Abstract
The pyrolysis process is a thermochemical conversion reaction that encompasses an intricate array of simultaneous and competitive reactions occurring in oxygen-depleted conditions. The final products of biomass pyrolysis are bio-oil, biochar, and some gases, with their proportions determined by the pyrolysis reaction conditions and technological pathways. Typically, low-temperature slow pyrolysis (reaction temperature below 500 °C) primarily yields biochar, while high-temperature fast pyrolysis (reaction temperature 700–1100 °C) mainly produces combustible gases. In the case of medium-temperature rapid pyrolysis (reaction temperature around 500–650 °C), conducted at very high heating rates and short vapor residence times (usually less than 1 s), the maximum liquid yield can reach up to 85 wt% (on a wet basis) or achieve 70 wt% (on a dry basis), with bio-oil being the predominant product. By employing the pyrolysis technique, valuable utilization of tobacco stem waste enriched with lignin can be achieved, resulting in the production of desired pyrolysis products such as transportation fuels, bio-oil, and ethanol. The present review focuses on catalytic pyrolysis, encompassing catalytic hydropyrolysis and catalytic co-pyrolysis, and meticulously compares the impact of catalyst structure on product distribution. Initially, we provide a comprehensive overview of the recent pyrolysis mechanism of lignin and tobacco waste. Subsequently, an in-depth analysis is presented, elucidating how to effectively design the catalyst structure to facilitate the efficient conversion of lignin through pyrolysis. Lastly, we delve into other innovative pyrolysis methods, including microwave-assisted and solar-assisted pyrolysis.
1. Introduction
China is a major tobacco-growing country, accounting for approximately one-third of the world’s annual tobacco production, with an annual output reaching 4.5–5 million tons [1]. After tobacco is harvested, a significant amount of tobacco waste is generated, including tobacco stems, shredded leaves, and stalks [2]. If these tobacco waste materials are not properly utilized, it not only leads to resource wastage but also pollutes water sources and soil, thereby causing environmental implications. Currently, the main methods for the utilization of tobacco waste, both domestically and internationally, include direct combustion for power generation, production of tobacco sheets after sorting, and extraction of high-value chemicals using organic solvents. However, the majority of these utilization methods have relatively low economic value. Pyrolysis is an intricate series of reactions that enables the conversion of tobacco stem waste into gas, bio-oil, and char in the absence of molecular oxygen [3]. The pyrolysis process is categorized into slow pyrolysis and fast pyrolysis, based on the differential removal rates of vapors and aerosol components from liquid formation and solid [4,5,6,7]. However, upgrading lignin in tobacco stem waste through pyrolysis is still highly challenging, as various chemical structures in lignin could significantly impact the thermal reactions and the condensed interunitary linkages could render the recalcitrance of depolymerization [8,9].
In recent times, there has been a notable emphasis on the advancement of sustainable technologies aimed at mitigating the energy crisis. Lignin, the second most abundant constituent of lignocellulosic biomass, exhibits the capability to undergo fast pyrolysis, leading to the conversion into phenolic products [10,11]. These phenolic products hold significant potential as viable precursors for the production of biofuels. However, the presence of phenolic oligomers often poses a challenge as they tend to deactivate the catalyst during the upgrading process, rendering the bio-oil less amenable to the production of high-value chemicals [12]. Consequently, the reduction of phenolic oligomers during the pyrolysis process presents a significant hurdle. Therefore, the promotion of valorization of lignin in tobacco waste through the pyrolysis process emerges as a feasible approach for the generation of high-value chemicals. Furthermore, renewable transportation fuels derived from biomass possess considerable potential for significant reductions in greenhouse gas emissions, contributing to the diversification of global fuel resources. Through thermal conversion employing fast pyrolysis, approximately 75% of the initial plant material and its associated energy content can be transformed into a bio-oil intermediate, offering suitability for subsequent upgrading into motor fuel. Notably, woody biomass, characterized by its low ash content and the high quality of bio-oil produced, stands out as the predominant and extensively researched material in thermochemical processes [13]. Furthermore, the utilization of biomass presents itself as a viable alternative for harnessing renewable energy sources in the construction of smart cities.
Several studies have explored various aspects of biomass pyrolysis. Mohan et al., for instance, concentrated on recent advancements in wood pyrolysis, elucidating the characteristics of the resultant bio-oils, the primary products of swift wood pyrolysis. The inquiry delves into the impact of wood composition and structure, heating rate, and residence time during pyrolysis on the comprehensive reaction rate and volatile yield [14]. Serrano-Ruiz and co-workers directed their attention towards the primary methodologies employed for profound chemical transformations, encompassing gasification, pyrolysis, and aqueous-phase catalytic processing. Special emphasis was placed on pathways that involve catalytic reactions in an aqueous phase [15]. Carpenter et al. recently provided a comprehensive overview, summarizing the present state of knowledge pertaining to the influence of feedstock and pretreatments on the yield, product distribution, and upgradability of bio-oil [13]. Growing apprehensions regarding depleting reserves of fossil fuels, coupled with the escalating impacts of global warming induced by rising atmospheric CO2 levels, are compelling society to explore alternative renewable energy sources. These sources are envisaged to serve as substitutes for coal, natural gas, and petroleum within the existing energy framework. Consequently, our focus is directed towards catalytic pyrolysis, specifically examining catalytic hydropyrolysis and catalytic co-pyrolysis, with a meticulous comparison of the influence of catalyst structure on product distribution.
The exploitation of economically viable carbon resources, such as biomass and industrial solid waste, for the production of hydrocarbon fuels and high-value chemicals holds significant application value and presents expansive market prospects [16,17].
For example, the pyrolysis of tobacco stem waste has garnered significant attention from both academic and industrial sectors [18,19,20]. However, due to the inherent structural differences of lignin or tobacco stem waste, the distribution of products during the pyrolysis process becomes complex [9]. Hence, catalytic pyrolysis of lignin is an important mean to enhance the thermal cracking performance of lignin and regulate product distribution. Understanding the pyrolysis mechanism is crucial for the design and optimization of catalysts, ultimately leading to the production of high-quality bio-oil. Previous research has demonstrated that the initial pyrolysis process follows a unimolecular decomposition mechanism [21,22]. Initially, various radicals are formed through the homolytic cleavage of C-O and C-C bonds in the biomass. These radicals then undergo hydrogenation reactions, resulting in the formation of primary products. Subsequently, these primary products undergo a series of secondary reactions, including homolysis, rearrangement, and further hydrogenation reactions, leading to the generation of secondary products [23]. Additionally, these radicals can also propagate chains by transferring to other species [24]. The chain reactions are eventually terminated upon collision between two radicals, resulting in the formation of stable compounds. However, the challenge lies in the control of product distribution and the attainment of high-quality bio-oil. To address these challenges, catalysts have been employed in the pyrolysis process, offering benefits such as reduced reaction time, decreased energy consumption, and the production of high value-added products [24]. Various catalysts have been explored in the pyrolysis process, including alkali halides [25], metallic oxides [26], zeolites [27], and metal-supported catalysts [28]. The presence of a catalyst enables the achievement of a high bio-oil yield at relatively lower temperatures. Consequently, catalytic pyrolysis has gained considerable attention in recent years. This review aims to provide a comprehensive examination of the impact of catalyst structure on product distribution during pyrolysis, along with prospects for designing efficient catalysts.
2. Biomass Pretreatment
Pretreatment is an indispensable procedural stage in the biochemical and thermochemical conversion of biomass, entailing structural modifications designed to address the intrinsic recalcitrance of biomass. This process is imperative for refining biomass characteristics, thereby augmenting the energy utilization efficiency of the biomass [29]. In heat-dependent pretreatment processes, the degradation capability of lignocellulosic biomass (LCB) is regulated by its polymeric and aromatic constituents (cellulose, hemicellulose, and lignin). In contrast, the heteroatoms and inorganic elemental components of non-lignocellulosic biomass (NLCB) serve as catalysts, expediting decomposition and resulting in the creation of a product characterized by a carbon framework and structural modifications. These alterations contribute to enhancing the performance of the pretreated material in bioconversion processes. Primary impediments confronting contemporary pretreatment technologies revolve around elevated costs and the challenge of securing a pretreated product while minimizing degradation of pivotal components. Pretreatment methods necessitate tailored customization in alignment with the biomass origin and its intended application in bioconversion and biorefinery processes. The comparison of different biomass pretreatment methods is provided in Table 1.

Table 1.
A brief overview of the advantages and disadvantages associated with various categories of pretreatment methods.
3. Pyrolysis Mechanism of Lignin
Fast pyrolysis of biomass demonstrates the capability to transform 75 wt% of the input material into bio-oil [10]. However, the bio-oil obtained is a complex mixture of organic compounds that requires further upgrading to yield valuable chemicals. Many researchers have also isolated lignin from tobacco stems or tobacco leaf waste and conducted thorough characterizations of its structure, including the utilization of techniques such as two-dimensional nuclear magnetic resonance (NMR) [18]. Additionally, they have extensively investigated the pyrolysis performance of lignin [9,18]. However, the yield of monomers per lignin remained relatively low, accompanied by a broad distribution of products.
In general, the addition of a catalyst can effectively modify the distribution of products during the pyrolysis process [30]. Additionally, variables such as vapor residence time (VRT), temperature, and heating rate can also impact the yields and distribution of bio-oil products [31]. The complex structure of lignin gives rise to a complex decomposition mechanism during pyrolysis. Lignin pyrolysis typically involves three stages: drying, fast degradation, and slow degradation. Among the main products derived from lignin pyrolysis are phenolic compounds, including phenol (P-) type, syringol (S-) type, catechol (C-) type, and guaiacol type (Figure 1). To investigate the pyrolysis mechanism and product distribution, the kinetics and mechanism of lignin pyrolysis have been explored using techniques such as thermogravimetric analysis-Fourier transform infrared spectroscopy (TG−FTIR) and pyrolyzers equipped with gas chromatography/mass spectrometry (Py-GC/MS) [32,33]. Furthermore, thermogravimetry/derivative thermogravimetry has been employed to examine the evaporation of moisture, the significant evolution of aromatic compounds, and the formation of char [34].
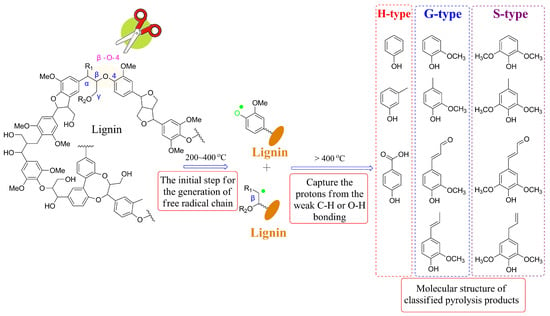
Figure 1.
Proposed reaction mechanism of pyrolysis of lignin.
The decomposition mechanism of lignin remains intricate due to its complex structure. In order to elucidate the lignin degradation process, various lignin models that represent key structural features found in lignin have been employed [35]. Haruo and colleagues conducted a study demonstrating the significant impact of side-chain hydroxyl groups on the pyrolytic β-ether cleavage of phenolic model dimers [36]. Specifically, the phenolic dimer with hydroxyl groups at the Cα- and Cβ-positions exhibited the highest yield of guaiacol and 1-phenylpropene (Figure 2). Conversely, the absence of hydroxyl groups at the Cα- and Cβ-positions in the phenolic dimer resulted in a low yield of guaiacol and 1-phenylpropene, indicating a considerably reduced reactivity of these models. Two distinct reaction routes were identified, leading to different products. The Cβ-O cleavage of these models yielded guaiacol and 1-phenylpropene, while the Cγ-elimination reaction routes generated vinyl ether. The complexity of the pyrolysis process contributes to a broad distribution of products, thereby limiting the utilization of bio-oil [37].
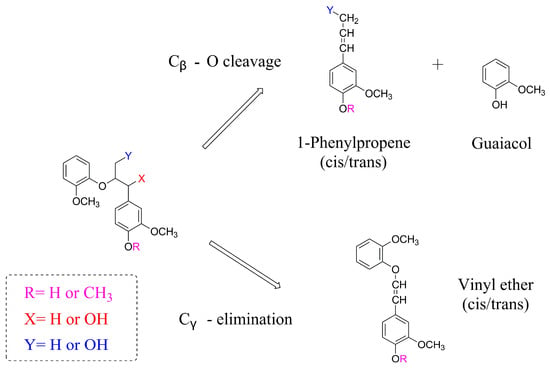
Figure 2.
Proposed pathways of the β-ether types of model compound.
Efforts have been made to enhance the quality of bio-oil through the utilization of catalysts in the process of catalytic biomass fast pyrolysis. Zeolites, active carbon, and metallic oxides have been developed as catalysts for this purpose [38]. For instance, Zheng et al. investigated the efficacy of bio-based activated carbon (B-AC) catalysts in upgrading biomass pyrolysis vapors to produce methylfurans and phenol [39]. The addition of B-AC catalysts demonstrated a notable impact on the generation of phenolic compounds during the biomass pyrolysis process. This effect can be attributed to the depolymerization, deoxidization, and demethylation reactions facilitated by B-AC (Figure 3). Given that the wide distribution of products is unfavorable for the pyrolysis process of lignin, the design of an effective catalyst becomes a crucial matter that requires significant attention.
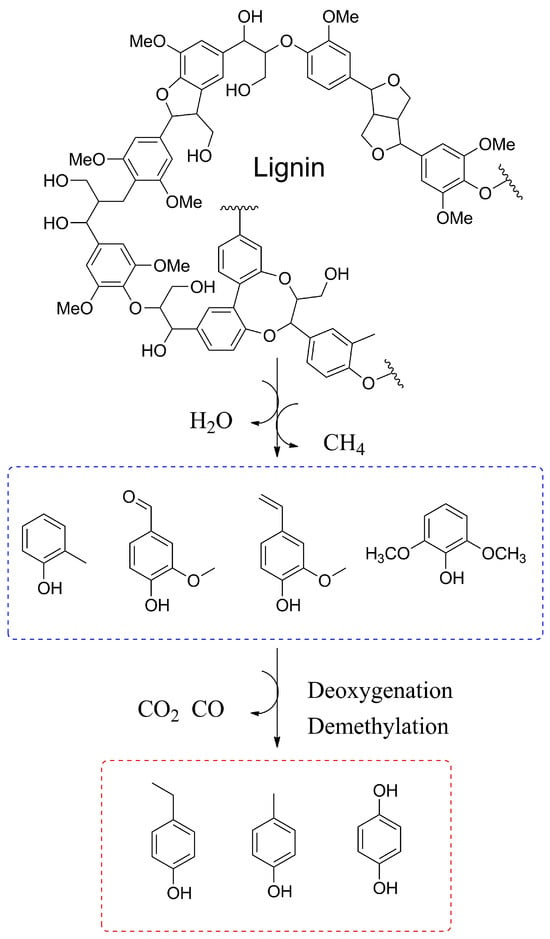
Figure 3.
Possible reaction pathways for the phenols from lignin catalytic fast pyrolysis upgrading over B-AC catalyst.
4. Catalytic Pyrolysis of Lignin
In order to attain effective production and enhancement of bio-oil during the pyrolysis process, researchers have employed catalytic pyrolysis to convert diverse phenolic compounds into phenolic-rich bio-oil. However, the occurrence of free radical reactions during pyrolysis results in a wide array of products [40]. Catalytic pyrolysis offers an alternative approach to obtain phenolic-rich bio-oil by promoting or inhibiting specific reactions [41]. To gain a comprehensive understanding of the structural relationship between catalysts and product distributions, various catalysts have been investigated to examine the impact of catalyst structure on the distribution of products obtained from lignin or tobacco pyrolysis. This section provides a detailed discussion on the performance of these catalysts/additives, including alkali or alkali salts, metal oxides, molecular sieve catalysts, and catalysts based on activated carbon (AC).
4.1. Pyrolysis with Alkali or Alkali Salts
It is evident that the presence of even a small quantity of alkali or alkaline earth metals (AAEMs) in lignin has a significant impact on pyrolysis, resulting in a modification of product distributions [42,43]. Density functional theory (DFT) calculations have revealed the existence of three distinct binding sites for metal ions on guaiacylglycerol-β-guaiacyl ether (GGE) (Figure 4) [44]. Among these binding sites, Model 2, in which the metal ion interacts with O(Cβ) and O(methoxy), emerges as the most probable position due to its lowest energy stabilization of the complex [44]. Experimental findings indicate that the metal cations of AAEMs can interact with the acidic functional groups present in biomass, thereby impeding the polymerization of pyrolysis intermediates through crosslinking of these acidic groups [45]. For instance, Zhou et al. discovered that Ca(OH)2 can react with hydroxyl groups (-OH) on the aromatic rings, resulting in the formation of the aryl-O-Cα structure, consequently hindering the agglomeration of lignin pyrolysis intermediates. Furthermore, the addition of Ca(OH)2 promotes further cracking of the pyrolysis intermediates into phenolic compounds. Brown conducted an investigation on the effect of a series of acetate metals on the fast pyrolysis of lignin [46]. The yield of volatile aromatics from lignin infused with alkaline earth metals was lower compared to that of alkali metals. Sodium, exhibiting higher reactivity than other alkali metals, demonstrated a higher yield of volatile aromatics during lignin fast pyrolysis.
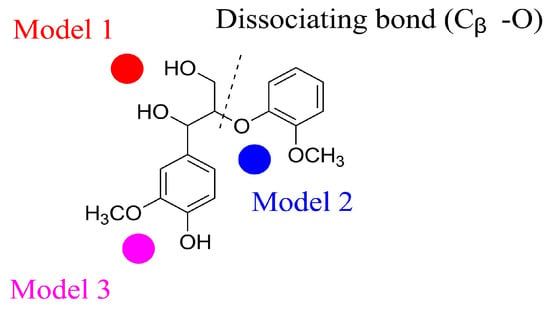
Figure 4.
Binding sites of AAMEs on GEE. Model 1: O(Cα)-M-O(Cγ), Model 2: O(Cβ)-M-O(methoxy), and Model 3: O(hydroxyl)-M-O(methoxy).
Furthermore, the anions associated with AAEMs also play a role in influencing the generation of phenolic compounds by providing varying degrees of basicity or hydrogen donors. Peng et al. conducted a comparative analysis of the effects of several alkalis, including NaOH, KOH, Na2CO3, and K2CO3, on lignin pyrolysis for the production of aromatic chemicals [47]. It was observed that all alkalis facilitated decarboxylation and decarbonylation reactions, as well as the elimination of unsaturated alkyl-branched chains from aromatic chemicals. Hydroxide ions were found to enhance deoxygenation, resulting in a higher yield of phenols devoid of -OCH3 groups, while carbonates predominantly promoted the production of methoxy-phenols. Moreover, during pyrolysis, anions such as HCOO−, HCO3−, HPO42−, and H2PO4− present in AAEMs decomposed to generate hydrogen donors, which stabilized the phenolic intermediates and facilitated the removal of side chains from the phenolic ring [48,49]. Cui et al. demonstrated that the addition of Ca(HCOO)2 promoted the decomposition of alkoxy-phenols into alkyl-phenols, leading to a relatively higher content of phenolic compounds (73.65% vs. 79.86%) [47].
4.2. Pyrolysis with Metal Oxide Catalysts
Metal oxides, characterized by their acid/base properties, have emerged as promising catalysts for the fast pyrolysis of biomass [50]. The basic nature of metal oxides facilitates the removal of oxygenated side chains attached to phenolic rings, thereby promoting the production of phenolic compounds. For instance, Lu et al. investigated various nano metal oxides (such as MgO, CaO, TiO2, Fe2O3, NiO, and ZnO) in the fast pyrolysis process [51]. Among these catalysts, nano NiO demonstrated the highest selectivity towards phenolic compounds through deoxygenation and decarboxylation during the catalytic upgrading of biomass pyrolysis vapors. Nair et al. explored the production of guaiacols through catalytic fast pyrolysis of alkali lignin with TiO2, ZrO2, and CeO2 [52]. The catalysts followed the order of TiO2-300, TiO2-500, TiO2-700 > ZrO2, CeO2 > Aeroxide TiO2 > non-catalytic in terms of guaiacol and its derivative yields. The enhanced production of phenolics can be attributed to the generation of valence band holes, which convert surface hydroxyl groups (-OH) in TiO2 into highly active hydroxyl radicals (·OH) [53]. Furthermore, Wang et al. discovered that activated red mud (consisting of Fe2O3, Al2O3, and TiO2) with hierarchical porosity exhibited excellent catalytic activity in lignin thermal decomposition [54]. The presence of activated red mud promoted the formation of alkyl-phenols and hydrocarbons. These findings highlight the synergistic effects of different metal oxides in facilitating efficient biomass conversion during pyrolysis.
4.3. Pyrolysis with Zeolites Catalysts
Zeolites have demonstrated exceptional catalytic performance owing to their high acidity and unique pore structure [55]. By adjusting the Si/Al mole ratio of HZSM-5, the acidity/basicity of the catalyst can be fine-tuned, subsequently influencing the distribution of products obtained from lignin pyrolysis oil [56]. Figure 5 illustrates that zeolites with a higher SiO2/Al2O3 mole ratio facilitate the cleavage of methoxyl groups, ether bonds, and aliphatic C-C bonds, as well as the dehydration of aliphatic hydroxyl groups. On the other hand, zeolites with a lower SiO2/Al2O3 mole ratio exhibit enhanced effectiveness in the decomposition of carboxylic acids [56]. The formation mechanism of phenolic compounds in the fast pyrolysis of biomass catalyzed by molecular sieves typically involves three distinct processes. Initially, lignin undergoes depolymerization, resulting in the formation of abundant large alkoxy-phenols or dimers, along with a smaller fraction of small alkoxy-phenols. Unfortunately, these alkoxy-phenols and dimers may undergo re-polymerization, leading to the formation of undesired chars. However, in the presence of zeolites, the Brønsted acid sites within the zeolite catalysts promote the cracking, de-alkylation, and dehydration reactions of these large alkoxy-phenols and dimers, facilitating the formation of small alkoxy-phenols. Ultimately, under the catalytic influence of Brønsted acid sites, the small alkoxy-phenols undergo further transformation into alkyl-phenols through promoted C-O cleavage and de-alkoxylation reactions [56].
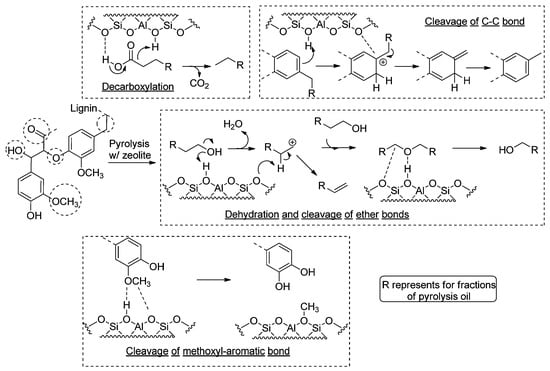
Figure 5.
Primary decomposed functional groups in lignin during pyrolysis.
Modifying the distribution of acid sites and pore structures has been recognized as an effective approach to significantly adjust the composition of pyrolysis products. In the study by Yaman et al., the incorporation of HZSM-5 and SBA-15 catalysts resulted in a reduction of heavier compounds and an increase in guaiacyl-, syringyl-, and catechol-type compounds through the thermal decomposition of lignin [57]. Furthermore, the introduction of Al or Fe metal impregnation into HZSM-5 and SBA-15 catalysts enhanced the deoxygenation of lignin-derived bio-oil, leading to a selective increase in the phenolic content of the bio-oil. Similarly, the addition of a zeolite catalyst has been shown to enhance the quality of bio-oil. For instance, Mulika et al. demonstrated that bio-oil could be upgraded using ferrierite, ZSM-5, Al2O3, Co-Mo/Al2O3, and Mo2C catalysts [58].
4.4. Pyrolysis with Activated Carbon
The utilization of activated carbon (AC) in catalytic fast pyrolysis has gained significant attention due to its extensive porous surface area, adjustable pore structure, surface chemistry, and excellent thermostability at elevated temperatures [59,60,61,62]. Notably, the structure of AC is greatly influenced by the chosen preparation method [63]. Yang et al. demonstrated the favorable catalytic activity of activated carbon in biomass pyrolysis for phenols [64]. The presence of a highly porous structure with acidic surface functionality exhibited remarkable selectivity towards simple phenols and furfurals. Furthermore, Chen et al. reported that N-doped AC also facilitated the formation of phenolic compounds during the catalytic fast pyrolysis of bamboo waste. Moreover, Lu et al. discovered that N-AC promoted the production of alkylphenols [65]. The potential formation mechanism of alkylphenols is depicted in Figure 6. Initially, the N-functional groups, such as pyridinic-N and pyrrolic-N, present in the N-doped AC adsorb the lignin molecule through the Lewis-base active sites on the surface. This is followed by the cleavage of ether bonds to yield phenolic monomers, which occurs due to electron-transfer or charge-transfer interactions between the adsorbed lignin and the catalyst. Subsequently, the active N-containing groups catalyze the conversion of these adsorbed products, leading to the formation of alkylphenols and aromatic hydrocarbons.
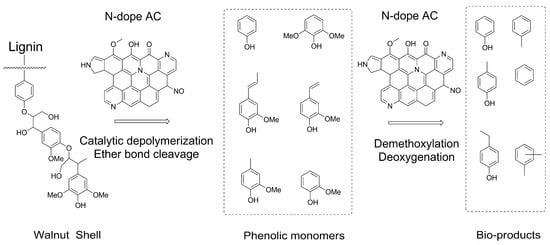
Figure 6.
The scheme of N-dope AC catalyzed lignin depolymerization for phenolic compounds.
5. Microwave-Assisted Pyrolysis of Lignin
Microwave pyrolysis (MP) has emerged as a highly promising method for the conversion of biomass into valuable biofuels, including biochar, bio-oil, and syngas [66], which is shown in Figure 7. The utilization of microwave technology in biomass pyrolysis enables the achievement of lower pyrolysis temperatures and induces distinct changes in product chemistry when compared to conventional methods [67]. This study on microwave pyrolysis is the first to unequivocally establish that the heating rate plays a crucial enabling role. As was shown in Figure 8, selective microwave-biomass pyrolysis is feasible, resulting in significantly higher quality and intrinsic value compared to conventional bio-oils. This presents an opportunity to selectively pyrolyze hemicellulose and cellulose instead of lignin, leading to the production of bio-oils with a narrower range of products and the absence of phenolic compounds. Looking from another perspective, the development of selective microwave-biomass pyrolysis technology holds great potential for the production of lignin-based chemicals with high quality from tobacco stem waste. This presents a new opportunity for the pyrolysis process to yield valuable outputs from tobacco waste.
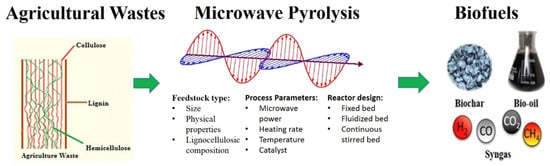
Figure 7.
Scheme for microwave pyrolysis (MP) from biomass to biofuels, adopted from Ref. [66]. The red arrows represent Electric Field Intensity and the blue arrows represent Magnetics Field Intensity.
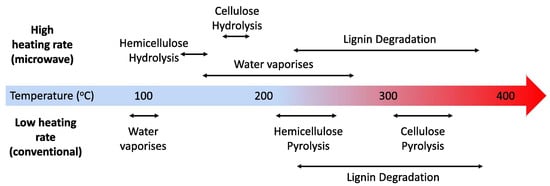
Figure 8.
Scheme for microwave and conventional pyrolysis, adopted from Ref. [67].
The microwave-assisted pyrolysis of lignin (MAP) has also been garnering increasing interest among researchers, including MAP of lignin without oxygen, under high temperatures, and with or without catalysts [68]. Farag et al. investigated the MAP of kraft lignin at different conditions, and up to 80% of the carbon atoms in the oil phase were aromatic carbons by the detailed compositional analysis [69]. Bu et al. also conducted the MAP of alkali lignin with activated carbons as catalyst [70]. The predominant chemical compounds found in bio-oils were phenols, guaiacols, hydrocarbons, and esters, constituting a significant portion ranging from 71% to 87% of the bio-oils, depending on various reaction conditions. Notably, bio-oils with notably high concentrations of phenol (45% in the bio-oil) were successfully obtained.
Frediani et al. investigated the MAP of kraft lignin using a multimode microwave oven with carbon as a microwave absorber, which was carried out at reduced pressure with a fractionating system (Figure 9) [71]. Two experimental configurations were employed, as illustrated in Figure 1. In both setups, samples were housed in a 1000 mL borosilicate Erlenmeyer flask within the oven and linked to two condensing systems cooled at 298 K and 263 K, respectively. The resulting liquids were collected in a flask, while gases were stored in a gas holder. The most significant findings were obtained when the MAP of kraft lignin was conducted at a residual pressure of 0.013 kPa. In this condition, a bio-oil yield of 37 wt% was achieved within a duration of 9 min. Additionally, Divine et al. developed a novel reactor system that consisted of a high-Q cylindrical microwave resonant cavity and a specially designed quartz reactor. This system enabled the investigation of the continuous mass loss kinetics of Kraft lignin pellets during pyrolysis, within the temperature range of 300–700 °C [72].
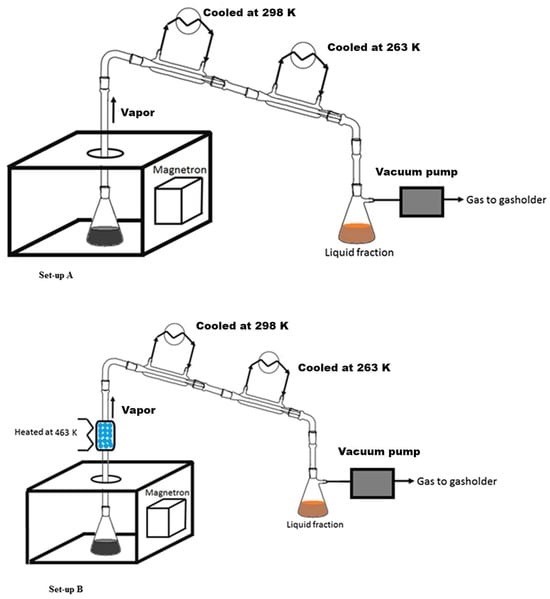
Figure 9.
Experimental set-up used to carry out the MAP at reduce residual pressure, adopted from Ref. [71].
6. Solar-Assisted Pyrolysis of Lignin
The underlying principles of solar pyrolysis and autothermal pyrolysis are highly comparable. However, in contrast to conventional autothermal pyrolysis setups that rely on external electric heaters or burning fuels to supply heat to the reactor, solar pyrolysis systems utilize concentrated solar radiation to directly heat the reactor [73]. Solar-assisted pyrolysis of biomass refers to the thermal decomposition of feedstocks in an inert environment, devoid of any oxidizing agent. This process entails a series of intricate exothermic and endothermic reactions. A schematic diagram of solar pyrolysis process with common feedstocks and their products is shown in Figure 10 [74]. Notably, the combination of solar assisted torrefaction and pyrolysis can effectively mitigate the presence of acidic compounds in bio-oil [75], and the formation of phenols and anhydrosugars was promoted if biomass was pretreated with aqueous phase bio-oil washing [76]. Additionally, Li et al. carried out solar pyrolysis experiments on pine sawdust, peach pit, grape stalk, and grape marc to investigate their potential for fuel gas production as valuable agricultural and forestry by-products in a laboratory-scale solar reactor [77]. Their findings indicated that the gas yield generally exhibits a positive correlation with both temperature and heating rate for different biomass residues. Conversely, the liquid yield showed an inverse relationship with these parameters.

Figure 10.
Schematic of solar pyrolysis processes with carbonaceous feedstocks and process products, adopted from Ref. [74].
For biomass, such as tobacco stem waste, the concept of lignin-first presents a promising avenue for the efficient utilization of lignocellulosic biomass in its entirety. Nevertheless, existing conversion approaches heavily rely on high-temperature hydrogenolysis facilitated by supported metal catalysts, resulting in the production of low-functionalized products or posing challenges in the separation of the solid catalyst from cellulose/hemicellulose components [78]. Notably, Wu et al. presented their outstanding findings on the fractionation and valorization of lignocellulose through solar energy-driven conversion of native lignin at ambient conditions, as shown in Figure 11 [79]. Interestingly, their system exhibited distinct mechanistic characteristics compared to the previously studied two-step photocatalytic systems, where it was established that the cleavage of β-O-4 bonds in lignin occurs through the pre-oxidation of Cα-OH to Cα=O [80,81,82]. They have discovered that the cleavage of the β-O-4 bond followed a photoredox electron-hole coupled (EHCO) mechanism. This mechanism involves the active participation of both photogenerated electrons and holes in the formation of products. Notably, a Cα radical is generated as an intermediate during the photochemical process, displaying a significantly reduced bond dissociation energy (BDE) in the β-O-4 bond (Figure 12).
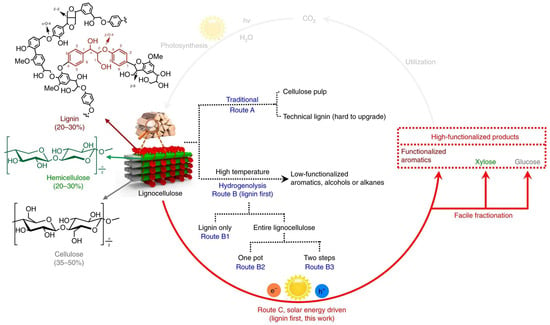
Figure 11.
Routes for the valorization of lignocellulosic biomass. The β-O-4 linkage is highlighted in the representative lignin fragment. Route A, traditional biorefinery leading to cellulose pulp and technical lignin. Route B, lignin-first approach with high-temperature hydrogenolysis by a supported metal catalyst for the conversion of native lignin. Route C, lignin-first approach with solar energy-driven photocatalytic conversion of lignin under mild conditions. Adopted from Ref. [79].
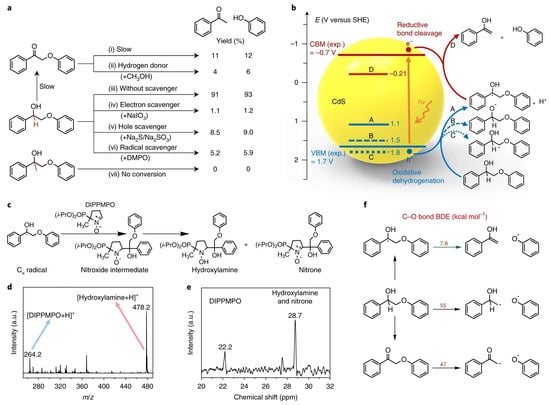
Figure 12.
Mechanism for solar energy-driven cleavage of β-O-4 bond. (a) Control experiments with different reactants or additives catalyzed by CdS QD−4.4 nm under visible light. Hydrogen donor in PP-one control reaction, CH3OH 0.5 mL. Scavenger concentrations: 2.0 mM NaIO3; 4.0 mM Na2S + 2.0 mM Na2SO3; 3.0 mM DMPO. Reaction time: 3 h. (b) Potentials of oxidative dehydrogenation of PP-ol via three different paths (A, B, C) and potential of reductive cleavage of β-O-4 bond in the Cα radical intermediate (D) against the positions of VBM and CBM of CdS. Experimental values of VBM and CBM were obtained through UV–visible and electrochemical measurements of bulk CdS. (c) Spin trapping reaction of Cα radical with DIPPMPO. (d) Positive-ion electrospray ionization mass spectrum. (e) 31P NMR spectrum of product mixture. The reaction was performed under standard conditions except for adding 10 mM DIPPMPO. (f) Comparison of BDE of β-O-4 bond in Cα radical with those in PP-ol and PP-one. Adopted from Ref. [79].
7. Summary and Outlook
The conversion of lignin derived from tobacco stem waste into high-value chemicals through pyrolysis has garnered increasing attention among researchers. Substantial progress has been achieved in the realms of thermal pyrolysis of lignin, microwave-assisted pyrolysis, and solar-assisted pyrolysis. Research efforts have been dedicated to comprehending the impact of biomass composition, catalyst properties, and various technologies on product distribution. The synthesis of diverse phenolic compounds with distinct substituents necessitates catalysts possessing versatile catalytic capabilities, encompassing hydrogenation, alkylation, decarbonylation, and demethylation. The acid/base active sites inherent in these catalysts play a pivotal role in the biomass pyrolysis process. These active sites not only lower the activation energy, but also impede the polymerization of pyrolysis intermediates. Moreover, the effective stabilization of precursors of phenolic compound can be achieved through the application of hydrogen donors. To enhance yields and selectivity of phenolic compounds, researchers must investigate the synergistic effects between the active sites on the catalyst surface and pyrolysis intermediate products. Such advancements would establish a robust theoretical foundation for the efficient conversion of lignin from tobacco stem waste into high-value chemical products.
Author Contributions
Conceptualization, Z.T., R.Z. and R.C.; literature review, J.L. and Y.L.; writing—original draft preparation, Z.T., Y.L. and J.Z.; writing—review and editing, R.C. and F.C.; revisions and final editing, R.Z., L.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21902053) and Open Funding Project of the State Key Laboratory of Biocatalysis and Enzyme Engineering (No. SKLBEE20220018).
Data Availability Statement
Data are contained within the article.
Acknowledgments
All authors are thankful to their representative universities/institutes for literature services.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, H.; Liu, Q.; Ma, J.; Liu, L.; Qu, Y.; Gong, Y.; Yang, S.; Luo, T. Heavy metal(loids) in typical chinese tobacco-growing soils: Concentrations, influence factors and potential health risks. Chemosphere 2020, 245, 125591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, J.; Liu, G.; Yang, H.; Liu, W.; Wang, L.; Kong, C.; Zheng, D.; Yang, J.; Deng, L.; et al. Co-digestion of tobacco waste with different agricultural biomass feedstocks and the inhibition of tobacco viruses by anaerobic digestion. Bioresour. Technol. 2015, 189, 210–216. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Jin, S.; Lin, Y.; Yang, H. Catalytic pyrolysis of tobacco rob: Kinetic study and fuel gas produced. Bioresour. Technol. 2011, 102, 11027–11033. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent-A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Calabuig, E.; Marcilla, A. Effect of a mesoporous catalyst on the flash pyrolysis of tobacco. Thermochim. Acta 2021, 705, 179032. [Google Scholar] [CrossRef]
- Liu, H.; Deng, Y.; Xie, C.; Zhu, H. Experimental study on pyrolysis characteristics of the tobacco stem based on microwave heating method. Appl. Therm. Eng. 2016, 106, 473–479. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, F.; Liu, H.; Hu, J.; Yang, S.; Wang, H. Comprehensive investigation on the slow pyrolysis product characteristics of waste tobacco stem: Pyrolysis reaction mechanism and conversion mechanism of N. Fuel 2023, 350, 128902. [Google Scholar] [CrossRef]
- Hu, J.; Wu, S.; Jiang, X.; Xiao, R. Structure-reactivity relationship in fast pyrolysis of lignin into monomeric phenolic compounds. Energy Fuels 2018, 32, 1843–1850. [Google Scholar] [CrossRef]
- Guo, G.; Liu, X.; Li, R.; Li, Q.; Yu, H.; Li, M. Characterization of tobacco stalk lignin using nuclear magnetic resonance spectrometry and its pyrolysis behavior at different temperatures. J. Anal. Appl. Pyrolysis 2019, 142, 104665. [Google Scholar] [CrossRef]
- Bai, X.; Kim, K.H.; Brown, R.C.; Dalluge, E.; Hutchinson, C.; Lee, Y.J.; Dalluge, D. Formation of phenolic oligomers during fast pyrolysis of lignin. Fuel 2014, 128, 170–179. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Li, J.; Guo, L.; Yang, H.; Ma, F.; Yu, H. Comparative study of the fast pyrolysis behavior of ginkgo, poplar, and wheat straw lignin at different temperatures. Ind. Crops Prod. 2018, 122, 465–472. [Google Scholar] [CrossRef]
- Bayerbach, R.; Meier, D. Characterization of the water-insoluble fraction from fast pyrolysis liquids (pyrolytic lignin). Part IV: Structure elucidation of oligomeric molecules. J. Anal. Appl. Pyrolysis 2009, 85, 98–107. [Google Scholar] [CrossRef]
- Carpenter, D.; Westover, T.; Czernik, S.; Jablonski, W. Biomass feedstocks for renewable fuel production: A review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16, 384–406. [Google Scholar] [CrossRef]
- Mohan, D.; Charles, U.; Pittman, J.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Qiu, B.; Yang, C.; Shao, Q.; Liu, Y.; Chu, H. Recent advances on industrial solid waste catalysts for improving the quality of bio-oil from biomass catalytic cracking: A review. Fuel 2022, 315, 123218. [Google Scholar] [CrossRef]
- Faix, O.; Bremer, J.; Meier, D.; Fortmann, I.; Scheijen, M.; Boon, J. Characterization of tobacco lignin by analytical pyrolysis and Fourier transform-infrared spectroscopy. J. Anal. Appl. Pyrolysis 1992, 22, 239–259. [Google Scholar] [CrossRef]
- Kibet, J.; Khachatryan, L.; Dellinger, B. Phenols from pyrolysis and co-pyrolysis of tobacco biomass components. Chemosphere 2015, 138, 259–265. [Google Scholar] [CrossRef]
- Muzyka, R.; Chrubasik, M.; Dudziak, M.; Ouadi, M.; Sajdak, M. Pyrolysis of tobacco waste: A comparative study between Py-GC/MS and fixed-bed reactors. J. Anal. Appl. Pyrolysis 2022, 167, 105702. [Google Scholar] [CrossRef]
- Elder, T.; Beste, A. Density functional theory study of the concerted pyrolysis mechanism for lignin models. Energy Fuels 2014, 28, 5229–5235. [Google Scholar] [CrossRef]
- Huang, J.; He, C. Pyrolysis mechanism of α-O-4 linkage lignin dimer: A theoretical study. J. Anal. Appl. Pyrolysis 2015, 113, 655–664. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.; Zhu, Y.; Wen, W.; Pan, Y.; Wu, J.; Wu, J. Pyrolysis mechanism study of lignin model compounds by synchrotron vacuum ultraviolet photoionization mass spectrometry. Energy Fuels 2016, 30, 2204–2208. [Google Scholar] [CrossRef]
- Chu, S.; Subrahmanyam, A.; Huber, G. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound. Green Chem. 2013, 15, 125–136. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Q.; Hu, B.; Chen, D.; Liu, J.; Dong, C. Influence of inherent alkali metal chlorides on pyrolysis mechanism of a lignin model dimer based on DFT study. J. Therm. Anal. Calorim. 2019, 137, 151–160. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Lee, D.H. Preparation of nano-NiO particles and evaluation of their catalytic activity in pyrolyzing biomass components. Energy Fuels 2008, 22, 16–23. [Google Scholar] [CrossRef]
- Singh, S.; Nandeshwar, K.; Ekhe, J. Thermochemical lignin depolymerization and conversion to aromatics in subcritical methanol: Effects of catalytic conditions. New J. Chem. 2016, 40, 3677–3685. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T. Catalytic pyrolysis of coffee grounds using NiCu-impregnated catalysts. Energy Fuels 2014, 28, 228–235. [Google Scholar] [CrossRef]
- Anukam, A.; Jonas, B. Biomass pretreatment and characterization: A review. In Biotechnological Applications of Biomass; IntechOpen: London, UK, 2021; pp. 1–17. [Google Scholar]
- Zou, Q.; Lin, W.; Xu, D.; Wu, S.; Mondal, A.K.; Huang, F. Study the effect of zeolite pore size and acidity on the catalytic pyrolysis of Kraft lignin. Fuel Process. Technol. 2022, 237, 107467. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Jiang, H.; Yu, H. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, H.; Park, J.; Oh, S.; Choi, J.W. Predicting structural change of lignin macromolecules before and after heat treatment using the pyrolysis-GC/MS technique. J. Anal. Appl. Pyrolysis 2014, 110, 305–312. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, Q.; Ye, J.; Yao, Q.; Zhao, C. Study on the thermal degradation behaviors and kinetics of alkali lignin for production of phenolic-rich bio-oil using TGA-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2016, 117, 116–124. [Google Scholar] [CrossRef]
- Shen, D.; Liu, G.; Zhao, J.; Xue, J.; Guan, S.; Xiao, R. Thermo-chemical conversion of lignin to aromatic compounds: Effect of lignin source and reaction temperature. J. Anal. Appl. Pyrolysis 2015, 112, 56–65. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhong, D.; Zeng, K.; Li, J.; Flamant, G.; Nzihou, A.; Yang, H.; Chen, H. Identifying at molecular scale the pyrolysis heavy components from two lignin monomers. Fuel 2022, 328, 125333–125343. [Google Scholar] [CrossRef]
- Kawamoto, H.; Horigoshi, S.; Saka, S. Effects of side-chain hydroxyl groups on pyrolytic β-ether cleavage of phenolic lignin model dimer. J. Wood Sci. 2007, 53, 268–271. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst deactivation and its mitigation during catalytic conversions of biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, K.B. Production of phenols by lignocellulosic biomass pyrolysis. In Production of Biofuels and Chemicals with Pyrolysis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 289–319. [Google Scholar]
- Li, W.; Wang, D.; Zhu, Y.; Chen, J.; Lu, Y.; Li, S.; Zheng, Y.; Zheng, Z. Efficient ex-situ catalytic upgrading of biomass pyrolysis vapors to produce methylfurans and phenol over bio-based activated carbon. Biomass Bioenergy 2020, 142, 105794–105804. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Wu, D.; Tong, H.; Ren, L. Density functional theory studies on pyrolysis mechanism of β-O-4 type lignin dimer model compound. J. Anal. Appl. Pyrolysis 2014, 109, 98–108. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Z.; Xie, W.; Liu, J.; Li, Y.; Zhang, W.; Fu, H.; Lu, Q. Advances on the fast pyrolysis of biomass for the selective preparation of phenolic compounds. Fuel Process. Technol. 2022, 237, 107465–107482. [Google Scholar] [CrossRef]
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of alkali and alkaline earth metals on in-situ catalytic fast pyrolysis of lignocellulosic biomass: A microreactor study. Energy Fuels 2016, 30, 3045–3056. [Google Scholar] [CrossRef]
- Eom, I.Y.; Kim, K.H.; Kim, J.Y.; Lee, S.M.; Yeo, H.M.; Choi, I.G.; Choi, J.W. Characterization of primary thermal degradation features of lignocellulosic biomass after removal of inorganic metals by diverse solvents. Bioresour. Technol. 2011, 102, 3437–3444. [Google Scholar] [CrossRef]
- Kim, K.H.; Jeong, K.; Kim, S.S.; Brown, R.C. Kinetic understanding of the effect of Na and Mg on pyrolytic behavior of lignin using a distributed activation energy model and density functional theory modeling. Green Chem. 2019, 21, 1099–1107. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Chang, J. Study on the product characteristics of pyrolysis lignin with calcium salt additives. Materials 2019, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Dalluge, D.L.; Kim, K.H.; Brown, R.C.J. The influence of alkali and alkaline earth metals on char and volatile aromatics from fast pyrolysis of lignin. J. Anal. Appl. Pyrolysis 2017, 127, 385–393. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of lignin for phenols with alkaline additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Bolatibieke, D.; Nan, D.; Lu, Q. Pyrolysis of biomass impregnated with ammonium dihydrogen phosphate for polygeneration of phenol and supercapacitor electrode material. Front. Chem. 2020, 8, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, W.; Yu, Y.; Chang, J.; Cai, L.; Shi, S. Adding nickel formate in alkali lignin to increase contents of alkylphenols and aromatics during fast pyrolysis. Bioresour. Technol. 2017, 227, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Naron, D.R.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. Influence of impregnated catalyst on the phenols production from pyrolysis of hardwood, softwood, and herbaceous lignins. Ind. Crop. Prod. 2019, 131, 348–356. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.; Dong, C.; Zhu, X. Catalytic upgrading of biomass fast pyrolysis vapors with nano metal oxides: An analytical Py-GC/MS study. Energies 2010, 3, 1805–1820. [Google Scholar] [CrossRef]
- Nair, V.; Vinu, R. Production of guaiacols via catalytic fast pyrolysis of alkali lignin using titania, zirconia and ceria. J. Anal. Appl. Pyrolysis 2016, 119, 31–39. [Google Scholar] [CrossRef]
- Mizuguchi, J.; Tsukada, Y.; Takahashi, H. Recovery and characterization of reinforcing fibers from fiber reinforced plastics by thermal activation of oxide semiconductors. Mater. Trans. 2013, 54, 384–391. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Bai, X.; Yi, W.; Fu, P. Catalytic pyrolysis of lignin with red mud derived hierarchical porous catalyst for alkyl-phenols and hydrocarbons production. J. Anal. Appl. Pyrolysis 2018, 136, 8–17. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Influence of Si/Al ratio of ZSM-5 zeolite on the properties of lignin pyrolysis products. ACS Sustain. Chem. Eng. 2013, 1, 316–324. [Google Scholar] [CrossRef]
- Yaman, E.; Yargic, A.S.; Ozbay, N.; Uzun, B.B.; Kalogiannis, K.G.; Stefanidis, S.D.; Pachatouridou, E.P.; Iliopoulou, E.F.; Lappas, A.A. Catalytic upgrading of pyrolysis vapours: Effect of catalyst support and metal type on phenolic content of bio-oil. J. Clean. Prod. 2018, 185, 52–61. [Google Scholar] [CrossRef]
- Mulika, S.; Duangchan, A. Upgrading of bio-oil derived from tobacco using ferrierite, ZSM-5 and Co-Mo/Al2O3 catalysts. Eng. Appl. Sci. 2015, 42, 101–106. [Google Scholar]
- Sun, K.; Huang, Q.; Ali, M.; Chi, Y.; Yan, J. Producing aromatic-enriched oil from mixed plastics using activated biochar as catalyst. Energy Fuels 2018, 32, 5471–5479. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Jandosov, J.M.; Kerimkulova, A.R.; Azat, S.; Zhubanova, A.A.; Digel, I.E.; Savitskaya, I.S.; Akimbekov, N.S.; Kistaubaeva, A.S. Nanostructured carbon materials for biomedical use. Eurasian Chem.-Technol. J. 2013, 15, 209–217. [Google Scholar] [CrossRef]
- Azat, S.; Busquets, R.; Pavlenko, V.V.; Kerimkulova, A.R.; Whitby, R.L.; Mansurov, Z.A. Applications of activated carbon sorbents based on greek walnut. Appl. Mech. Mater. 2014, 467, 49–51. [Google Scholar] [CrossRef]
- Kerimkulova, A.R.; Azat, S.; Velasco, L.; Mansurov, Z.A.; Lodewyckx, P.; Tulepov, M.I.; Kerimkulova, M.R.; Berezovskaya, I.; Imangazy, A. Granular rice husk-based sorbents for sorption of vapors of organic and inorganic matters. J. Chem. Technol. Metall. 2019, 54, 578–584. [Google Scholar]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Yang, Z.; Lei, H.; Zhang, Y.; Qian, K.; Villota, E.; Qian, M.; Yadavalli, G.; Sun, H. Production of renewable alkyl-phenols from catalytic pyrolysis of Douglas fir sawdust over biomass-derived activated carbons. Appl. Energy 2018, 220, 426–436. [Google Scholar] [CrossRef]
- Ma, S.; Li, H.; Zhang, G.; Iqbal, T.; Li, K.; Lu, Q. Catalytic fast pyrolysis of walnut shell for alkylphenols production with nitrogen-doped activated carbon catalyst. Front. Environ. Sci. Eng. 2020, 15, 25. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.Y.; Cheng, Y.W.; Xia, C.; Mahari, W.A.W.; Liew, R.K.; Peng, W.; Yuan, T.Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Robinson, J.; Binner, E.; Vallejo, D.B.; Perez, N.D.; Mughairi, K.A.; Ryan, J.; Shepherd, B.; Adam, M.; Budarin, V.; Fan, J.; et al. Unravelling the mechanisms of microwave pyrolysis of biomass. Chem. Eng. J. 2022, 430, 132975. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, L.L.; Fan, L.L.; Shan, S.Q.; Liu, Y. Review of microwave-assisted lignin conversion for renewable fuels and chemicals. J. Anal. Appl. Pyrolysis 2016, 119, 104–113. [Google Scholar]
- Farag, S.; Fu, D.; Jessop, P.G.; Chaouki, J. Detailed compositional analysis and structural investigation of a bio-oil from microwave pyrolysis of kraft lignin. J. Anal. Appl. Pyrolysis 2014, 199, 249–257. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yadavalli, G.; Tang, J. Bio-based phenols and fuel production from catalytic microwave pyrolysis of lignin by activated carbons. Bioresour. Technol. 2014, 162, 142–147. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Frediani, P.; Frediani, M. Bio-oils from microwave assisted pyrolysis of kraft lignin operating at reduced residual pressure. Fuel 2020, 278, 118175. [Google Scholar] [CrossRef]
- Nde, D.B.; Muley, P.D.; Sabliov, C.M.; Nokes, S.E.; Boldor, D. Microwave assisted pyrolysis of Kraft lignin in single mode high-Q resonant cavities: Degradation kinetics, product chemical composition, and numerical modeling. Energy Convers. Manag. 2021, 230, 113754. [Google Scholar] [CrossRef]
- Rahman, M.A.; Aziz, M.A. Solar pyrolysis of scrap tire: Optimization of operating parameters. J. Mater. Cycles Waste Manag. 2018, 20, 1207–1215. [Google Scholar] [CrossRef]
- Rahman, M.A.; Parvej, A.M.; Aziz, M.A. Concentrating technologies with reactor integration and effect of process variables on solar assisted pyrolysis: A critical review. Therm. Sci. Eng. Prog. 2021, 25, 100957. [Google Scholar] [CrossRef]
- Parvej, A.M.; Rahman, M.A.; Reza, K.M.A. The combined effect of solar assisted torrefaction and pyrolysis on the production of valuable chemicals obtained from water hyacinth biomass. Clean. Waste Syst. 2022, 3, 100027. [Google Scholar] [CrossRef]
- Chen, D.Y.; Cen, K.H.; Cao, X.B.; Zhang, J.; Chen, F.; Zhou, J. Upgrading of bio-oil via solar pyrolysis of the biomass pretreated with aqueous phase bio-oil washing, solar drying, and solar torrefaction. Bioresour. Technol. 2020, 305, 123130. [Google Scholar] [CrossRef]
- Li, R.; Zeng, K.; Soria, J.; Mazza, G.; Gauthier, D.; Rodriguez, R.; Flamant, G. Product distribution from solar pyrolysis of agricultural and forestry biomass residues. Renew. Energy 2016, 89, 27–35. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Lancefeld, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of functionalized phenolic monomers through selective oxidation and C–O bond cleavage of the β-O-4 linkages in lignin. Angew. Chem. Int. Ed. 2015, 54, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Wang, M.; Li, H.; Zhang, J.; Hou, T.; Chen, H.; Zhang, X.; Lu, J.; Wang, F. Visible-light-driven self-hydrogen transfer hydrogenolysis of lignin models and extracts into phenolic products. ACS Catal. 2017, 7, 4571–4580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).