic acid) microspheres prepared using the solid-in-oil-in-water method for the transarterial chemoembolization of a liver tumor. Colloids Surf. B Biointerfaces 2015, 132, 305–312. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Huang, Y.; Chen, Y.; Wang, J.; Xu, L.; Zhang, F.; Zhuge, Y.; Zou, X. Preparation of microspheres encapsulating sorafenib and catalase and their application in rabbit VX2 liver tumor. Biomed. Pharmacother. 2020, 129, 110512. [Google Scholar] [CrossRef] [PubMed]
- Qiu, E.; Liu, F. PLGA-based drug delivery systems in treating bone tumors. Front. Bioeng. Biotechnol. 2023, 11, 1199343. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F. Management of vasospastic angina. Heart 2022, 109, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Chaitman, B. Angina and Its Management. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.A.; Miaskowski, C. The symptom experience of angina in women. Pain. Manag. Nurs. 2000, 1, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, D.W.; Beatty, A.L.; Meyer, C.S.; Whooley, M.A. Longitudinal Association Between Angina Pectoris and Quality of Life. Am. J. Cardiol. 2022, 164, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Joshi, G.; Sawant, K. Improvement in antihypertensive and antianginal effects of felodipine by enhanced absorption from PLGA nanoparticles optimized by factorial design. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Misra, S.K.; Moitra, P.; Zhang, X.; Jeong, S.J.; Stitham, J.; Rodriguez-Velez, A.; Park, A.; Yeh, Y.S.; Gillanders, W.E.; et al. Use of acidic nanoparticles to rescue macrophage lysosomal dysfunction in atherosclerosis. Autophagy 2023, 19, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Lalani, J.; Rathi, M.; Lalan, M.; Misra, A. Protein functionalized tramadol-loaded PLGA nanoparticles: Preparation, optimization, stability and pharmacodynamic studies. Drug Dev. Ind. Pharm. 2013, 39, 854–864. [Google Scholar] [CrossRef]
- Davda, J.; Labhasetwar, V. Characterization of nanoparticle uptake by endothelial cells. Int. J. Pharm. 2002, 233, 51–59. [Google Scholar] [CrossRef]
- Smith, D.J.; Trantolo, D.J.; King, W.F.; Gusek, E.J.; Fackler, P.H.; Gresser, J.D.; De Souza, V.L.; Wise, D.L. Induction of secretory immunity with bioadhesive poly (D,L-lactide-co-glycolide) microparticles containing Streptococcus sobrinus glucosyltransferase. Oral. Microbiol. Immunol. 2000, 15, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.K.; Bøgevig, S.; Balchen, T.; Springborg, A.H.; Royal, M.A.; Storgaard, I.K.; Lund, T.M.; Møller, K.; Werner, M.U. Dose safety and pharmacodynamics of subcutaneous bupivacaine in a novel extended-release microparticle formulation: A phase 1, dose-ascending study in male volunteers. Basic Clin. Pharmacol. Toxicol. 2024, 134, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.C.; Fillingim, R.B.; Ohrbach, R.; Patel, K.V. Assessment of Psychosocial and Functional Impact of Chronic Pain. J. Pain. 2016, 17 (Suppl. S9), T21–T49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).