Determining the Role of Water Molecules in Sodalite Formation Using the Vapor Phase Crystallization Method
Abstract
1. Introduction
2. Material and Experimental Design
2.1. Resources
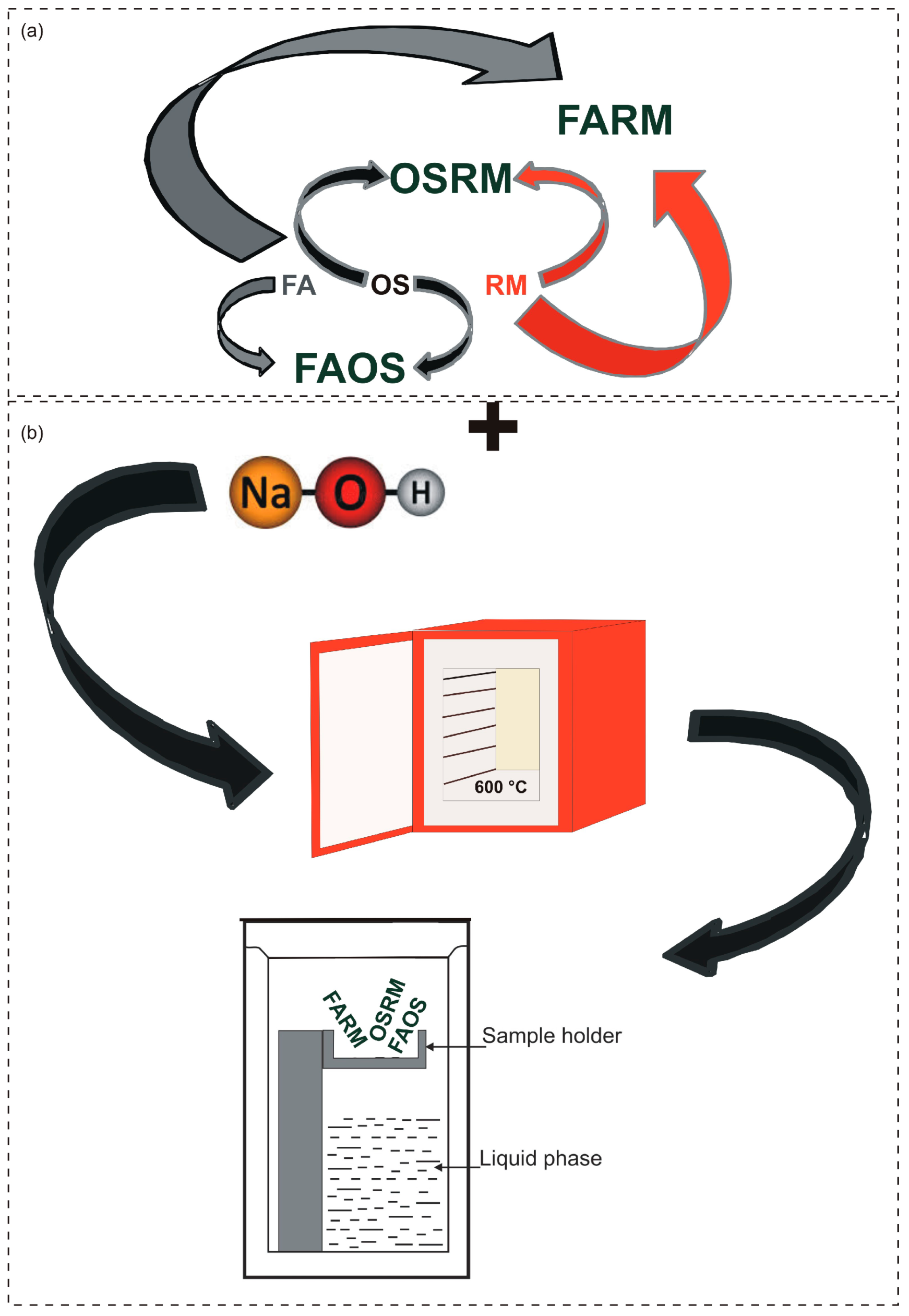
2.2. Experimental Procedure
2.3. Raw Material and Final Product Characterization
3. Results
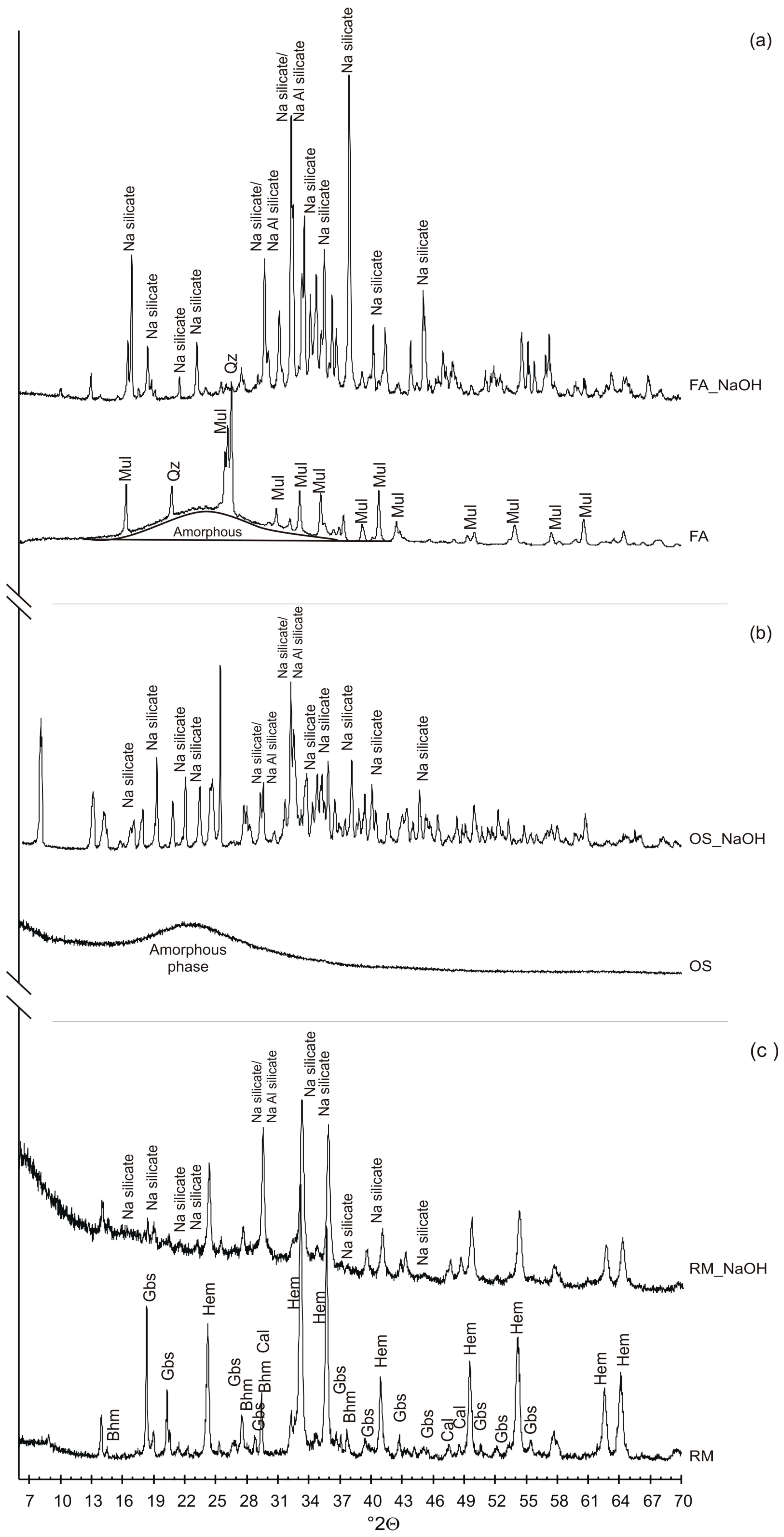
3.1. Raw Material and Pre-Fused Products
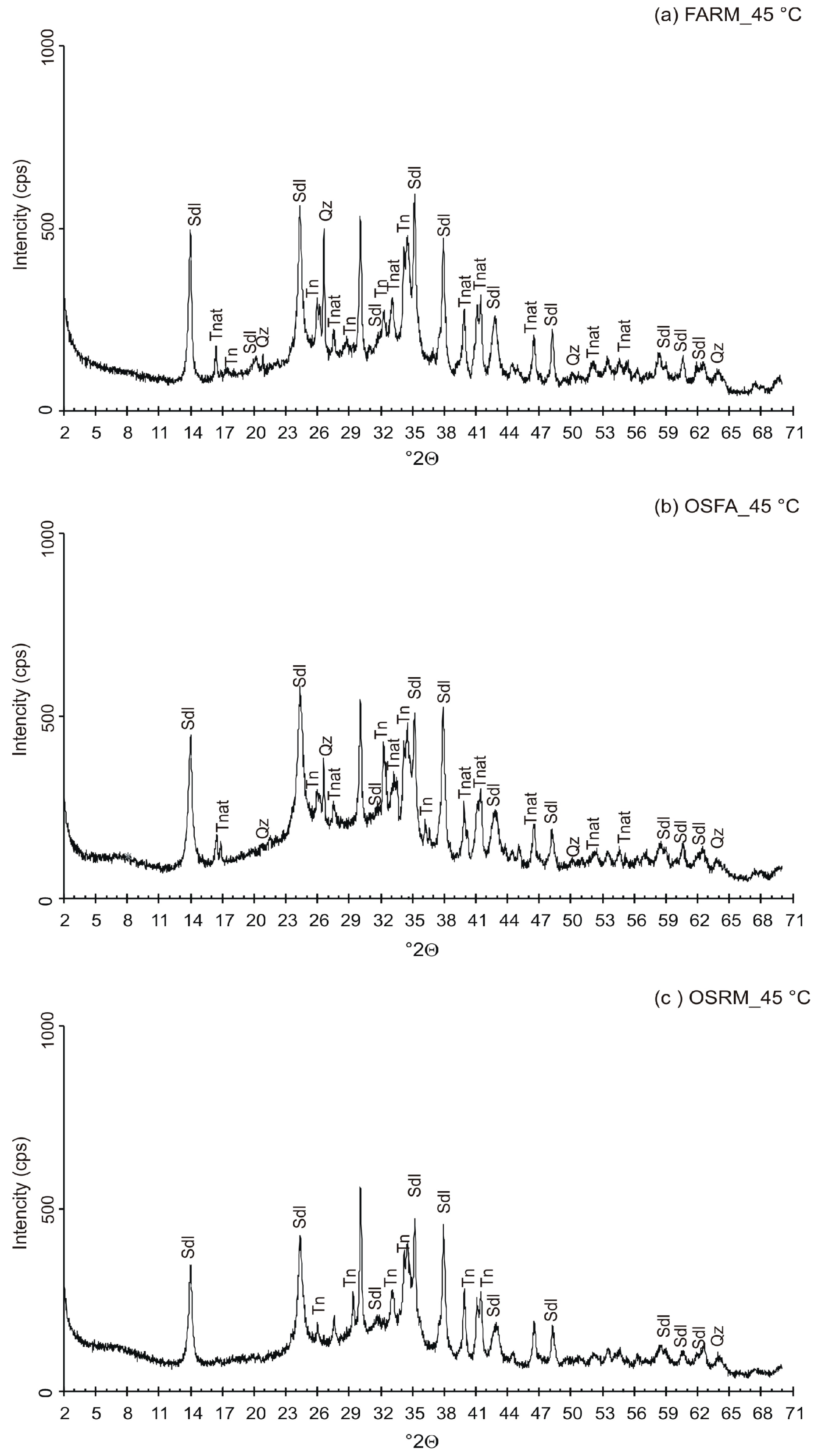
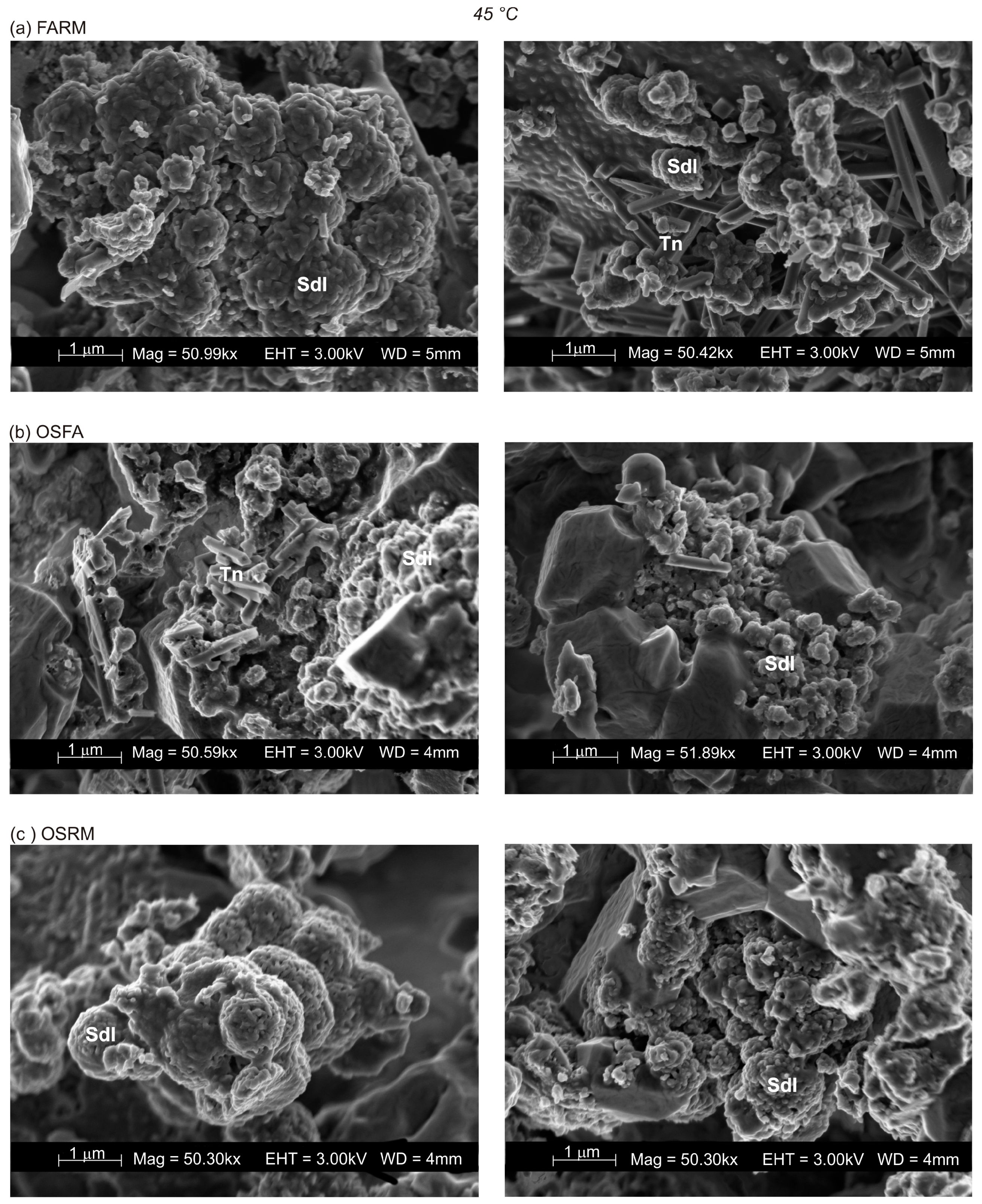
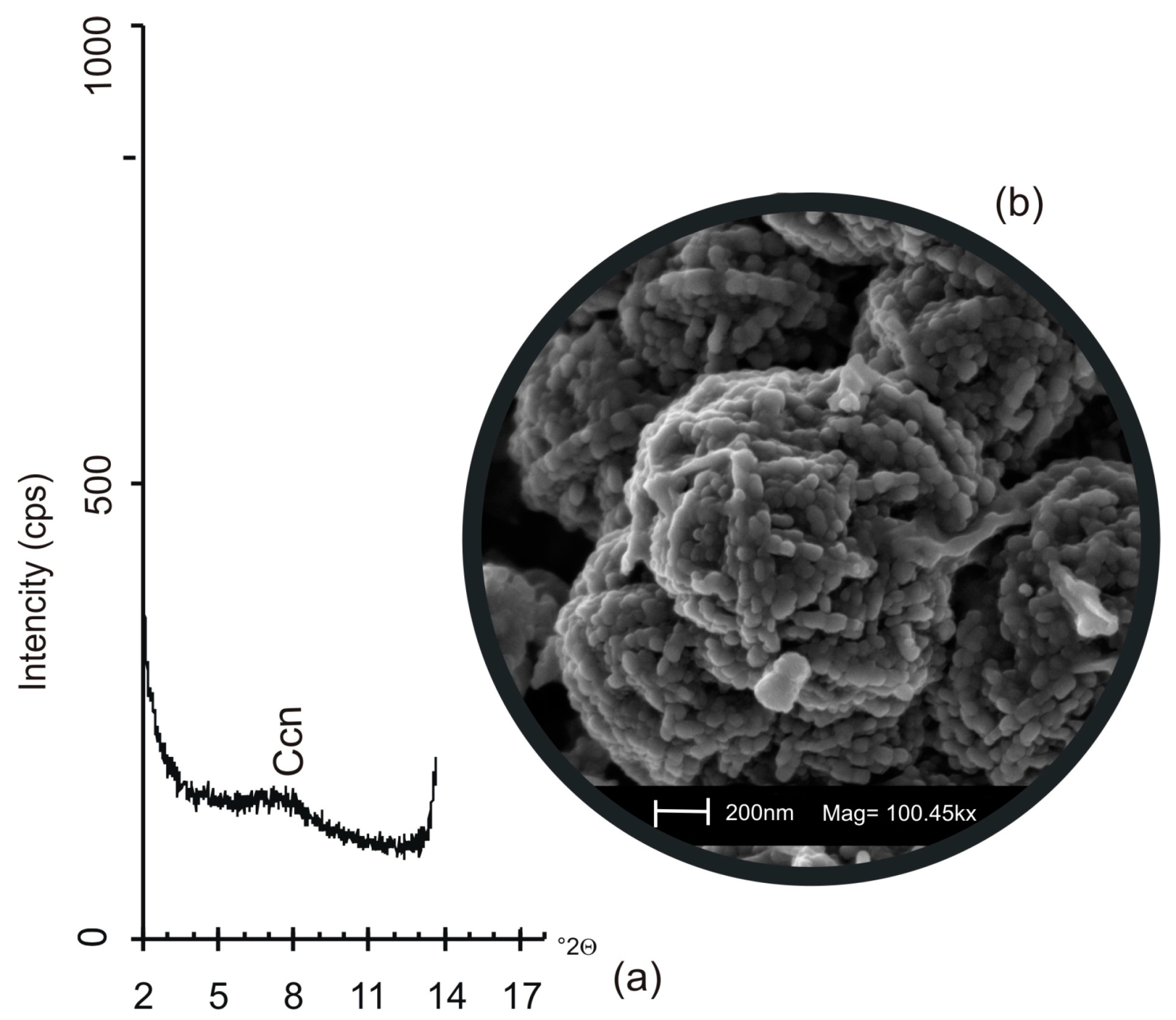
3.2. VPC Treatment at 45 °C
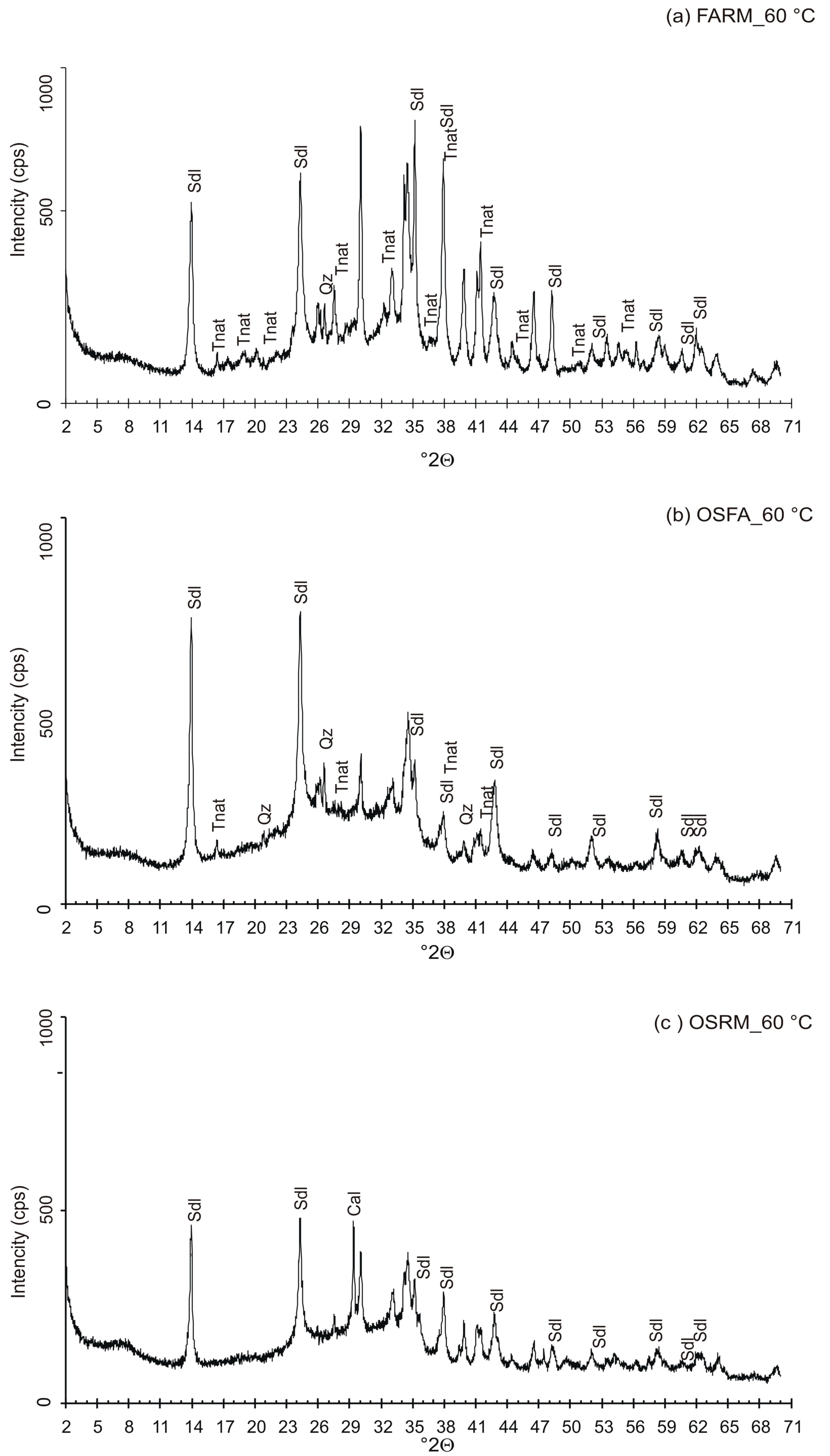
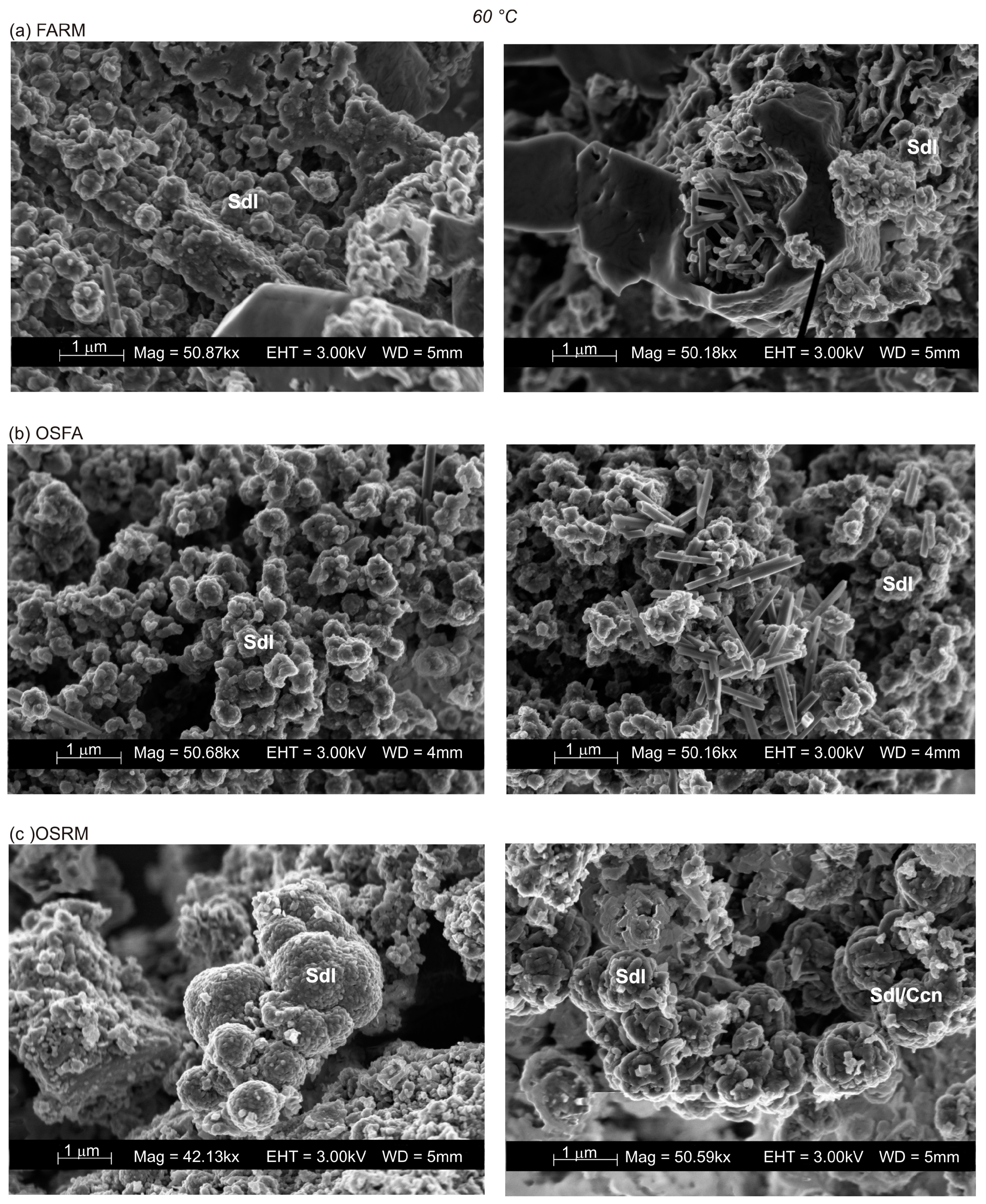
3.3. VPC Treatment at 60 °C
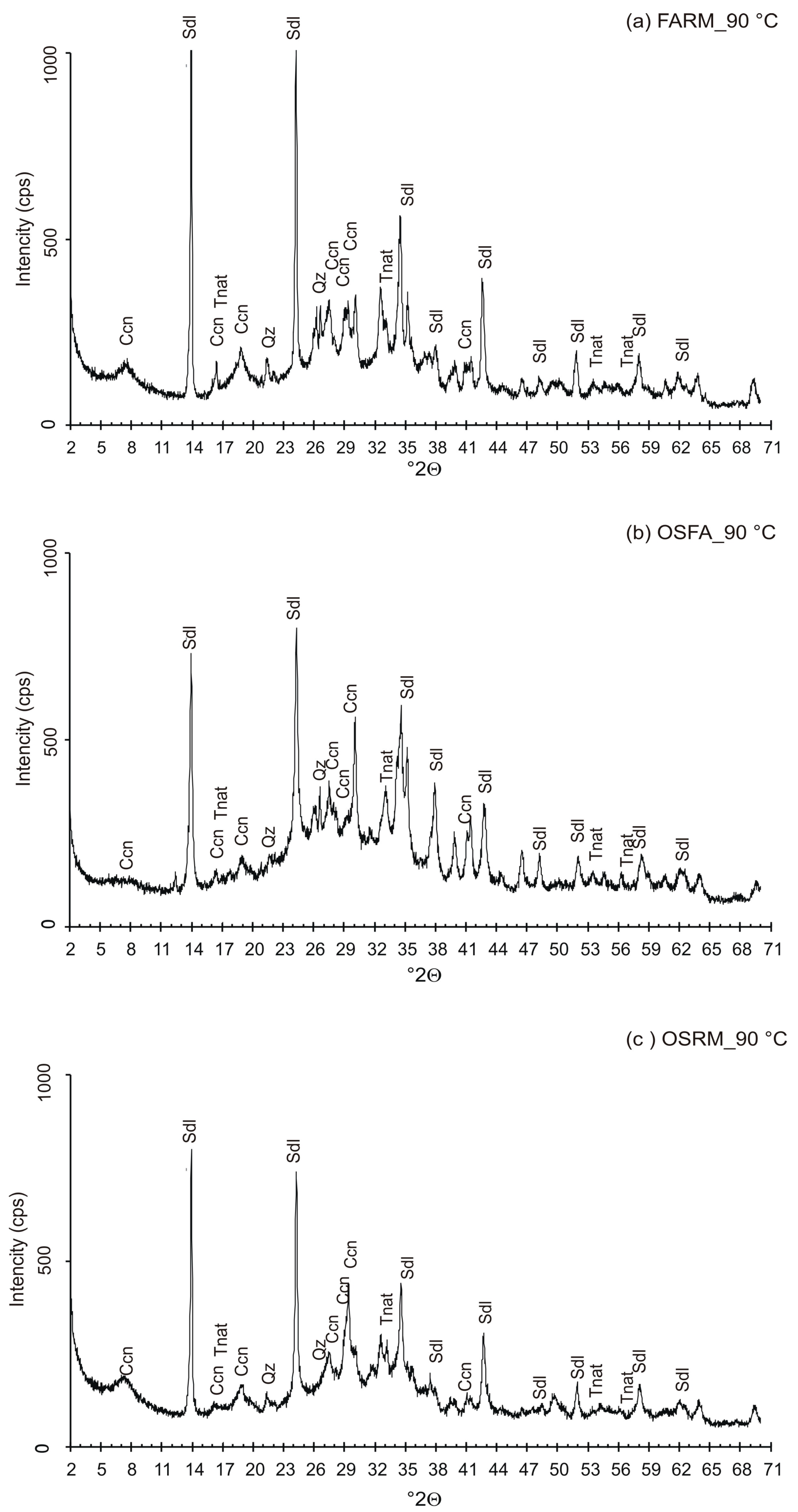
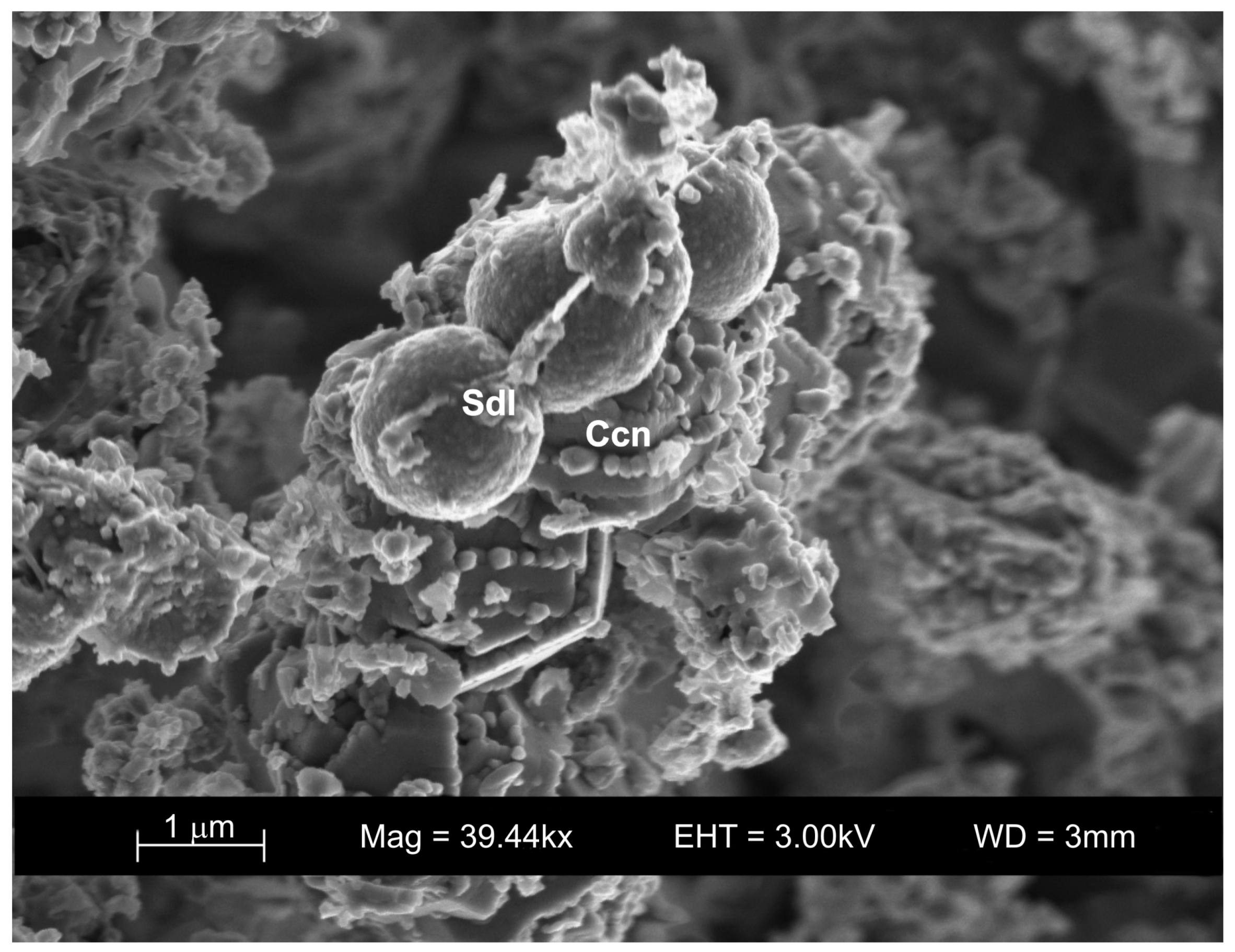
3.4. VPC Treatment at 90 °C
4. Discussion
5. Concluding Remarks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holler, H.; Wirsching, U. Zeolite formation from fly-ash. Fortschr. Mineral. 1985, 63, 21–43. [Google Scholar]
- Mondragon, F.; Rincon, F.; Sierra, L.; Escobar, J.; Ramirez, J.; Fernandez, J. New perspectives for coal ash utilization: Synthesis of zeolitic materials. Fuel 1990, 69, 263–266. [Google Scholar] [CrossRef]
- Shigemoto, N.; Hayashi, H.; Miyaura, K. Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Längauer, D.; Čablík, V.; Hredzák, S.; Zubrik, A.; Matik, M.; Danková, Z. Preparation of synthetic zeolites from coal fly ash by hydrothermal synthesis. Materials 2021, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Panek, R.; Madej, J.; Bandura, L.; Słowik, G. Recycling of waste solution after hydrothermal conversion of fly ash on a semi-technical scale for zeolite synthesis. Materials 2021, 14, 1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, P.; Wang, Y.; Peng, W.; Ren, Z.; Li, Y.; Chu, B.; Zhu, Q. Research progress on synthesis of zeolites from coal fly ash and environmental applications. Front. Environ. Sci. Eng. 2023, 17, 149. [Google Scholar] [CrossRef]
- Iqbal, A.; Sattar, H.; Haider, R.; Munir, S. Synthesis and characterization of pure phase zeolite 4A from coal fly ash. J. Clean. Prod. 2019, 219, 258–267. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Methods for characterization of composition of fly ashes from coal-fired power stations: A critical overview. Energy Fuels 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Yang, L.; Qian, X.; Yuan, P.; Bai, H.; Miki, T.; Men, F.; Li, H.; Nagasaka, T. Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. J. Clean. Prod. 2019, 212, 250–260. [Google Scholar] [CrossRef]
- Guozhi, L.; Zhang, T.; Cheng, C.; Zhang, W.; Wang, L.; Wang, Y.; Zhang, Z. Zeolite a preparation from high alumina fly ash of China using alkali fusion and hydrothermal synthesis method. Mater. Res. Express 2019, 6, 65049. [Google Scholar] [CrossRef]
- Hussain, T.; Hussain, A.I.; Chatha, S.A.S.; Ali, A.; Rizwan, M.; Ali, S.; Ahamd, P.; Wijaya, L.; Alyemeni, M.N. Synthesis and characterization of Na-zeolites from textile waste ash and its application for removal of lead (Pb) from wastewater. Int. J. Environ. Res. Public Health 2021, 18, 3373. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T. Synthesis of zeolite from waste LCD panel glass. Appl. Chem. Eng. 2017, 28, 521–528. [Google Scholar]
- Lin, G.; Zhuang, Q.; Cui, Q.; Wang, H.; Yao, H. Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid. Chin. J. Chem. Eng. 2015, 23, 1768–1773. [Google Scholar] [CrossRef]
- Ng, E.P.; Awala, H.; Tan, K.H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice 5husk. Microporous Mesoporous Mater. 2015, 204, 204–209. [Google Scholar] [CrossRef]
- Wajima, T.; Ikegami, Y. Synthesis of zeolitic materials from waste porcelain at low temperature via a two-step alkali conversion. Ceram. Int. 2007, 33, 1269–1274. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Wang, Q.; Ahn, W.S. Co- and Mn-coimpregnated ZSM-5 prepared from recycled industrial solid wastes for low-temperature NH-SCR. Ind. Eng. Chem. Res. 2019, 58, 22857–22865. [Google Scholar] [CrossRef]
- Tehubijuluw, H.; Subagyo, R.; Yulita, M.F.; Nugraha, R.E.; Kusumawati, Y.; Bahruji, H.; Hartati, J.A.A.; Prasetyoko, D. Utilization of red mud waste into mesoporous ZSM-5 for methylene blue adsorption-desorption studies. Environ. Sci. Pollut. Res. 2021, 28, 37354–37370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Zhao, Y.; Jow, J.; Gong, B.; Yang, T.-X.; Li, W.; Zhang, Y.; Niu, Y.-C.; Zeng, Y.-P. Study on synthesized zeolite Na with red mud and treatment of the ammonium in wastewater. Bull. Chin. Ceram. Soc. 2018, 37, 3700–3706. [Google Scholar]
- Wu, R.; Xiao, Y.; Zhang, P.; Lin, J.; Cheng, G.; Chen, Z.; Yu, R. Asphalt VOCs reduction of zeolite synthesized from solid wastes of red mud and steel slag. J. Clean. Prod. 2022, 345, 131078. [Google Scholar] [CrossRef]
- Celoria, G.; Begni, F.; Paul, G.; Boccaleri, E.; Merlo, V.; Marchese, L.; Bisio, C. Zeolites Derived from Natural Kaolinite for CO2 Adsorption. Processes 2024, 12, 194. [Google Scholar] [CrossRef]
- Ganbarov, D.; Amirov, S.; Zulfugarov, Z.; Mamedov, K. Synthesis of zeolites from obsidian under hydrothermal conditions. Inorg. Mater. 1977, 13, 282–284. [Google Scholar]
- Youcef, L.D.; López-Galindo, A.; Verdugo-Escamilla, C.; Belaroui, L.S. Synthesis and characterization of zeolite LTA by hydrothermal transformation of a natural Algerian palygorskite. Appl. Clay Sci. 2020, 193, 105690. [Google Scholar] [CrossRef]
- Wu, T.L.; Chen, Y.H.; Hsu, W.D. Phase transition pathway of hydrothermal zeolite synthesis. Phys. Chem. Miner. 2021, 48, 1. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva-Filipova, D.; Popov, C.; Lazarova, H.; Popova, M. Plasma-Modified Coal Fly Ash Zeolites with Enhanced Catalytic Efficiency toward the Total Oxidation of Volatile Organic Compounds as Low-Cost Substitutes for Platinum Group Metals Catalysts. Phys. Status Solidi A-Appl. Res. 2022, 219, 2100632. [Google Scholar] [CrossRef]
- Chang, H.L.; Shih, W.H. Synthesis of zeolites A and X from fly ashes and their ion-exchange behavior with cobalt ions. Ind. Eng. Chem. Res. 2000, 39, 4185–4191. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsumura, S.; Hino, R. Formation process of Na-X zeolites from coal fly ash. J. Mater. Sci. 2004, 39, 1677–1682. [Google Scholar] [CrossRef]
- Belviso, C.; Kharchenko, A.; Agostinelli, E.; Cavalcante, F.; Peddis, D.; Varvaro, G.; Yaacoub, N.; Mintova, S. Red mud as aluminium source for the synthesis of magnetic zeolite. Microporous Mesoporous Mater. 2018, 270, 24–29. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, F.; Bi, X.; Chen, D.; Li, J.; Sun, S.; Liu, J.; Chen, X. Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions. J. Hazard. Mater. 2018, 358, 441–449. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, L.; Jiang, Z.; Liu, C.; Zhang, Q.; Zou, Y.; Chen, Y.; Li, J.; Liu, X. Feasible low-cost conversion of red mud into magnetically separated and recycled hybrid SrFe12O19@ NaP1 zeolite as a novel wastewater adsorbent. Chem. Eng. J. 2021, 417, 128090. [Google Scholar] [CrossRef]
- Belviso, C.; Agostinelli, E.; Belviso, S.; Cavalcante, F.; Pascucci, S.; Peddis, D.; Varvaro, G.; Fiore, S. Synthesis of magnetic zeolite at low temperature using a waste material mixture: Fly ash and red mud. Microporous Mesoporous Mater. 2015, 202, 208–216. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, J.; Liu, D.; Meng, X.; Guo, Y.; Cheng, F. Feasible synthesis of magnetic zeolite from red mud and coal gangue: Preparation, transformation and application. Powder Technol. 2023, 423, 118495. [Google Scholar] [CrossRef]
- Belviso, C. EMT-type zeolite synthesized from obsidian. Microporous Mesoporous Mater. 2016, 226, 325–330. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Jakevičius, L.; Kantautas, A.; Vaitkevičius, V.; Vaičiukynas, V.; Dvořák, K. Conversion of silica by-product into zeolites by thermo-sonochemical treatment. Ultrason. Sonochem. 2021, 72, 105426. [Google Scholar] [CrossRef]
- Woolard, C.D.; Strong, J.; Erasmus, C.R. Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl. Geochem. 2002, 17, 1159–1164. [Google Scholar] [CrossRef]
- Askari, S.; Alipour, S.M.; Halladj, R.; Davood Abadi Farahani, M.H. Effects of ultrasound on the synthesis of zeolites: A review. J. Porous Mater. 2013, 20, 285–302. [Google Scholar] [CrossRef]
- Aldahri, T.; Behin, J.; Kazemian, H.; Rohani, S. Synthesis of zeolite Na-P from coal fly ash by thermo-sonochemical treatment. Fuel 2016, 182, 494–501. [Google Scholar] [CrossRef]
- Dewes, R.M.; Mendoza, H.R.; Pereira, M.V.L.; Lutz, C.; Van Gerven, T. Experimental and numerical investigation of the effect of ultrasound on the growth kinetics of zeolite A. Ultrason. Sonochem. 2022, 82, 105909. [Google Scholar] [CrossRef] [PubMed]
- Ojumu, T.V.; Du Plessis, P.W.; Petrik, L.F. Synthesis of zeolite A from coal fly ash using ultrasonic treatment—A replacement for fusion step. Ultrason. Sonochem. 2016, 31, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Microporous Mesoporous Mater. 2021, 323, 111262. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Tauanov, Z.; Azat, S.; Baibatyrova, A. A mini-review on coal fly ash properties, utilization and synthesis of zeolites. Int. J. Coal Prep. Util. 2022, 42, 1968–1990. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; López-Soler, A.; Plana, F. A fast method for recycling fly ash: Microwave-assisted zeolite synthesis. Environ. Sci. Technol. 1997, 31, 2527–2533. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Nxumalo, E.N.; Musyoka, N.M.; Mhlanga, S.D. Microwave irradiation-assisted synthesis of zeolites from coal fly ash: An optimization study for a sustainable and efficient production process. ACS Omega 2020, 5, 25000–25008. [Google Scholar] [CrossRef]
- Al-dahri, T.; AbdulRazak, A.A.; Rohani, S. Preparation and characterization of Linde-type A zeolite (LTA) from coal fly ash by microwave-assisted synthesis method: Its application as adsorbent for removal of anionic dyes. Int. J. Coal Prep. Util. 2022, 42, 2064–2077. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Chen, X.; Li, H.; Xu, X.; Dou, J.; Yu, J. Synthesis of a Coal Fly Ash-Based NaP Zeolite Using the Microwave-Ultrasonic Assisted Method: Preparation, Growth Mechanism, and Kinetics. Chem. Sel. 2023, 8, 202204353. [Google Scholar] [CrossRef]
- Wang, H.; Chang, Q.; Zhou, F.; Liu, Y.; Zeng, L.; Yan, C. Synthesis and characterization of a single phase zeolite X from high silicon fly ash. Sci. Adv. Mater. 2019, 11, 60–67. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Investigation of mechanical and thermal activation on metal extraction from red mud. Sustain. Mater. Technol. 2021, 27, e00246. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Shen, Y.; Li, W.; Zhao, S.; Zhao, Q.; Zhang, Y. A Study on the Removal Characteristics and Mechanism of Phosphorus from Simulated Wastewater Using a Novel Modified Red-Mud-Based Adsorption Material. Separations 2023, 10, 562. [Google Scholar] [CrossRef]
- Belviso, C.; Lettino, A.; Cavalcante, F. Zeolite Synthesis and Steam: Preliminary data Using coal fly ash as raw Material. J. Clust. Sci. 2023, 34, 3041–3046. [Google Scholar] [CrossRef]
- Belviso, C.; Mancinelli, M.; Lettino, A. A green process for zeolite synthesis: Low-temperature vapor phase treatment of natural bauxites. J. Mater. Sci. 2022, 57, 16619–16631. [Google Scholar] [CrossRef]
- Xu, W.; Dong, J.; Li, J.; Li, J.; Wu, F. A novel method for the preparation of zeolite ZSM-5Chem. Soc. Chem. Commun. 1990, 10, 755–756. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Mamedova, G.A. Synthesis of Zeolite with a Gmelinite Structure in the Dolomite-Halloysite-Obsidian System. Glass Phys. Chem. 2016, 42, 518–521. [Google Scholar] [CrossRef]
- Belviso, C.; Lucini, P.; Mancinelli, M.; Abdolrahimi, M.; Martucci, A.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Sturini, M. Lead, zinc, nickel and chromium ions removal from polluted waters using zeolite formed from bauxite, obsidian and their combination with red mud: Behaviour and mechanisms. J. Clean. Prod. 2023, 415, 137814. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Krznaric, I.; Antonic, T.; Bronic, J.; Subotic, B.; Thompson, R.W. Influence of Silica Sources on the Chemical Composition of Aluminosilicate Hydrogels and the Results of Their Hydrothermal Treatment. Croat. Chem. Acta 2003, 76, 7–17. [Google Scholar]
- Inada, M.; Eguchi, Y.; Enomoto, N.; Hojo, J. Synthesis of zeolite from coal fly ashes with different silica–alumina composition. Fuel 2005, 84, 299–304. [Google Scholar] [CrossRef]
- Tang, L.-J.; Xie, X.-Z.; Huang, Y.-X.; Pan, Y.; Mi, J.-X. Phase diagram for hydrothermal alkali activation of kaolin and quartz: Optimal digestion for the synthesis of zeolites. Mater. Chem. Phys. 2022, 290, 126570. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Huertas, F.J.; Lettino, A.; Ragone, P.; Fiore, S. The crystallisation of zeolite (X- and A-type) from fly ash at 25 °C in artificial sea water. Microporous Mesoporous Mater. 2012, 162, 115–121. [Google Scholar] [CrossRef]
- Dimitrov, L.; Valtechev, V.; Nihtianova, D.; Kalvachev, Y. Submicrometer zeoite A crystals formation: Low-temperature crystallization versus vapor phase gel transformation. Cryst. Growth Des. 2011, 11, 4958–4962. [Google Scholar] [CrossRef]
- Thoma, S.G.; Neno, T.M. Vapor phase transport synthesis of zeolites from sol-gel precursors. Microporous Mesoporous Mater. 2000, 41, 295–305. [Google Scholar] [CrossRef]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. The mechanism of the sodalite-to-cancrinite phase transformation in synthetic spent Bayer liquor. Microporous Mesoporous Mater. 1999, 31, 287–302. [Google Scholar] [CrossRef]
- Eugster, H.P. Origin and deposition of trona. Rocky Mt. Geol. 1971, 10, 49–55. [Google Scholar]
- Eugster, H.P. Sodium carbonate-bicarbonate minerals as indicators of PCO2. J. Geophys. Res. 1966, 71, 3369–3377. [Google Scholar] [CrossRef]
- Liu, X.; Fleet, M.E. Phase relations of nahcolite and trona at high P-T conditions. J. Mineral. Petrol. Sci. 2009, 104, 25–36. [Google Scholar] [CrossRef]
- Belviso, C.; Perchiazzi, N.; Cavalcante, F. Zeolite from fly ash: An investigation on metastable behavior of the newly formed minerals in a medium-high-temperature range. Ind. Eng. Chem. Res. 2019, 58, 20472–20480. [Google Scholar] [CrossRef]
| Element | FA | OS | RM |
|---|---|---|---|
| Na2O | 0.54 | 4.31 | 4.03 |
| MgO | 1.43 | 0.15 | 0.21 |
| Al2O3 | 28.21 | 13.20 | 11.46 |
| SiO2 | 46.8 | 74.20 | 7.89 |
| P2O5 | 0.78 | 0.02 | 0.09 |
| K2O | 1.26 | 4.94 | 0.45 |
| CaO | 5.57 | 0.96 | 3.53 |
| TiO2 | 1.49 | 0.09 | 4.82 |
| MnO | 0.06 | 0.08 | 0.21 |
| Fe2O3 | 5.23 | 1.76 | 36.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belviso, C. Determining the Role of Water Molecules in Sodalite Formation Using the Vapor Phase Crystallization Method. Processes 2024, 12, 486. https://doi.org/10.3390/pr12030486
Belviso C. Determining the Role of Water Molecules in Sodalite Formation Using the Vapor Phase Crystallization Method. Processes. 2024; 12(3):486. https://doi.org/10.3390/pr12030486
Chicago/Turabian StyleBelviso, Claudia. 2024. "Determining the Role of Water Molecules in Sodalite Formation Using the Vapor Phase Crystallization Method" Processes 12, no. 3: 486. https://doi.org/10.3390/pr12030486
APA StyleBelviso, C. (2024). Determining the Role of Water Molecules in Sodalite Formation Using the Vapor Phase Crystallization Method. Processes, 12(3), 486. https://doi.org/10.3390/pr12030486







