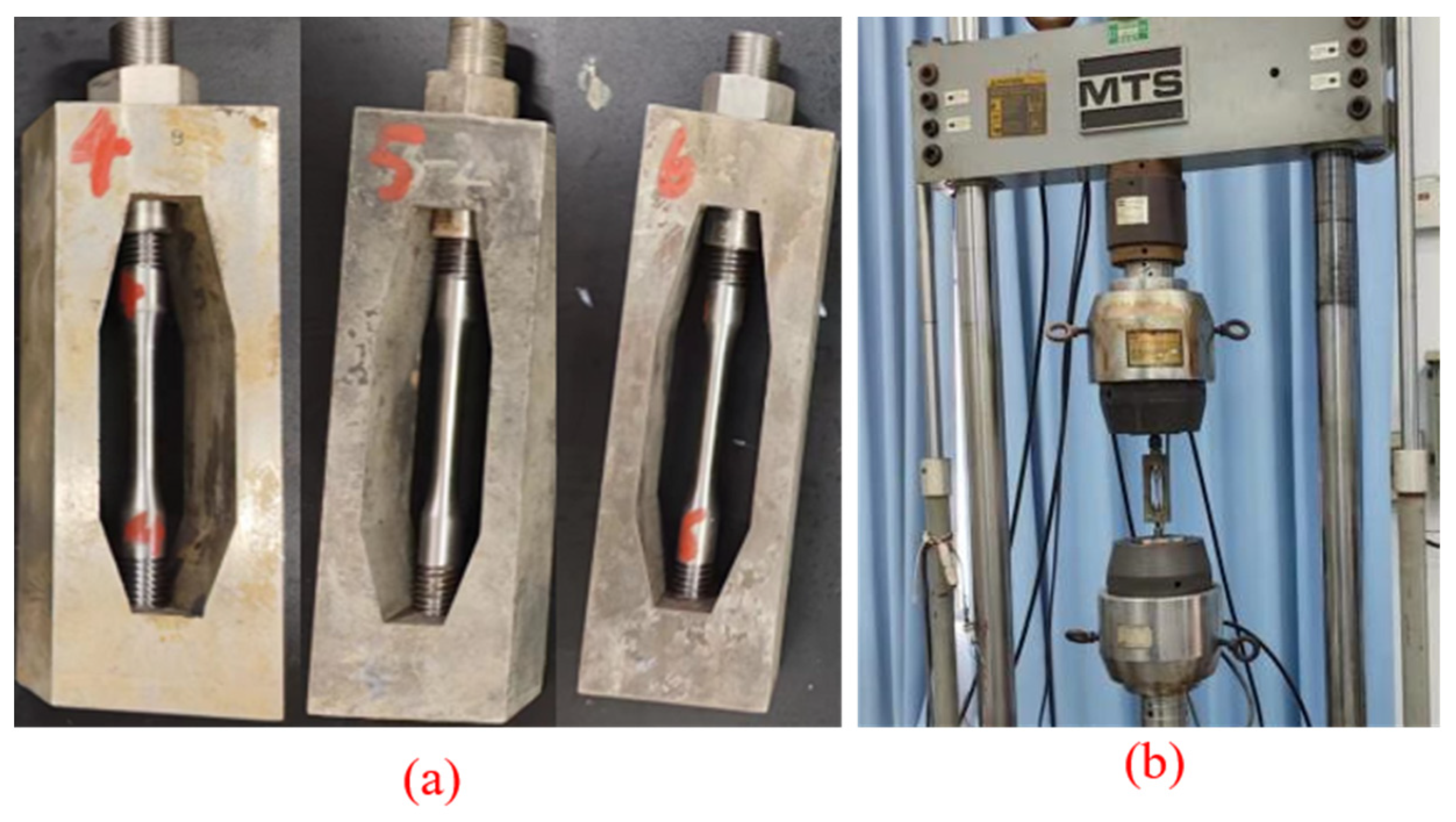
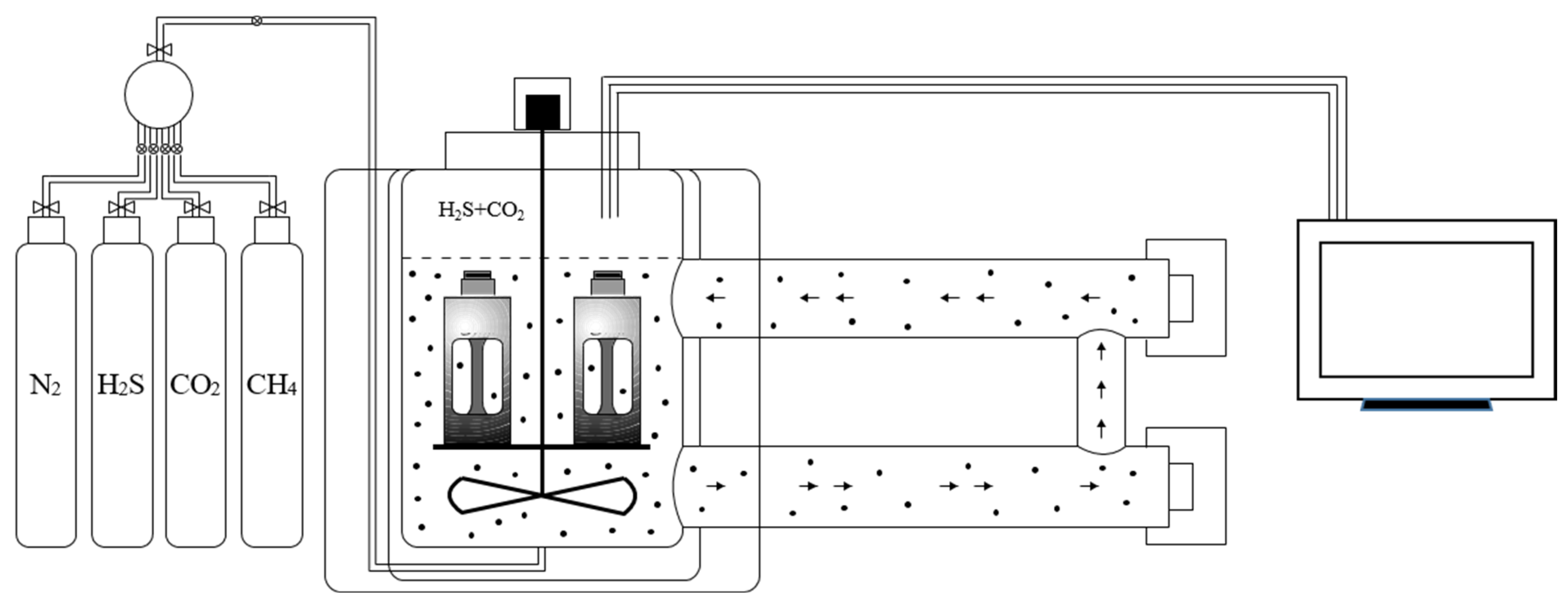
3.1. Analysis of Corrosion-Morphology Characteristics
- (1)
Analysis of corrosion-morphology characteristics of G105 material
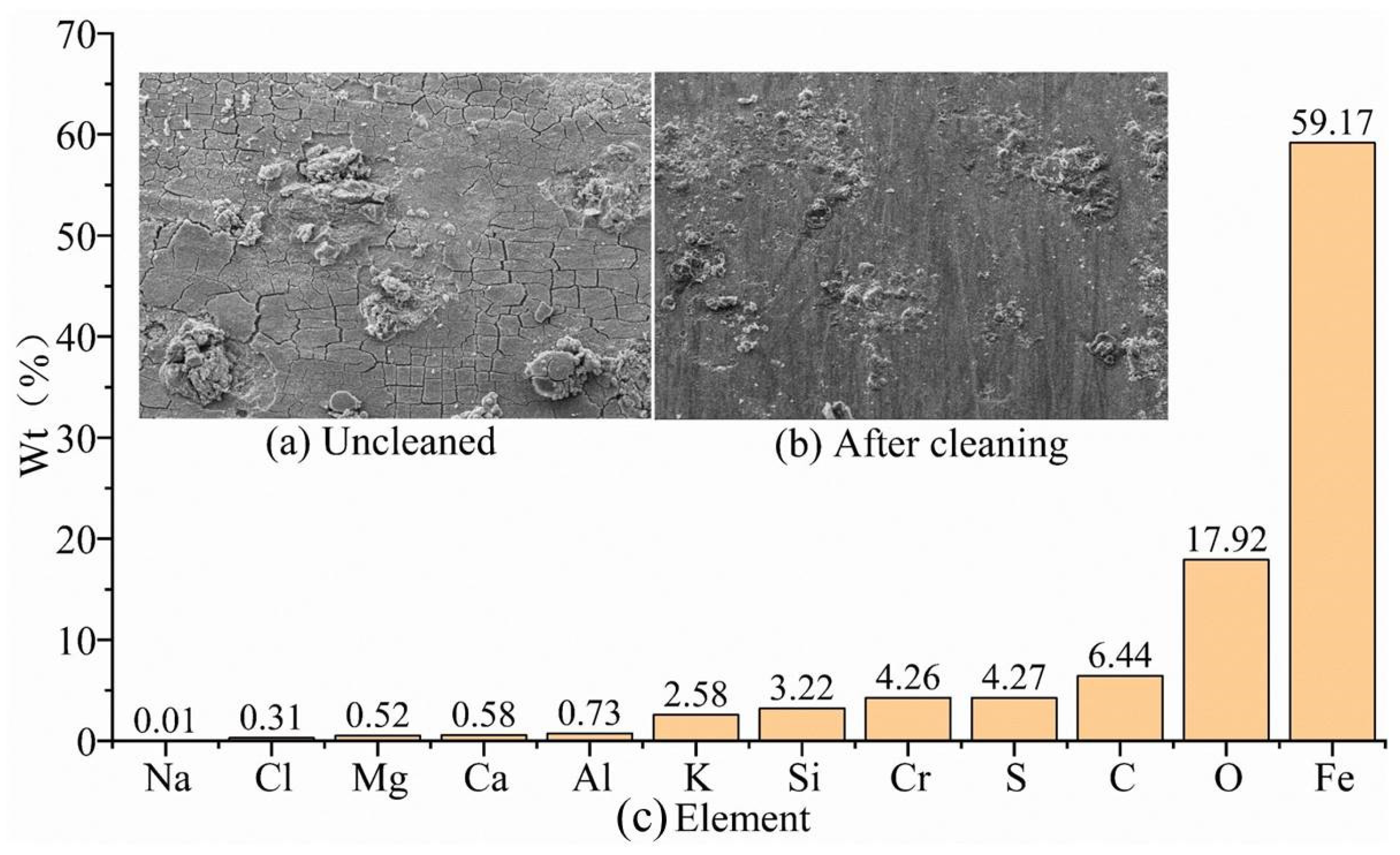
We use a scanning electron microscopy to observe the G105 drill pipe material in corrosive environments at the wellhead, well-middle, and bottomhole levels, and to conduct SEM-morphology analysis of the corrosion products of the uncleaned sample and the cleaned specimen. The mass fractions of each element are obtained by EDS spectroscopy analysis of the corrosion-product components.
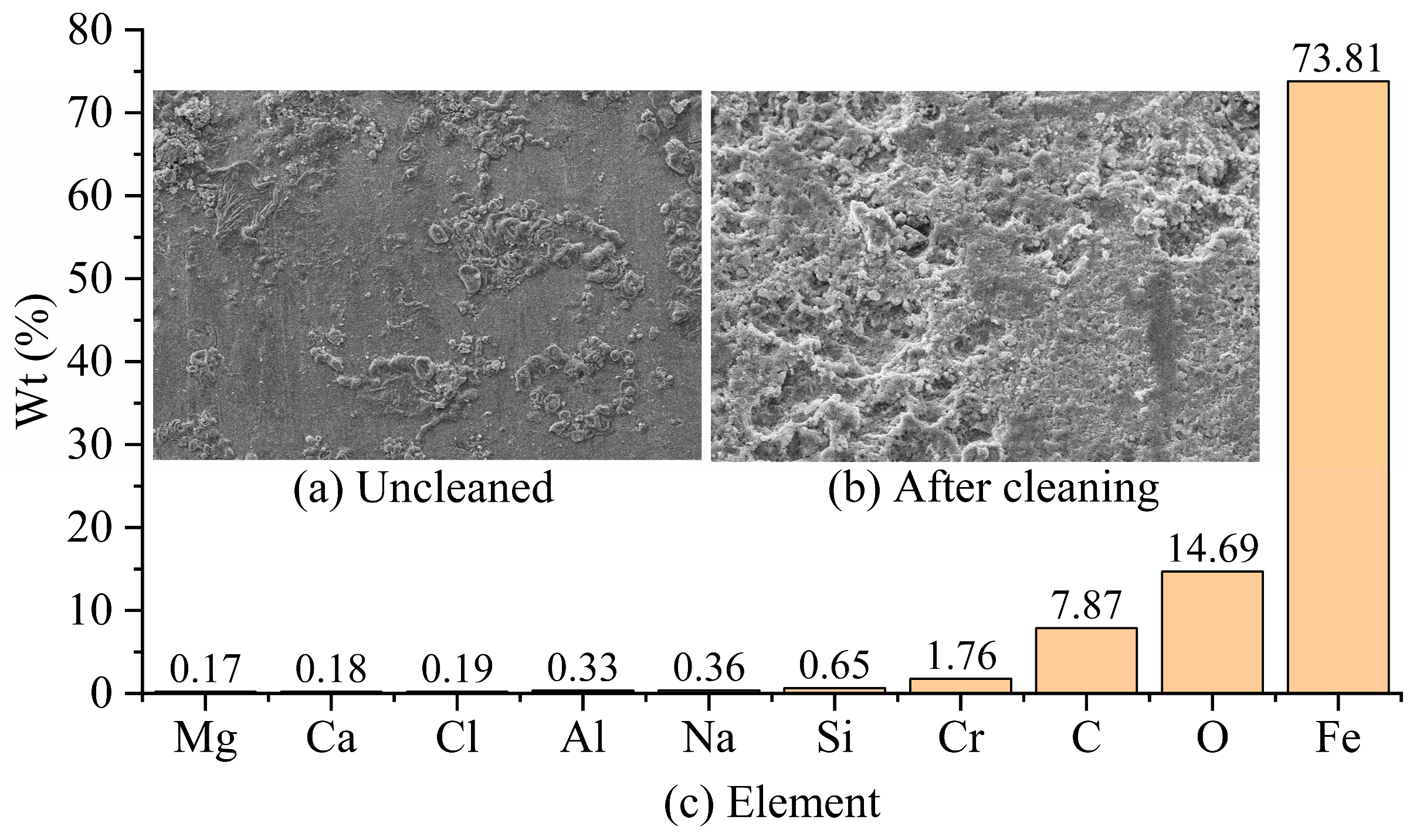
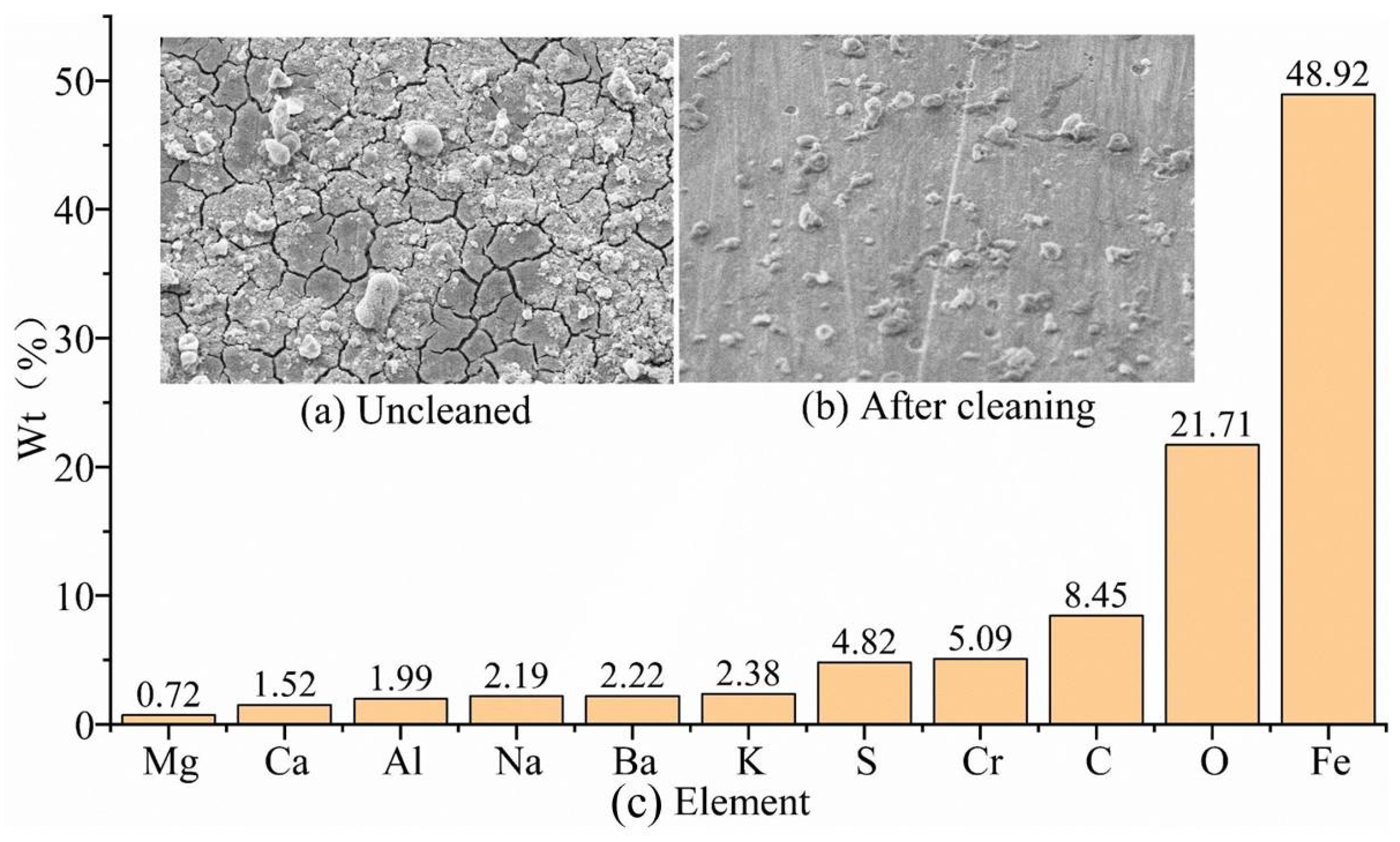
The corrosion-product morphology of the G105-material sample in the simulated wellhead location corrosion environment without cleaning is shown in
Figure 3a. It can be seen that the corrosion product is dense and uneven in thickness, with the existence of micro-cracks in local areas. The corrosion morphology of the G105-material specimen after cleaning is shown in
Figure 3b, and the sample shows serious uniform corrosion. In addition to the chemical composition of the G105-material drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 3c. The main elements of the corrosion products of the G105 material after corrosion are C, O, Fe, and Cr, with a small amount of Si, Na, Al, Cl, Ca, and Mg elements. Fe, Cr, Si, and Al elements come from the metal matrix, while the other elements from the drilling fluid and CO
2 gas, mainly resulting from CO
2 corrosion. However, due to the lower temperature and pressure at the wellhead, the corrosion products are loose and do not easily form a film, leading to intensified corrosion.
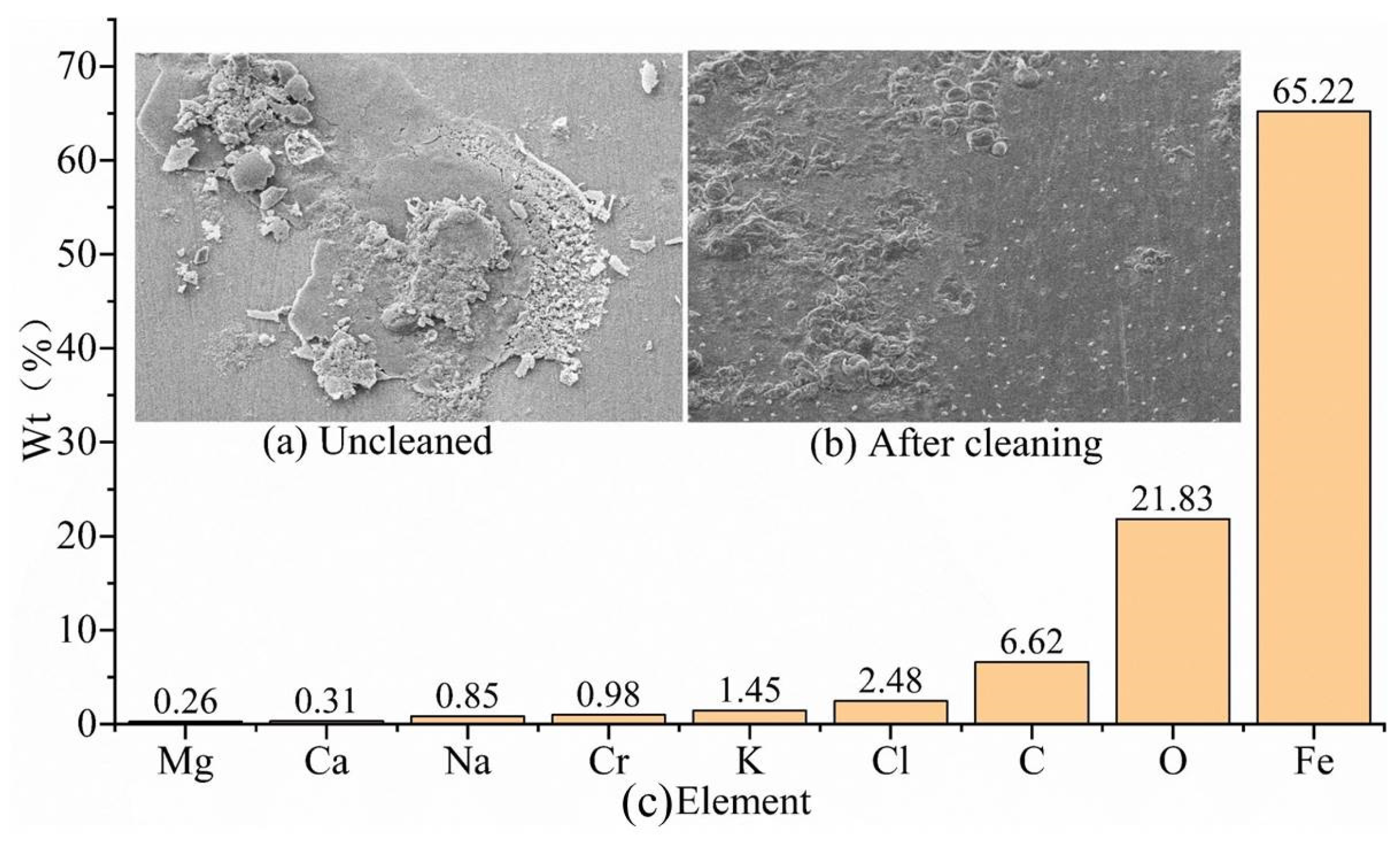
The corrosion morphology of the uncleaned G105-material sample in the simulated well-middle corrosion environment is shown in
Figure 4a, with loose and porous corrosion products and locally visible cracks visible. The corrosion morphology of the G105-material specimen after cleaning is shown in
Figure 4b, with slight corrosion traces localized on the surface of the sample and with residual drilling fluid adhering. In addition to the chemical composition of the G105 drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 4c. The corrosion products of the G105 material mainly contain Fe, O, C, Cl, K, and Cr elements, in addition to a small amount of Cr, Na, Ca, and Mg elements. The Fe and Cr elements come from the metal matrix, and the remaining elements come from drilling fluid and CO
2 gas, mainly resulting from CO
2 corrosion. Compared with the simulated corrosion environment at the wellhead location, the increase in the mass fraction of Cl in the corrosion environment at the well-middle location promotes the initiation and development of local corrosion.
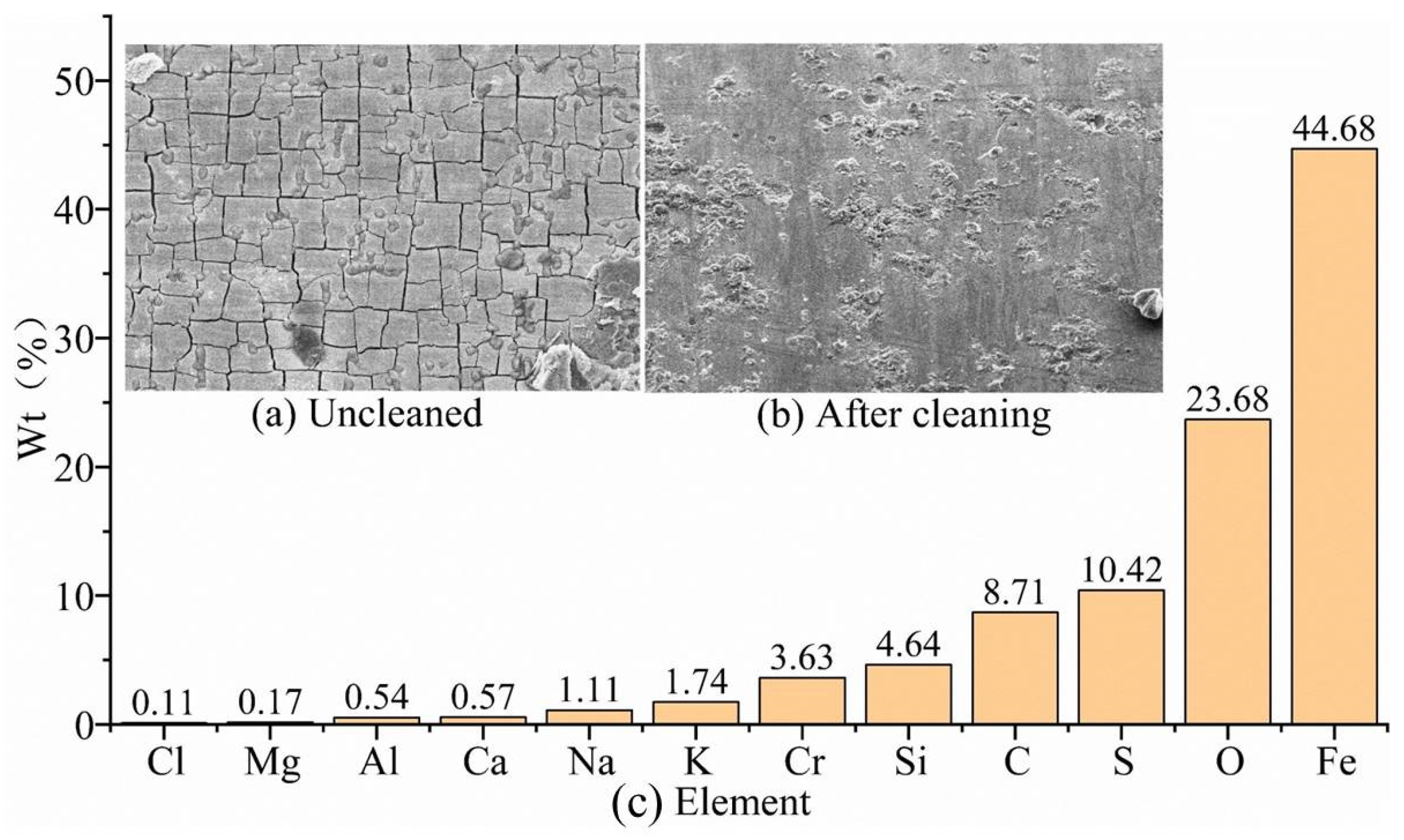
In the simulated corrosion environment at the bottomhole, the corrosion morphology of the uncleaned G105-material specimen, shown in
Figure 5a, the corrosion products show typical turtle-shaped cracks. The reason for this is that after the sample is taken out of the high-temperature and high-pressure reactor, Cr(OH)
3 in the corrosion products is easily dehydrated to form Cr
2O
3 [
23]. The corrosion morphology of the G105-material specimen after cleaning is shown in
Figure 5b, and a small amount of corrosion products are still locally attached. In addition to the chemical composition of the G105 drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 5c. The corrosion products of the G105 material mainly contain Fe, O, C, Cr, S, K, Ba, Na, Al, and Ca elements, in addition to a small amount of Mg element. The Fe, Si, Cr, and Al elements come from the metal substrate, while the other elements come from drilling fluid and CO
2/H
2S gas, and they are mainly produced by CO
2 corrosion and H
2S corrosion. Compared to the simulated environment at the wellhead and well-middle, a large number of cations were detected at the bottomhole, indicating an increase in the electrical conductivity of the simulated drilling fluid. The highly active Cr element will passivate to produce relatively dense Cr(OH)
3 films in CO
2-corrosion environments [
24]. The mass fraction of Cr at the bottom of the well is the highest, and the cracking phenomenon of the corrosion products also proves that it forms a relatively dense Cr(OH)
3 film at the bottomhole, which can effectively block the ions from contacting the metal substrate.
- (2)
Analysis of corrosion-morphology characteristics of S135 material
We use scanning electron microscopy to observe the S135 drill pipe material in corrosive environments in the wellhead, well-middle, and bottomhole simulated environments, and we conduct SEM-morphology analysis of the corrosion products of the uncleaned sample and the cleaned sample. The mass fraction of each element is obtained through EDS-energy-spectrum analysis of the corrosion-product components.
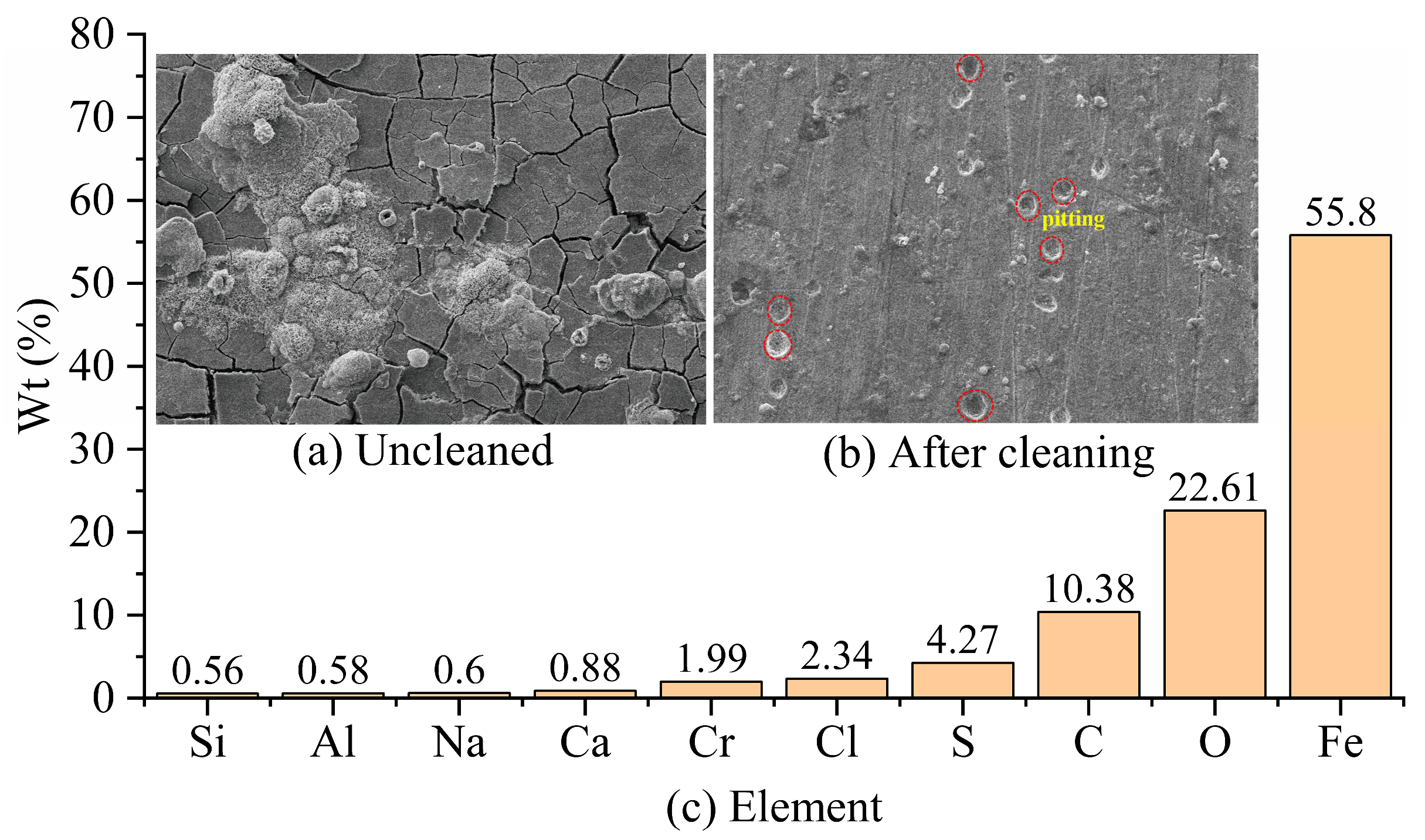
The corrosion morphology of the S135 material in the simulated wellhead-corrosion environment without cleaning is shown in
Figure 6a. It can be seen that the corrosion products present large-sized cracks, with local areas appearing in the shape of hairy balls. The corrosion morphology of the S135-material specimen after cleaning is shown in
Figure 6b, with clearly visible but sparsely distributed pitting. In addition to the chemical composition of the S135-material drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 6c. The corrosion products mainly contain Fe, O, C, S, Cl, and Cr elements, in addition to a small amount of Ca, Na, Al, and Si elements. Fe, Cr, and Al elements come from the S135 material, while the other elements come from the drilling fluid and the CO
2/H
2S gas. The S135 material is mainly corroded by CO
2 accompanied by H
2S at the wellhead, and the effect of Cl
− intensifies the occurrence of pitting corrosion.
The corrosion morphology of the S135 material from the simulated well-middle location corrosion environment and without cleaning is shown in
Figure 7a, with loose corrosion products, local accumulation, and uneven thickness. The corrosion morphology of the S135-material specimens after cleaning is shown in
Figure 7b, with corrosion pits of varying size scattered throughout the sample. In addition to the chemical composition of the S135-material drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 7c. The corrosion products of the S135 material mainly contain Fe, O, C, Si, Ba, Al, Na, Ca, K, and Mg elements, in addition to a small amount of S and Cr elements. The Fe, Si, Al, and Cr elements are from the S135 material, while the other elements are from the drilling fluid and the CO
2/H
2S gas. The S135 material in the well-middle is mainly corroded by CO
2 and slightly corroded by H
2S. When the types and contents of elements are detected as increasing, the conductivity of the solution increases, exacerbating the corrosion of the S135 material’s metal matrix. Compared with the wellhead, a large number of S135-material components and drilling fluid components were detected with corrosion products in the well-middle, with the lowest content of Cr proving that there is more serious corrosion under this working condition.
The corrosion morphology of the S135 material from the simulated bottomhole corrosive environment without cleaning is shown in
Figure 8a. The corrosion products are densely cracked and there is also a small amount of residual drilling fluid attached to the surface. The corrosion morphology of the S135 material after cleaning is shown in
Figure 8b, and there are still corrosion products attached to the surface of the sample, with an obvious pitting phenomenon visible locally. In addition to the chemical composition of the S135-material drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 8c. The corrosion products of the S135 material mainly contain Fe, O, S, C, Si, Cr, K, and Na elements, in addition to a small amount of Ca, Al, Mg, and Cl elements. The elements Fe, Si, Cr, and Al come from the metal substrate, while the other elements come from drilling fluid and CO
2/H
2S gas, mainly causing CO
2 corrosion and H
2S corrosion. Compared with the wellhead and well-middle positions, the S135 material exhibits more serious H
2S corrosion at the bottomhole.
- (3)
Analysis of corrosion-morphology characteristics of V150 material
We use scanning electron microscopy to observe the V150 drill pipe material in corrosive environments at the wellhead, well-middle, and bottomhole, and conduct SEM-morphology analysis of the corrosion products of the uncleaned sample and the cleaned sample. The mass fraction of each element is obtained through EDS-energy-spectrum analysis of the corrosion-product components.
The corrosion morphology of the uncleaned V150-material specimen in a simulated wellhead-corrosion environment is shown in
Figure 9a, with accumulation of the corrosion products and uneven thickness visible. The corrosion morphology of the V150-material specimen after cleaning is shown in
Figure 9b, with a large number of corrosion products still adhering and slight pitting corrosion observed. In addition to the chemical composition of the V150 drill pipe shown in
Table 2, the uncleaned corrosion products are shown in
Figure 9c. The corrosion products of the V150 material mainly contain Fe, O, and C elements, with very little content from other elements. Fe and a small amount of Cr coming from the metal matrix, while the other elements are from drilling fluids and CO
2/H
2S gas, mainly resulting in CO
2 corrosion.
The corrosion morphology of the V150 material in a simulated wellhead-corrosion environment is shown in
Figure 10a, with locally visible large-sized cracks in the corrosion products. The corrosion morphology of the V150-material specimen after cleaning is shown in
Figure 10b, and obvious homogeneous corrosion traces can be seen on the sample. In addition to the chemical composition of V150 drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 10c. In the simulated well-middle-location corrosion environment, the corrosion products of the V150 material mainly contain Fe, O, S, C, Cr, Na, K, Si, Ca, and Al elements, in addition to a small amount of Mg elements. Fe, Cr, Si, and Al elements come from the metal matrix, while the other elements come from the drilling fluid and CO
2/H
2S gas. CO
2 corrosion and H
2S corrosion co-exist. A large amount of Cr was detected and the corrosion products were in a cracked state, indicating that there is a dense Cr(OH)
3 film that has formed on the surface of the metal matrix, which can slow down corrosion. Compared with the wellhead simulation, in the V150 material we detected a large number of S elements in the well-middle simulation, resulting in severe H
2S corrosion and an increase in Cr content.
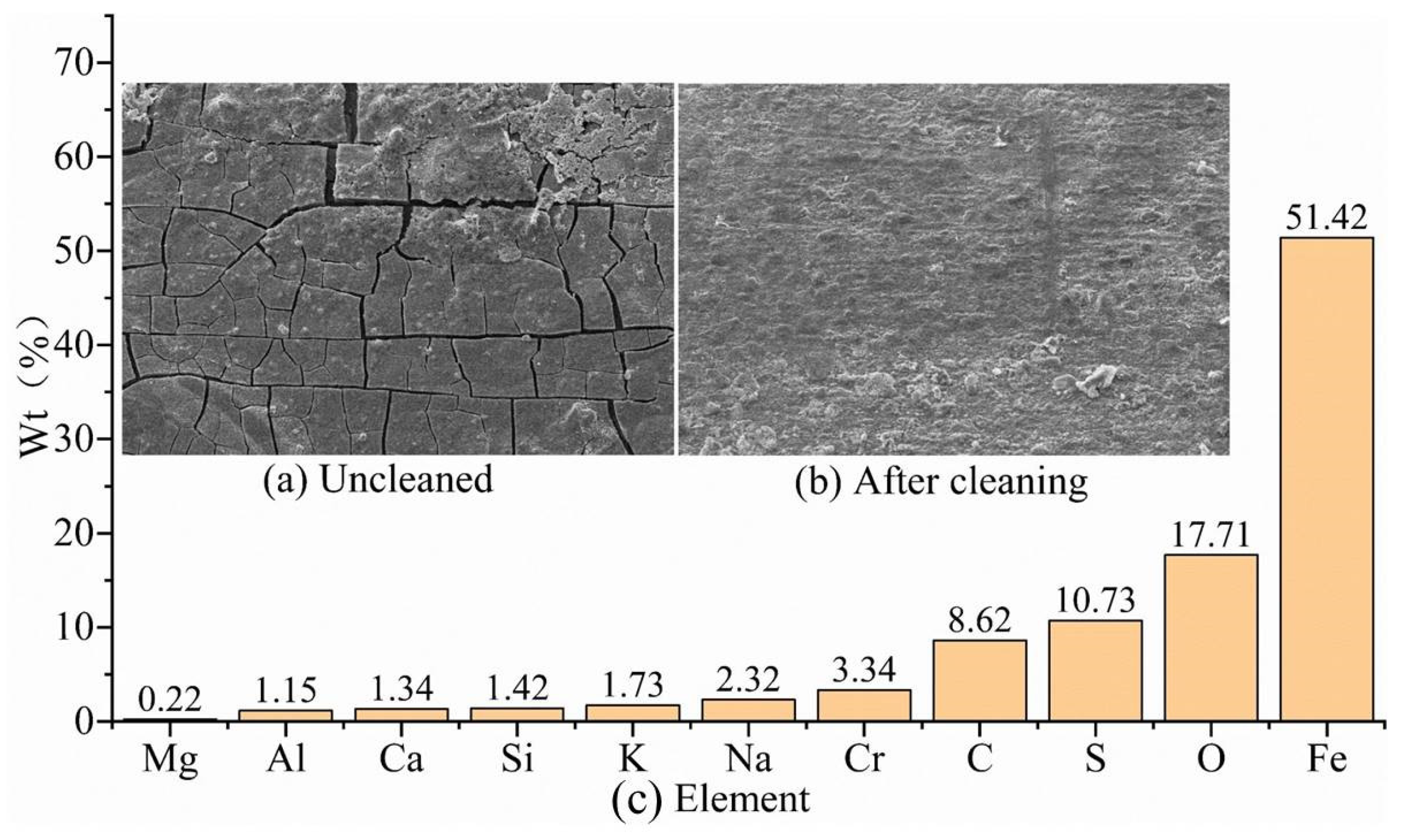
The corrosion morphology of the uncleaned V150 material in a simulated bottomhole corrosion environment is shown in
Figure 11a. The corrosion products are ruptured and uneven in thickness, with local accumulation of products. The corrosion morphology of the V150 material specimen after cleaning is shown in
Figure 11b, and there is no obvious corrosion phenomenon with local residual corrosion products. In addition to the chemical composition of the V150-material drill pipe shown in
Table 2, the components of the uncleaned corrosion products are shown in
Figure 11c. The corrosion products of the V150 material mainly contain Fe, O, C, S, Cr, Si, and K elements, in addition to a small amount of Al, Ca, Mg, Cl, and Na elements. Fe, Cr, Si, and a small amount of Al elements come from the metal matrix, while the other elements come from drilling fluid and CO
2/H
2S gas. CO
2 corrosion and H
2S corrosion co-exist; a large number of Cr elements were detected and the corrosion products were cracked, indicating that a dense Cr(OH)
3 film can slow down the corrosion of the metal matrix. Compared with the wellhead and well-middle, the types of corrosion-product elements in the bottomhole simulation have increased, but their content has decreased compared to the well-middle.
According to SEM of corrosion products and EDS and SEM of corrosion morphology after cleaning, it can be concluded that CO2 corrosion mainly occurs in the V150 drill pipe material under the three corrosion-simulation conditions of the wellhead, well-middle, and bottomhole locations. H2S corrosion is most severe in the V150 drill pipe material in the well-middle. From the wellhead to the bottomhole, the content of Cr in the corrosion products are constantly increasing, indicating that the protective effect of Cr(OH)3 film on the metal substrate is continuously increasing. Only slight CO2 corrosion occurred at the wellhead. CO2 and H2S corrosion co-exist in the well-middle, indicating a clear uniform-corrosion phenomenon. The types and contents of corrosion-product elements increase, and the solution conductivity increases, prompting the rapid formation of a dense Cr(OH)3 film by the Cr element, effectively impeding the contact of corrosive media with the metal substrate that intensifies corrosion. Although CO2 and H2S corrosion co-exist at the bottomhole, the presence of a dense Cr(OH)3 protection film can hinder the contact of the corrosive medium with the metal matrix, so the V150 material has slight corrosion at the bottomhole.
3.2. Analysis of Mechanical Performance
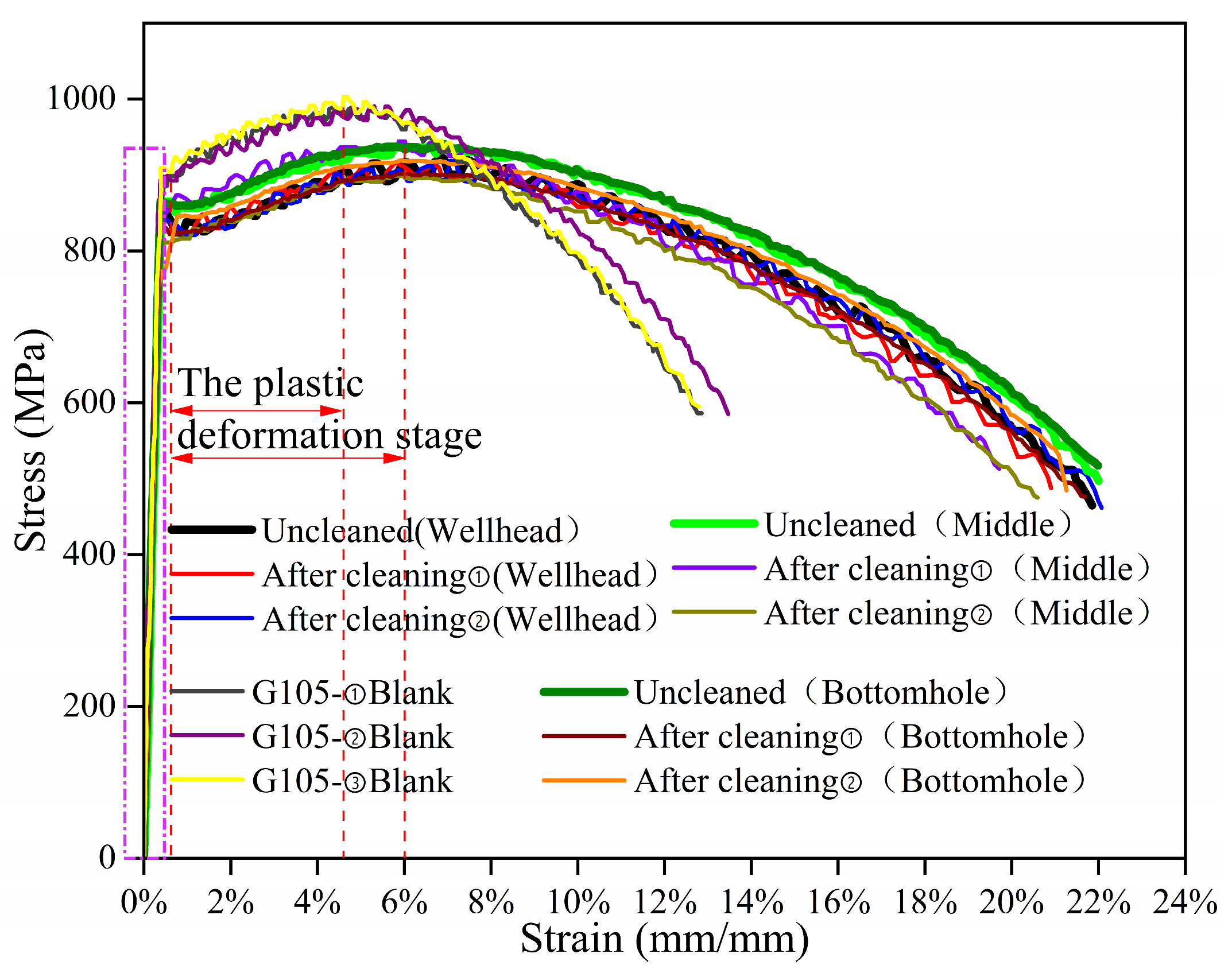
As shown in
Figure 12, the tensile stress–strain curve of G105-material specimens was obtained under simulated corrosion environments at the wellhead, well-middle, and bottomhole positions, as well as without corrosion. The variation trend of the tensile stress–strain curves of the uncleaned specimen at the wellhead is consistent with that of the cleaned samples, while the tensile stress–strain curves of the uncleaned specimen in the well-middle and at the bottomhole are slightly higher than those of the cleaned samples. From this, it can be seen that when the temperature is low, the corrosion product becomes loose and easily falls off under the action of force; when the temperature is high, the corrosion products are dense, which can to some extent slow down the fracture of the specimen under constant load. After constant-load-stress-corrosion, the average strain values of G105-material fracture under simulated corrosion environments at the wellhead, well-middle, and bottomhole positions are 21.7%, 20.1% and 22.0%, respectively. The average strain value of the uncorroded specimen fracture is 13.0%. The average maximum stress value (average tensile strength value) of G105-material fracture in the simulated corrosion environments at the wellhead, well-middle, and bottomhole positions are 907 MPa, 908 MPa, 901 MPa, respectively, and the average maximum stress value of the uncorroded specimen fracture is 986 MPa. During the elastic deformation stage (rectangular dashed line in
Figure 12), the stress-increase trend of the corrosion-simulation environment of the three well-section locations and the uncorroded samples under the action of constant-load-tensile stress is consistent. It can be seen that the elastic deformation ability of the G105 material decreases after the constant-load-stress-corrosion experiments; in the plastic deformation stage (the area indicated by the red double arrows in
Figure 12), the plastic deformation ability of the G105 material decreased compared to the uncorroded specimen after simulated corrosion experiments at the wellhead, well-middle, and bottomhole. After the stress value reaches the tensile strength (red dotted line), the decreasing trend of the stress–strain curve is slower compared with the uncorroded specimen under three simulated conditions.
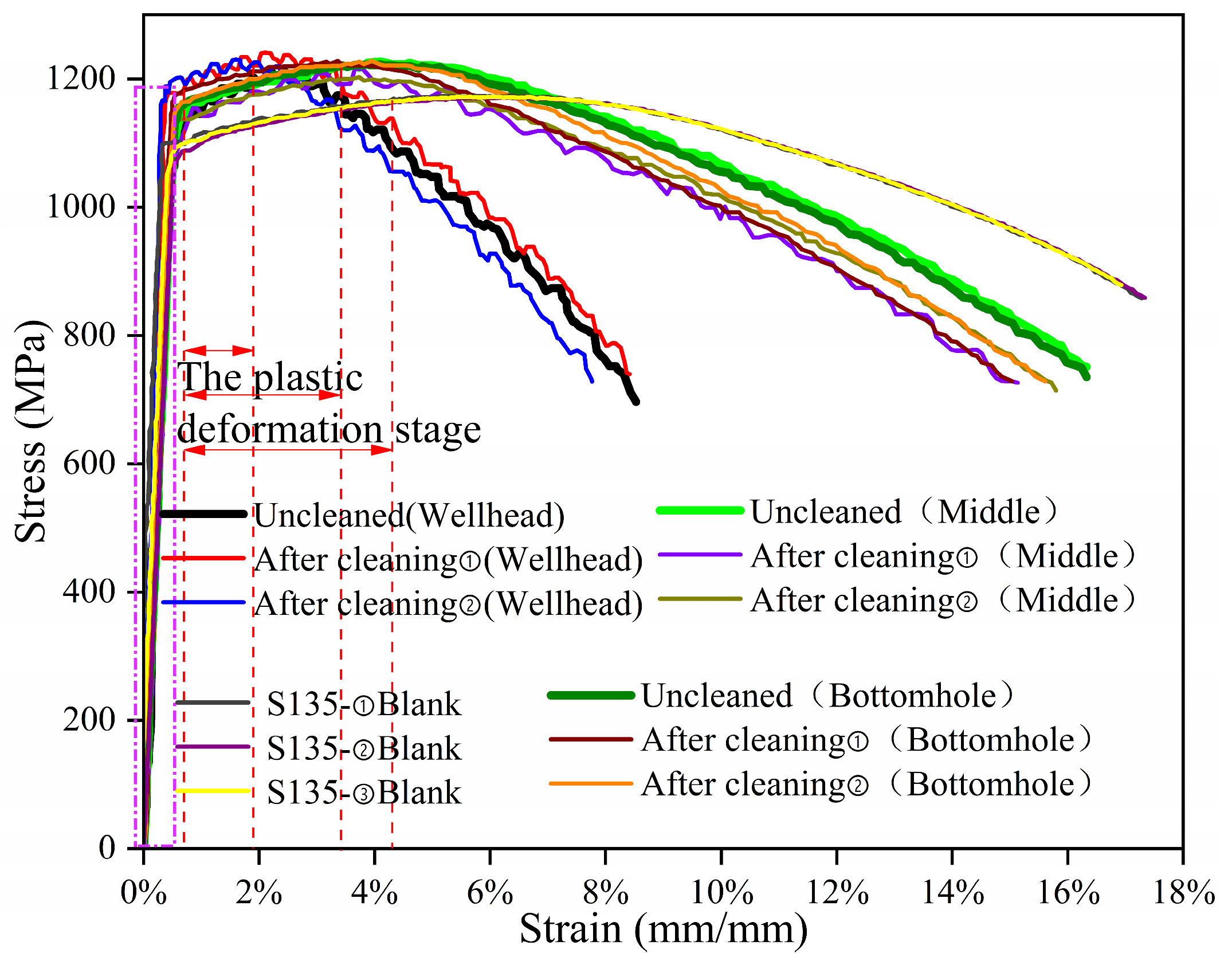
As shown in
Figure 13, the tensile stress–strain curves of S135-material specimens were obtained in corrosion-simulation environments for the wellhead, the middle, and the bottomhole locations, as well as without corrosion. The variation trend of the tensile stress–strain curve of the uncleaned specimen at the wellhead is consistent with that of the cleaned specimen, while the tensile stress–strain curves of the uncleaned specimen at the well-middle and at the bottomhole are slightly higher than those of the cleaned specimen; it can be inferred that when the temperature and pressure at the wellhead are low, the corrosion products formed are loose and easily detached under the action of the force. The increasing temperature, pressure, and corrosive media in the well-middle and at the bottomhole can result in denser corrosion products, which can slow down the fracture of the sample to a certain extent under constant load. After constant-load-stress corrosion, the average strain value of S135-material fracture under simulated corrosion environments at the wellhead, well-middle, and bottomhole locations are 16.4%, 15.7%, and 15.8%, respectively. The average strain value of the uncorroded specimen’s fracture is 17.1%; The average maximum stress values (the average tensile strength values) of the S135 material under corrosion-simulation environments at the wellhead, well-middle, and bottomhole locations are 1191 MPa, 1190 MPa, and 1203 Mpa, respectively. The average maximum stress value of the uncorroded specimen fracture is 1166 MPa. During the elastic deformation stage (dashed rectangle line in
Figure 13), under constant-load tension, the stress-increase trend of the corrosion-simulation environment and the uncorroded specimen at the three well-section locations is consistent. It can be seen that the stress value of the S135 material increases after the constant-load-stress-corrosion experiment. In the plastic deformation stage (indicated by the red double arrows in
Figure 13), the plastic deformation capacity of the S135 material increased compared to the uncorroded specimen after the corrosion-simulation experiments at the wellhead, in the well-middle, and at the bottomhole, and the stress during the plastic deformation stage was the highest at the wellhead. After the stress value reaches the tensile strength (red dashed line), the decreasing trend of the stress–strain curve increases compared with the uncorroded specimens under the three simulated corrosion conditions, that is the susceptibility to SSCC increases, and the decreasing trend of the stress–strain curve is steepest at the wellhead. In the SEM of the cleaned specimen, it can be seen that the S135 material exhibits obvious pitting corrosion at the wellhead. In addition, and H
2S corrosion effect makes the S135 material the most SSCC-sensitive at the wellhead.
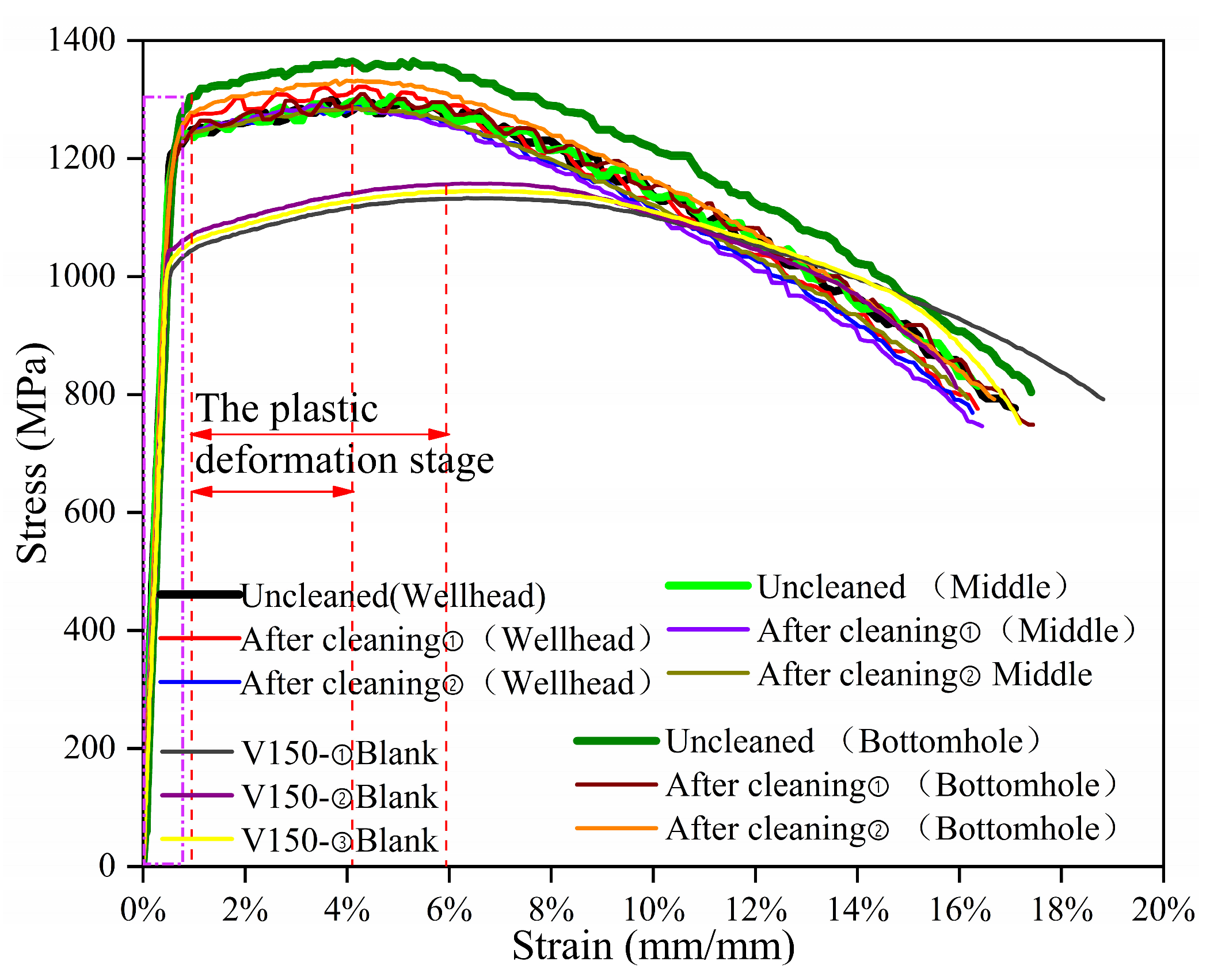
As shown in
Figure 14, the tensile stress–strain curves of the V150 material were obtained under the corrosion-simulation environments at the wellhead, in the well-middle, and at the bottomhole, as was obtained for uncorroded V150 material. The variation trend of the tensile stress–strain curves of the specimens at the wellhead, well-middle, and bottomhole are consistent, but the tensile stress–strain curve of the uncleaned specimen at the bottomhole are highest; it can be seen that the dense corrosion products at the bottomhole have to some extent slowed down the fracture of the sample under constant load. After constant-load stress corrosion, the average strain values of V150-material fracture under the corrosion-simulation environment at the wellhead, well-middle, and bottomhole positions were 16.6%, 16.8% and 16.9%, respectively. The average strain value of the uncorroded specimen fracture was 17.4%. The average maximum stress values (average tensile strength values) of V150-material fracture under the corrosion-simulation environment at the wellhead, well-middle, and the bottomhole location are 1288 MPa, 1271 MPa, and 1304 MPa, respectively. The average maximum stress value of the uncorroded specimen fracture is 1148 MPa. During the elastic deformation stage (rectangular dashed line in
Figure 14), under constant-load tension, the stress-increase trend of the corrosion-simulation environment and the uncorroded specimen at the three well sections is consistent. It can be seen that the elastic deformation capacity of the V150 material increases after the constant-load-stress-corrosion experiment; in the plastic deformation stage (indicated by the red double arrows in
Figure 14), the plastic deformation capacity of the V150 material increases compared to uncorroded specimen after simulated corrosion experiments at the wellhead, in the well-middle, and at the bottomhole. After the stress value reaches the tensile strength (red dashed line), the decreasing trend of the stress–strain curve is steeper compared to the uncorroded specimen under the three simulated working conditions.
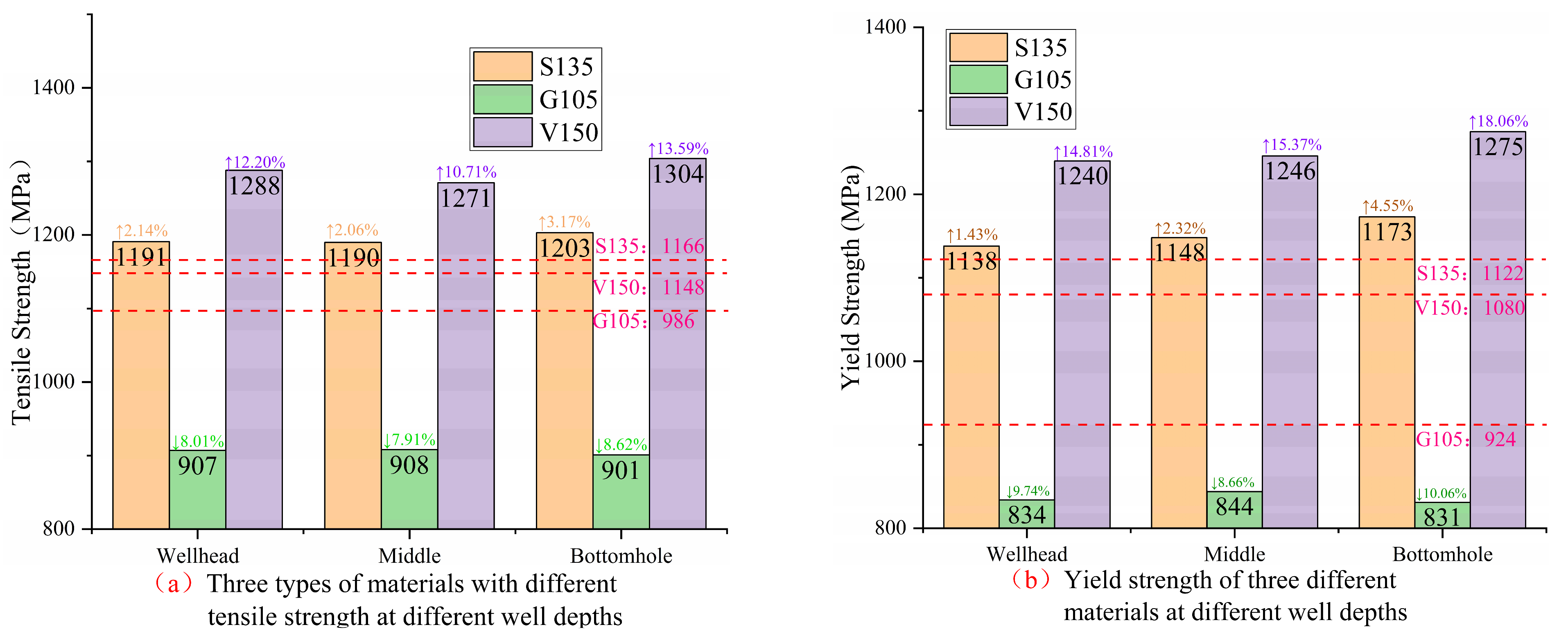
Referring to the standard API Spec 5DP-2020 “Drill pipe specification”, the tensile strength and yield-strength requirements for S135, G105, and V150 three materials are specified in
Table 4. As shown in
Figure 15, the tensile strength, yield strength, yield ratio, and fracture elongation parameters of three materials under different corrosion environments in different well sections were obtained. The red dashed line in the figure represents the measured tensile-performance parameter values of the uncorroded specimens. According to
Figure 15a,b, the strength of the three materials meets the standard requirements.
As can be seen from
Figure 15a, compared to the uncorroded specimens, the tensile strength of S135, G105, and V150 drill pipe materials increased by 2.06~3.17%, decreased by 7.91~8.62%, and increased by 10.71~13.59%, respectively. The S135 and V150 materials undergo strain hardening under the influence of H
2S in the process of tensile stress. The increase in tensile strength implies a decrease in their plastic deformation ability and toughness, whereas the G105 drill pipe material is affected by CO
2 corrosion, resulting in a decrease in its fracture resistance. The change in tensile strength of three materials varies from largest to smallest as follows: V150 material > G105 material > S135 material.
According to
Figure 15b, compared with the uncorroded specimens, the yield strength of the S135, G105, and V150 drill pipe materials increased by 1.43~4.55%, decreased by 8.66~10.06%, and increased by 14.81~18.06%, respectively. The increase in yield strength of S135 and the V150 material proves an increase in their susceptibility to stress corrosion cracking under H
2S action, and the V150 material has a greater sensitivity to hydrogen embrittlement. Although the G105 material has no risk of hydrogen embrittlement-sensitive fracture, its yield strength is damaged after corrosion. The change in yield strength of three materials ranges from largest to smallest as follows: V150 material > G105 material > S135 material.
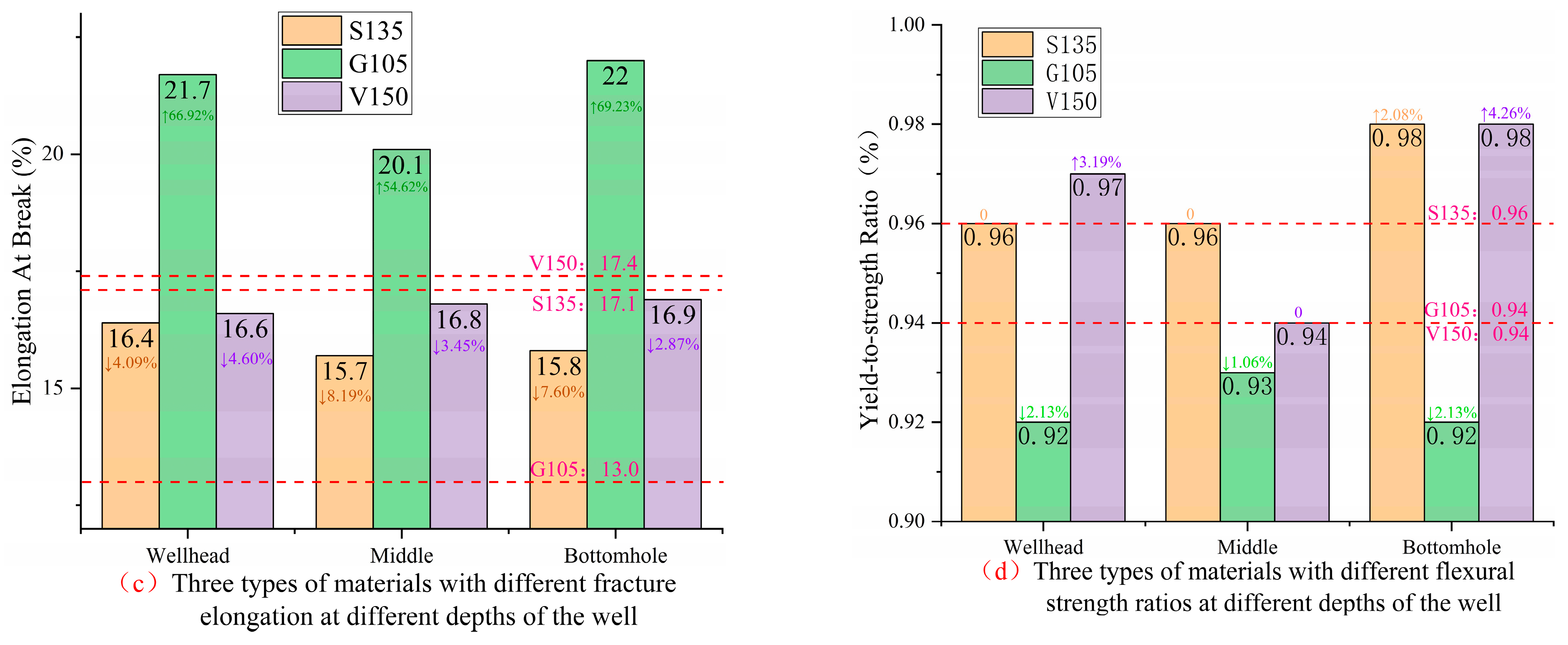
As can be seen from
Figure 15c, compared with the uncorroded specimen, the fracture elongation of the S135, G105, and V150 drill pipe materials decreased by 4.09~8.19%, increased by 54.62~69.23%, and decreased by 2.87~4.60%, respectively. Fracture elongation is used to evaluate the plasticity of pipes. The decrease in elongation of S135 and V150 drill pipe materials indicates the existence of strain hardening, while the G105 material has good plastic deformation ability. The change in fracture elongation of the three materials ranges from largest to smallest as follows: G105 material > S135 material > V150 material.
According to
Figure 15d, it can be seen that the flexural strength ratio of the S135 drill pipe material increases by 2.08% at the bottomhole, the yield ratio of the G105 drill pipe material decreases by 1.06~2.13%, and the yield ratio of the V150 drill pipe material increases by 3.19~4.26% at the wellhead and the bottomhole. The increases in flexural strength ratio of the S135 and V150 materials indicates an increased risk of brittle damage for both materials.