Abstract
The process of manufacturing drug delivery systems (DDSs) by fused deposition modeling (FDM) with 3D printing requires the availability of a polymeric filament containing the drug of interest. This filament is fused in the printer heating system and used to print polymer/drug volumetric parts. Polymers with pH-dependent solubility are widely known in the literature for their controlled release and drug dissolution-enhancing properties, biocompatibility, and variety of release profiles. Given these characteristics, the study of pH-responsive 3D printing filaments appears as a potential alternative for the development of new 3D printing functional materials for healthcare area applications. In this sense, this work aimed at the preparation and characterization of pH-dependent filaments of the Eudragit E 100 copolymer (E100) containing the model drug Amlodipine (Aml) for potential application in the manufacturing of DDSs by 3D printing. The E100/Aml filaments with two distinct drug concentrations were produced by hot-melt extrusion at 105 °C. The posterior chemical protonation treatment of the filaments for 60 min provided a significant improvement in their flexibility. Microstructural analysis (SEM, XRD, FTIR, and DLS) and thermal studies by DSC proved the feasibility of producing the filaments by hot-melt extrusion without the degradation of their constituent materials. The in vitro dissolution profiles of the E100/Aml samples were evaluated in simulated gastric and intestinal fluids. The facilitated solubility of the polymer in an acidic medium (pH = 1.2) was preserved in the filament form, with rapid and reproducible drug release from the polymer matrix. The saturation of the drug concentration in the medium occurred after 30 min of testing for E100/Aml models. A customized 3D part with geometry and fill control was also printed from E100/Aml filaments as proof of concept.
1. Introduction
The 3D printing techniques represent a set of technologies developed for the manufacture of three-dimensional objects. The parts production process involves the deposition of successive layers of the molten material of interest on the working surface of a 3D printer, based on the user’s design, using software associated with the equipment [,]. Currently, selective laser sintering (SLS), stereolithography (SLA), and fused deposition modeling (FDM) are the most common methods used for the development of devices such as sensors and biosensors, supercapacitors, flexible circuits, biomedical implants, and functional and customized drug delivery devices [,,,]. Polymers are the first choice of materials used in these applications since they present a great diversity of synthetic and natural types with desirable mechanical and thermal characteristics and compatibility with other chemical materials [,].
FDM printing stands out for its versatility and diversity of fabricated materials for applications in various areas of science and technology. In the process of manufacturing a three-dimensional polymeric part using this technique, a cylindrical solid filament (generally based on thermoplastic polymers and/or their composites) with a millimeter in diameter is required. This filament is heated to a temperature higher than its glass transition point in the 3D printer extrusion nozzle, making it reach a fluid state. With the computer-assisted movement of the extruder nozzle and maintaining constant temperature and extrusion flow, it is possible to form overlaid layers (layers deposited sequentially on top of the previous one) of the material on the printer working surface until the 3D part is completely produced [,].
Currently, the development of functional and pharmacologically active FDM 3D printing filaments is on the rise, and an open field of research, both in academia and industry, has formed [,]. Synthetic polymers such as polyethylene glycol, polyethylene oxide, polyvinylalcohol, and polyvinylpyrrolidone, have been the preferred choice to produce these filaments, due to their biocompatibility, biodegradability, and low melting point in comparison to other polymers, including their interaction with drugs, and different release profiles based on their formation with other polymers and/or additives characteristics [,,].
The use of FDM polymeric filaments proves to be an efficient and economical option for the three-dimensional printing of dosage forms [] due to the possibility of producing pharmaceutical forms with different geometries, formulations with new functional materials, and production scalability in comparison with other processes and (or) protocols that have been adopted for this purpose, such as rotary manufacturing processes []. The development of controlled drug release devices, with improved drug bioavailability and solubility at a desired pH, is also achievable based on the interaction between the drug and the selective polymeric matrices [,].
In particular, the development of unconventional FDM polymer/drug filaments with pH-dependent solubility is justified by the constant search for new materials, methods, designs, and personalized formulations that provide the programmed action of the active ingredient, with greater precision regarding the aimed body region, and increased administration interval concerning user’s life quality improvement []. Other aspects, such as specific permeability, porosity, hydrophobicity/hydrophilicity, and drug release kinetics, also justify the exploration of these filaments for application in the 3D printing of drug delivery systems (DDSs) [].
Among the polymers with pH medium solubility dependency, it is possible to highlight the cationic block copolymer poly(dimethylaminoethyl methacrylate/butyl methacrylate/methyl methacrylate) in a 2:1:1 monomer ratio, which belongs to the Eudragit family of thermoplastic pH-soluble polymers (commercially known as ) []. The is soluble in acidic media (below pH 5.0), biocompatible, biodegradable, and non-genotoxic. It has no irritation and/or sensitization potential, and it is classified as safe for use in pharmaceutical formulations by the United States Department of Food and Drugs (with acceptable daily intakes of 20 mg/kg bw) [].
In the case of , the hydration of dimethylamino groups and their molecules in an acidic environment (for example, in gastric fluid) causes a protonation reaction, which favors repulsion between these units. Subsequently, these units can interact with anions in the media to form salts, breaking the original molecule and, consequently, facilitating the polymer’s solubility (in contrast to what happens in a basic environment, where its polymer chain tends to become immobilized, and the material functions as a device to protect the drug from contacting the external environment [,]).
This polymer is already used in the pharmaceutical industry as a coating material in conventional pharmaceutical forms for oral administration, such as capsules and tablets, masking the taste and odor of medicines []. It is also used in the production of micro/nanocapsules, where polymeric matrices act as a support for active ingredients to be released after the ingestion of capsules [,]. However, studies showing its use in the development of FDM 3D printing filaments for applications in drug delivery systems are scarce in the literature [,], largely due to the difficulty of overcoming their brittle and sticky aspect. Cardoso and his collaborators [] developed FDM 3D printing filaments of this copolymer containing hesperidin, a flavonoid extracted from citrus fruits. In the work of Choudhury et al. [], FDM 3D printing filaments containing the berberine chloride drug were fabricated. The authors cited the difficulty of producing the filaments due to the brittle and sticky aspect of the polymer, but they overcame this problem by using an optimized combination of additives. The filaments were used to print custom capsules of the drug. In vitro release tests showed that the capsule models maintained the selective solubility of the polymer in simulated gastric fluid (pH = 1.2), for which the first contact of the drug with the medium took place at around 15 min, while the release of approximately 70% of the active ingredient occurred after 4 h. In addition, the printed parts proved to be matrices for slow drug release in the phosphate buffer (pH = 6.8), with capsule swelling for up to 12 h, with 90% of the drug released after 36 h. The authors concluded that the prototypes were potential candidates for the production of personalized pharmaceutical forms for the programmed release of active substances.
Aiming to significantly contribute to this field of research, this work proposes the preparation and characterization of pH-soluble FDM 3D printing filaments based on the combination of the copolymer and of the model drug Amlodipine Besylate (indicated as a first-choice medication in the treatment of hypertension and angina pectoris), and free of chemical and graft additives.
Polymer/drug composite filaments with different concentrations of Amlodipine were prepared by the hot-melt extrusion process. Microstructural, thermal, and mechanical characterizations, combined with the drug release profiles in different media, were performed. The main objective of the present work was to demonstrate the feasibility of developing filaments containing both components (a support polymer and the drug of interest) and their ability to maintain their original characteristics even after the printing process and, consequently, their potential application in personalized drug delivery systems.
2. Materials and Methods
2.1. Materials
Poly(dimethylaminoethyl methacrylate/butyl methacrylate/methyl methacrylate) (E100, 2:1:1, Figure 1a), in granules, and pH-soluble (below 5.0) was purchased from Evonik Industries, Essen, Germany. The model drug Amlodipine Besylate (Aml, Figure 1b) (C20H25ClN2O5C6H5SO3H, white powder, purity ≥ 98%, the molecular weight of 567.05, saturation solubility of 7.4 µg/mL (in water), LD50 oral-rat at 393 mg/kg with acute toxicity (Category 4, H302-Regulation (EC) 1272/2008 of the European Parliament and Council), and monopotassium phosphate salt (MKP, powder) were purchased from Sigma Aldrich, St. Louis, MO, USA. Sodium hydroxide was obtained from Êxodo Científica, Sumaré, Brazil. All reagents were used as received.
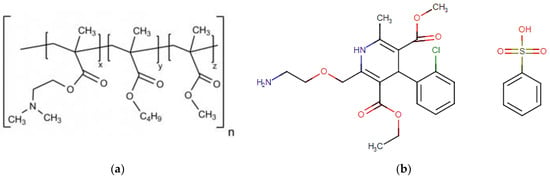
Figure 1.
The 2D representation of the molecular structures of (a) Eudragit® E100 (E100) and (b) Amlodipine Besylate (Aml).
2.2. Sample Preparation
Initially, variable amounts of the model drug (100 and 200 mg) were mechanically mixed with 24 g of the polymer (samples were named and , respectively) in a beaker until a homogeneous dispersion of the materials was obtained (Figure 2a). Samples of the unmixed polymer were also used for comparative purposes.
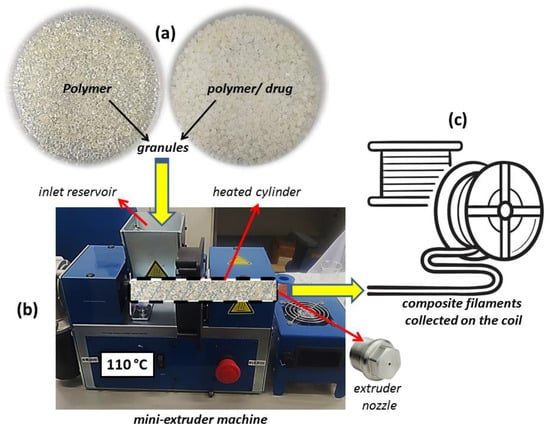
Figure 2.
(a) Unmixed polymer and polymer/drug granules; (b) mini-extruder machine details; and (c) illustration of the filaments collected in a circular coil.
Then, each sample was taken to a Filmaq3D CV mini-extruder (Filmaq3D, Curitiba, Brazil) so that the composite filaments could be produced by the hot-melt extrusion process (Figure 2b). In this step, the materials were physically mixed and inserted into the extrusion machine reservoir, which was connected to a cylindrical galvanized metal tube. During the procedure, a screw inside the tube pushed the material (rotation speed of 10 rpm) to the other end of the cylinder (connected to the extrusion nozzle possessing a diameter of 1.75 mm). The cylindrical extruder tube was initially maintained at 110 °C for 1 h before starting the extrusion process. Then, the extrusion process was activated at 105 °C, with an extrusion rate of 30 cm·min−1. Three independent samples of each filament model were produced and then collected in a circular coil (Figure 2c) for subsequently receiving chemical treatment (for 15, 30, 45, or 60 min) to increase their flexibility. The obtained materials were placed in a plastic reservoir (30 × 40 × 30 cm) to which a humid air flow terminal was initially connected (60% relative humidity concentration) and subsequently subjected to the vapor produced from a solution of 6.8 g of MKP in 0.2 M sodium hydroxide (1 L of deionized water, pH = 6.5). Finally, the samples were left to dry for 24 h at room temperature.
2.3. Bending Testing
The bending resistance of the filaments was evaluated in terms of the elastic modulus (E = ∆σ/∆ε, Young’s modulus) and rupture time (RT), both using data obtained from the elastic zones of the stress (strain) plots and utilizing an EMIC DL 10000 universal mechanical testing machine (INSTRON, Brazil). For the tests, the samples were fixed using two supports separated from each other by 4 cm, and a load cell speed (maximum normal force of 500 N) of 10 mm·min−1 was applied in accordance with the ASTM D790 standard []. Three samples of each filament configuration were considered when analyzing the results.
2.4. Microstructural Properties
2.4.1. Scanning Electron Microscopy (SEM)
Scanning electron microscopy (SEM) images were obtained on a Hitachi TM-1000 SEM device (Hitachi, Ltd., Chiyoda, Japan) with an accelerating voltage of 20 kV. Samples were previously covered with a gold layer applied by a Q150R Quorum device (Quorum Technologies, Lewes, UK) using a 5 nm·min−1 rate for 5 min.
2.4.2. X-Ray Diffraction (XRD)
X-ray diffraction patterns of the drug and filament samples were achieved on a Miniflex Rigaku device (Rigaku Corporation, Osaka, Japan), Kα radiation, with a scanning rate of 0.02°·s−1 and diffraction angle range of 5–50°.
2.4.3. Fourier-Transform Infrared (FTIR) Spectroscopy
Fourier-transform infrared (FTIR) spectroscopy was performed on a Shimadzu Prestige 21 device (Shimadzu Corporation, Kyoto, Japan). FTIR spectra were established in the wavenumber range of 500 to 3500 cm−1 (90 scans and a resolution of 16 cm−1). The samples for testing were prepared from a mixture of 10 mg of the filament and 1 g of KBr, which was soaked and pressed into the pellets.
2.4.4. Dynamic Light Scattering (DLS) Measurements
The mean hydrodynamic size of the particle collection (Z-average size) was obtained by the dynamic light scattering (DLS) technique (in triplicate) on a Malvern Zetasizer Nano Analyzer (Malvern Panalytical Ltd., Malvern, UK). The z-average value is a central and stable measurement parameter for particle size quality control analysis, as defined in the ISO13321 standard [] (particle size analysis–photon correlation spectroscopy).
2.5. Thermal Analysis
The thermal behavior of the samples was studied by Differential Scanning Calorimetry (DSC). A Shimadzu DSC-60 Plus device (Shimadzu Corporation, Japan) was used for the experiments. Aluminum mini pans with lids (2.5 mm in diameter, Shimadzu Corporation) were used to dispose of 2 mg of each type of filament. DSC tests were conducted (i) with one heating cycle in the temperature range of 30 to 285 °C (heating rate of 10 °C.min−1) and (ii) with two continuous heating and cooling cycles (in the range of 30 to 150 °C, using the same heating rate as above).
2.6. Statistical Analysis
ImageJ software (version 13.0.6.) was used to estimate the drug particle size and diameter of each filament type from the SEM micrographs. Data were presented as the mean ± standard deviation, calculated from thirty observations (). The hypothesis test for the difference between means with unknown population deviation was performed as an inferential statistics test, with a significance level () of (the hypothesis of statistically equal means is accepted for p-values greater than α).
2.7. In Vitro Release Kinetics
The in vitro kinetics of the Amlodipine drug’s release from the polymeric filament-shaped matrices was analyzed based on the results of the temporal variation in the drug concentration in gastric () and intestinal () simulated fluids. These acidic and basic solutions were prepared according to the procedures established in the United States Pharmacopeia [,]. The gastric fluid was prepared from a solution of 3 g of NaCl dissolved in 1.5 L of deionized water. The pH of the solution was adjusted to 1.2 ± 0.1 using dilute hydrochloric acid. The intestinal fluid was prepared from a solution of 10 g of potassium phosphate dissolved in 1 L of deionized water. The pH of the solution was altered to 7.2 ± 0.1 using 1 N sodium hydroxide. For testing, 30 mg of each filament sample was immersed in 100 mL of each type of simulated fluid at 36.5 °C (arranged in a 250 mL beaker) while being kept under constant stirring (rotation speed of 100 rpm) [,]. The concentration of Amlodipine in each fluid medium as a function of time was determined from the drug calibration curve (absorbance versus concentration), established at its maximum absorption peak (λ = 243 nm). Three independent samples of each /Aml system were evaluated. For the tests, 2 mL aliquots of each solution containing the filaments were analyzed at fixed time intervals in a spectrophotometer Kasvi K-37 (Kasvi, Pinhais, Brazil). The equivalent absorbance intensities were converted into relative drug concentration, , where and are the initial and time-dependent concentrations, respectively. The drug release kinetics in the simulated media were analyzed for a 165 min period.
2.8. FDM 3D Printing
A commercial direct drive 3D printer (model Kywoo3D Tycoon was used to print the test part. In this printer, the filament is directed into a PTFE tube with an internal diameter of 2.0 mm in contact with the hot end of the extruder nozzle. Thus, the filament takes a short path (about 10–15 cm) to the hot end (compared to Bowden-type printers), minimizing the clogging of the printing system due to possible filament breaks and, consequently, considerable material loss. A three-dimensional dosage form model of Amlodipine (cylindrical geometry, with 6 mm in base diameter and 12 mm in height) was designed in a CAD environment. Thus, the part was printed using the Repetier Host slicer software v. 2.2.4, with a grid-type infill pattern (24 layers, 20% inner density) and an extrusion temperature of 145 °C.
3. Results
3.1. Structural and Morphological Characterization
Samples of the /Aml composite filaments are shown in Figure 2. The amount of material used in the preparation stage resulted in a production of approximately 3.8 m of continuous filament for each /Aml model (Figure 3a) without significant material loss during the hot-melt extrusion (HME) process.
Figure 3.
(a) Sample of E100/Aml continuous filament resulting from the extrusion process at 105 °C. (b) Photo image of small segments of E100/Aml filaments under bending after the chemical treatment.
It is important to highlight that the heating cylinder of the mini-extruder machine (Figure 2), which received the material for extrusion, is composed of two heating electrical resistors and that the entire system, including the extruder, is covered by a thermal blanket (15 cm in length, from the extruder nozzle). With this arrangement, the temperature gradient along the cylinder tends to decrease considerably compared to other extrusion systems (where heating occurs only at the end of the cylinder, close to the extruder nozzle). This directly results in a more homogeneous heat conduction through the material over a greater range of the tube length [].
Thus, it was possible to initially stabilize the temperature of the cylindrical extruder tube at 110 °C for 1 h before starting extruding the /Aml mixtures using a lower temperature of 105 °C. Improvements noted in the filament production process included the reduction in the sticky aspect of the (acrylic copolymer) inside the tube, which would be observed at higher extrusion temperatures [] and the guarantee of its fluidity and the prevention of the thermal expansion of the filament at the exit of the extruder nozzle, which would negatively influence the expected diameter [,].
Then, the polymeric filaments were subjected to the chemical process of protonation []. Protonation is the action of adding a proton to an atom, molecule, or ion and differs from hydrogenation, which consists of a process by which hydrogen atoms are added to unsaturated fats and oils (unsaturated molecules are those that contain double bonds, while saturated ones contain only single bonds, and, consequently, these double bonds have the potential to accept hydrogen atoms and, thus, become hydrogenated); additionally, during protonation, a change in the charge of the protonated species takes place (and mass and chemical properties are altered), while in the hydrogenation process, it is unaffected.
In this final phase of filament preparation, they are exposed to a vaporized protonated solution (pH = 6.5), allowing the dimethylamino groups of their constituent molecules to receive H+ protons from the solution. This causes the molecules to repel each other due to electrical repulsion from these neighboring protonated groups. This effect occurs radially, from the surface to the bulk of the material and, as a consequence, the filaments acquire greater flexibility when no chemical treatment is applied (Figure 3b).
In this sense, the improvement in the filaments’ flexibility was quantified based on the analysis of the elastic modulus (E) variations as a function of the sample’s exposure time, t, to chemical treatment (Figure 4).
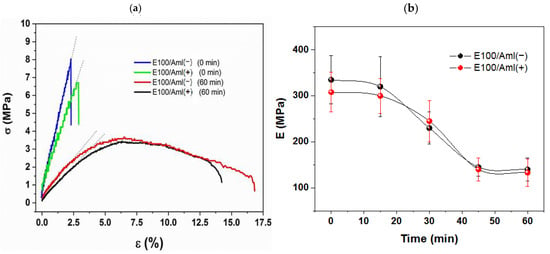
Figure 4.
(a) The σ versus ε curves for E100/Aml(−) and E100/Aml(+) filament samples without (0 min) and with 60 min of chemical treatment; (b) elastic modulus (E) of the E100/Aml filaments as a function of the chemical reaction time.
E100/Aml(−) and E100/Aml(+) filaments without chemical treatment displayed purely elastic behavior (characterized by their respective σ vs. ε linear curves (0 min), Figure 4a), with high stiffness and brittleness compared to the normal stress applied during the bending tests. These characteristics were confirmed by the estimated average values of the elastic modulus, E, of approximately 335 and 308 MPa, and of the rupture time, RT, at around 43.2 and 48.4 s, for the E100/Aml(−) and E100 /Aml(+) filaments, respectively. On the other hand, the samples that received the chemical treatment for 60 min had their elastic behavior reduced, with the emergence of a plastic deformation phase (Figure 4a) and a longer time for the parts to rupture.
With the increasing exposure time, from 15 to 60 min, for the polymeric matrix to the protonation reaction, a progressive decrease in the E values was observed. The inflection point of the E vs. t curves (Figure 4b) was observed at about 30 min of exposure time, with E = 230 MPa and RT = 75.8 s for the /Aml(−) filaments and E = 245 MPa and RT = 69.6 s for the /Aml(+) filaments. This represents a significant variation in these parameters when compared with the initial mechanical properties displayed by the filaments. The lower values of E were found for protonation reaction times in the interval between 45 and 60 min. For the last measured reaction time, the values of E = 140 MPa and E = 133 MPa (approximately 58% and 57% of reduction in relation to the initial E values), and RT = 104.5 s and RT = 95.2 s (with a 141% and 98% increase for samples with low- and high-drug concentrations, respectively) support the claim that there is an improvement in flexibility for filaments for the proposed use.
SEM micrographs of the Amlodipine Besylate (Aml) particles and of the flexible E100, E100/Aml(−), and E100/Aml(+) filaments are shown in Figure 5. Amlodipine particles (Figure 5a) are characterized by a microplate-type geometry and have irregular dimensions [,]. From the analysis of the SEM micrographs, using the ImageJ software, it was possible to verify two distinct particle size populations, centered mostly at 23.45 ± 5.23 µm and at 2.15 ± 0.74 µm. This micrometer-scale size distribution of the Amlodipine particles is one of the main contributing factors to its low solubility in water [,].
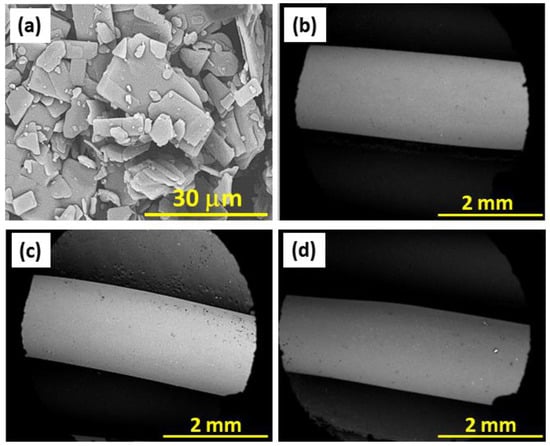
Figure 5.
SEM images of the (a) Amlodipine (Aml) particles; (b) E100, (c) E100/Aml(−), and (d) E100/Aml(+) filaments.
Drug-free filaments ( sample, Figure 5b) were also produced and analyzed for comparison purposes with the /Aml(−) (Figure 5c) and /Aml(+) (Figure 5d) filaments. They all exhibited a surface without apparent structural defects (no bubbles, no cracks, and/or abrupt variations in diameter resulting from heating are visibly observable). The absence of these geometric discontinuities is a determining factor for the usability of these filaments in conventional 3D printers [,].
In addition, the pristine E100 filament presented an average sample diameter of 1.79 ± 2.01 mm (n = 30), while the estimated diameters of the /Aml(−) and /Aml(+) ones were 1.81 ± 2.08 mm (n = 30) and 1.80 ± 1.25 mm (n = 30), respectively. The statistical evaluation demonstrated that the average diameter of the filaments does not present significant variations (p-value > α for all sample comparisons). This indicates that the presence of the drug in the bulk of the filament does not cause evident changes in the volume/unit length relationship and, consequently, in the surface area of the samples in contact with the dissolution media for the study of drug release kinetics.
It is important to highlight that a low-mass concentration of the drug was used in the mixtures to prepare the filaments, which corroborates the invariability of the filament diameters. In other words, the control of these parameters shows that the drug concentration variability, which can be released from the polymer matrix in the gastrointestinal fluids, can be directly related to specific parameters, such as the pH-dependent solubility of the copolymer, and to the quantity and dispersion of the drug mass in the polymeric matrix [].
Figure 6 highlights the XRD spectra obtained for the pristine Aml and for , /Aml(−), and /Aml(+) filament samples. The Aml spectrum confirmed the crystalline nature of the drug, associated with the presence of its characteristic diffraction peaks in the range of the 2θ angles studied (at 5.9°, 10.4°, 11.7°, 13.1°, 14.5°, 18.1°, 19.3°, 20.2°, 23.4°, and 24.4°) [,].
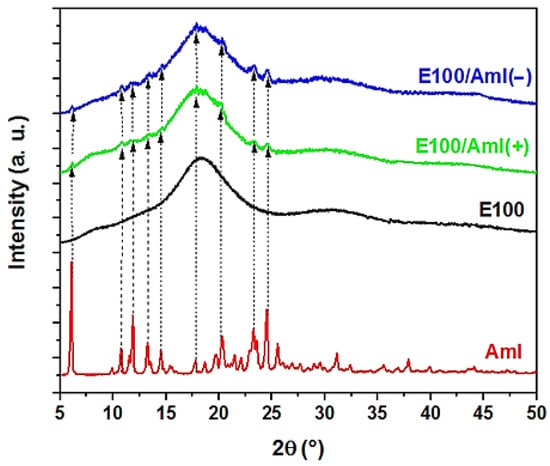
Figure 6.
Characteristic X-ray diffraction patterns of the Amlodipin (Aml) drug and E100, E100/Aml(−), and E100/Aml(+) filament samples.
The X-ray spectrum of the type filaments showed a broad peak, with the vertical displacement of the baseline, in a wide 2θ range between 10 and 25°, as a signature of an amorphous solid matrix. This result is in agreement with the XRD data of the polymer in its original form [].
The XRD spectra of the /Aml filaments showed similar characteristics, as displayed in Figure 6. The diffractograms of the /Aml(−) and /Aml(+) filament samples showed a broad peak in the same 2θ angle region as observed for the pure E100 filament, which could be attributed to the predominance of the polymeric phase in the polymer/drug sample. In addition, in this 2θ range, although the Aml peaks were not as explicit as in the spectrum of the pure drug sample, it was possible to distinguish small peaks over the trace, indicating the presence of the Aml crystalline phase. This is probably due to the small amount of drug in the samples and/or the decrease in the degree of crystallinity of the drug due to its interaction with the polymeric matrix.
The molecular vibrations of the filament samples were studied by FTIR spectroscopy (Figure 7). The spectrum of pristine Amlodipine (as provided by the manufacturer) exhibited all of its characteristic vibrational bands, as follows: 3305 cm−1 for the molecular vibration of valence electrons of the N-H bond; 3159 cm−1 for valence electrons of the C-H bond (aromatic); 2982 cm−1 for valence electrons of the C-H bond (aliphatic); 1695 cm−1 for valence electrons of the bond (aliphatic); 1675 cm−1 for valence electrons of the bond (aliphatic); 1614 cm−1 for valence electrons of the bond (aromatic); 1497 cm−1 for the out-of-plane folding of N-H bonds; 1304 cm−1 for the coplanar folding of pyridine; 1267 cm−1 for symmetric stretching of the C-O bond; 1207 cm−1 for asymmetric stretching of the bond; 1090 cm−1 for symmetric stretching of the bond; 1015 cm−1 for symmetrical stretching of the C-O-C bond; 758 cm−1 for the out-of-plane folding of the C-H bond; 730 cm−1 for the vibration of valence electrons of the C-S bond (aliphatic); 690 cm−1 for the coplanar folding of the C-S bond; and 565 cm−1 for the out-of-plane folding of the C-N bond [,,,,,,,].
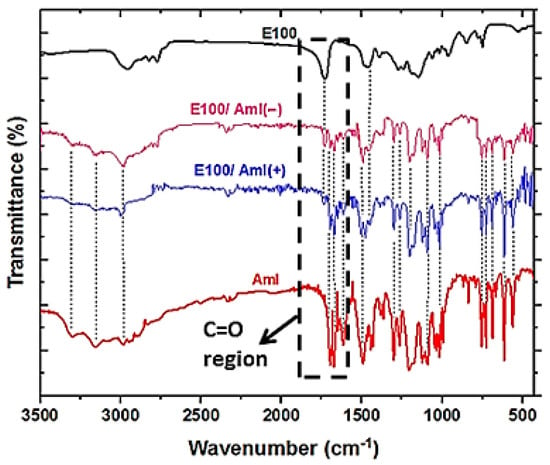
Figure 7.
FTIR spectra of the pristine Aml, E100, and E100/Aml filaments. The details show the region of the polymer and drug C=O vibrational bands.
The FTIR spectrum of the filament sample showed the typical bands of the copolymer in its original form, centered at 2956 cm−1 (with asymmetric stretching of the C-H bond), 2875 cm−1 (with symetric stretching of the C-H bond), 2820 cm−1 (with asymmetric stretching of the H3C-N-CH3 bond), 2772 cm−1 (with symmetric stretching of the H3C-N-CH3 bond), 1730 cm−1 (with symmetric stretching of the bond of the ester group), 1460 cm−1 (with asymmetric stretching of the H-C-H bond), 1388 cm−1 (with the coplanar folding of the C-H bonds), and 1150 cm−1 (with the asymmetric stretching of the C-O bond of the ester group) [,,,].
In addition, the FTIR spectra of the /Aml(+) and /Aml(−) filaments exhibited molecular vibration bands through the whole wavenumber range of interest (without significant differences). Whether using a high or low concentration of the drug dispersed in the polymer matrix, it is possible to discern the presence of a considerable number of vibrational bands for both constituent materials. In other words, the presence of most of the vibration bands for both the polymer and drug in the E100/Aml samples, located at the respective characteristic wavenumbers of the isolated substances, highlights the predominance of the physical interaction between the polymer and drug molecules. In fact, the molecular structures of these components (Figure 1) do not support significant chemical interactions for the formation of chemical complexes between these materials []. The most evident change in the FTIR spectrum of the E100/Aml filament samples is the intensity decrease in the carbonyl vibrational bands (at 1730 cm−1 for the polymer and at 1695 cm−1 for the drug) in relation to the spectrum of the pristine Amlodipine and the E100 filament. This characterizes polar interactions between the drug and the polymer, which are important to demonstrate the physical interaction concerning the constituent materials and the molecular stability of the drug after the HME process.
The thermal characteristics of the pristine Amlodipine drug (Aml), , /Aml(−), and /Aml(+) filaments were analyzed by DSC (Figure 8). The DSC spectrum of the Aml sample shows a narrow and well-defined peak at 210 °C, already known as its melting point (Figure 8a) [,]. This result is characteristic of a crystalline material, which was already validated by the XRD results. In addition, Aml degradation signals were not detected in the spectrum, demonstrating the thermal stability of the component in the analyzed temperature range.
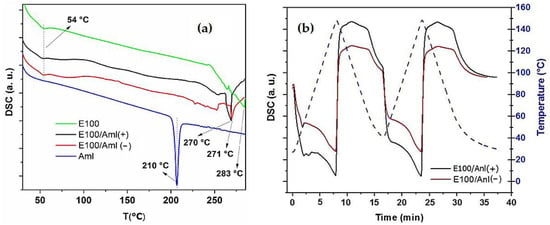
Figure 8.
(a) DSC spectra (heating) of the Aml (pristine drug) and
E100, E100/Aml(−), and E100/Aml(+) filaments; (b) DSC curves, with two continuous heating and cooling cycles for the E100/Aml(−) and E100/Aml(+) filaments (temperature range of 30–150 °C).
The DSC spectra of the and polymer/drug filaments showed an endothermic signal at 54 °C due to the glass transition temperature of the copolymer (Figure 8a) [,]. In these samples, the peaks observed at 283, 271, and 270°C are a signature of the degradation point of the copolymer in , /Aml(−) and /Aml(+) filaments, respectively []. These data indicate that the extrusion process of the polymer/drug filaments at 105 °C (which exposes the materials thermally) is adequate without altering the properties of the drug and polymer molecules.
It is also possible to observe the absence of the drug melting point in the DSC spectra of the /Aml(−) and /Aml(+) filaments. Thus, as the presence of Amlodipine in these samples was confirmed by XRD and FTIR, the absence of its melting point in the DSC spectra suggests the better dispersion of the drug in the amorphous matrix. Specifically, a significant portion of the micrometric particles of Amlodipine (as confirmed by SEM analysis) tend to dissolve and decrease in size in the bulk of the filament, increasing the drug contact area []. This behavior is mentioned in the literature for solid dispersions of Amlodipine with other polymers, such as poly(vinyl pyrrolidone), chitosan, microcrystalline cellulose, and poly(lactic acid-co-glycolic acid), all aiming to facilitate the dissolution of the drug in the medium of interest [,,,].
The analysis of the hydrodynamic particle size of the Amlodipine drug obtained by DLS (Figure 9) in the acidic medium confirms this hypothesis. The drug particles, as supplied by the manufacturer, showed a heterogeneous particle size distribution, with three main populations at 175.5 60.71 nm, 607.6 ± 203.2 nm, and 5380.0 ± 322.9 nm (with 31%, 23%, and 46% intensity, respectively). In addition, DLS data showed that the Z-average size for the drug particles was estimated to be 691.7 nm.
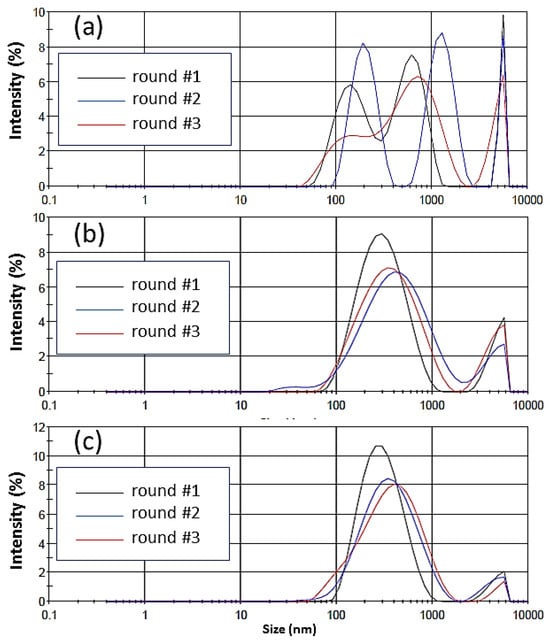
Figure 9.
Hydrodynamic particle size in accordance with intensity of the (a) Amlodipine drug supplied by the manufacturer in an acidic medium and (b) Amlodipine drug released from the bulk of the E100/Aml(−) and (c) E100/Aml(+) filaments in an acidic medium. Three rounds of measurements were performed for each sample.
On the other hand, the Amlodipine particles that were released from the E100/Aml(−) and E100/Aml(+) matrices in the same medium presented two well-defined particle size populations, centered at 327.6 173.2 nm (87% intensity) and 4626.0 823.8 nm (13% intensity) for the E100/Aml(−) model, and centered at 321.5 157.0 nm (93% intensity) and 4590.0 838.8 nm (7% intensity) for the E100/Aml(+) model. In addition, their Z-mean calculated values were 332.4 nm and 284.8 nm for the filaments with low and high drug concentrations, respectively. Thus, it is possible to infer from the results that the interaction of the drug with the polymer promotes not only a significant reduction in the intensity of the micrometric particles of Amlodipine but also makes the majority of the population particles, around 90% of the intensity of the measurements, display at about 320 nm, and as a consequence, a reduction in the mean hydrodynamic size (Z-mean value) of the drug in the fluid medium occurs.
In fact, the pressure that disintegrates particles is significantly higher the smaller their size. Drugs characterized by a smaller particle size are known to have greater interfacial solubility due to a thinner diffusion layer and greater surface area, which is generally preferable for the oral administration of drugs []. The encapsulation of Amlodipine under these conditions in the pH-dependent matrix of the E100 polymer is another differentiating factor regarding the programmed release of the drug. It is known that the dispersion of drugs poorly soluble in water and with a low dissolution rate (such as Amlodipine) in amorphous solid solutions of the copolymer can abruptly increase the drug dissolution [,]. A higher dissolution of Amlodipine can be expected from solid dispersion, probably due to the presence of amorphous Aml-particles incorporated into the E100 matrix. This indicates that the polymer can inhibit the recrystallization of the drug molecules [].
In this sense, the choice of the polymer for the production of 3D printing filaments can be justified, firstly, because it increases the surface area of action of the Amlodipine particles dispersed in the matrix and, secondly, since it provides 3D printing filaments of amorphous solid dispersions of the drug with much smaller particles than those supplied by the manufacturer, with a consequent improvement in its dissolution and bioactivity.
Figure 8b shows the DSC results of the /Aml filament samples subjected to two complete and continuous cycles of heating and cooling, in the range of 30–150 °C. It is important to highlight that the components of these samples (polymer and drug) had, previously, been subjected to a heating process at 105 °C in the heated tube of the extrusion machine. The thermal behavior study of the /Aml samples through two new cycles allowed the temperature gradient with which these filaments were subjected in conventional FDM 3D printers to be stimulated during the three-dimensional printing of the engineered solid pharmaceutical products, and/or on the possible reuse of the waste materials after FDM 3D in the production of new filaments (in both cases, considering a second and/or a third heating/cooling moment).
The results show that, in both cycles, the thermal signatures of the /Aml(−) and /Aml(+) samples are preserved in relation to the DSC spectrum obtained for the same samples after the first heating process (in the same temperature range). In the spectra, the original amorphous characteristic of the copolymer is kept, and no degradation signal of the constituent materials is observed []. The data obtained are in agreement with the earlier reported results, which demonstrated that the initial mass loss and subsequent complete degradation of the drug [] and polymer [,,] only occurs at high temperatures (above 200 °C, and reaching up to 600 °C, to attain the total degradation of the drug).
3.2. Drug Release Evaluation
The drug release profiles from the polymeric matrix of the filaments in simulated gastric (pH = 1.2, acidic medium) and intestinal (pH = 7.2, basic medium) fluids are presented in Figure 10. It is possible to observe the kinetics of the average concentration of Amlodipine available in the acidic environment, which in the first few minutes of the trials displayed an exponential behavior for both /Aml(−) and /Aml(+) filaments. The low variability of the concentrations observed at each evaluated time indicates the homogeneous and reproducible distribution of the drug in the bulk of the filaments since the average concentration was obtained for independent samples of each /Aml system.
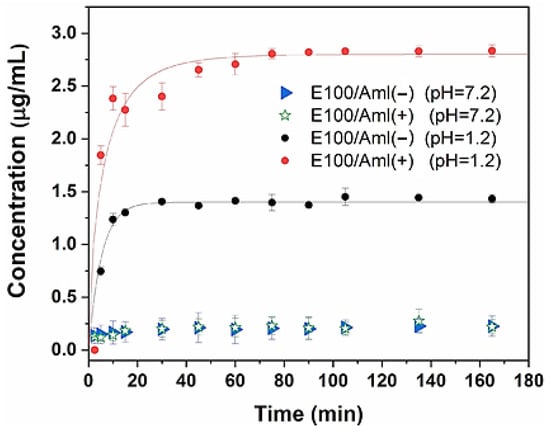
Figure 10.
In vitro release kinetics of Amlodipine in acidic (pH = 1.2) and basic (pH = 7.2) media from the polymeric matrix of the E100/Aml(−) and E100/Aml(+) filaments.
The drug concentrations released into the medium as a function of time assumed an asymptotic behavior (saturation value) around 30 min after the tests were initiated for both hybrid filaments, achieving saturation concentrations of 1.38 μg/mL and 2.81 μg/mL for the /Aml(−) and /Aml(+) filaments, respectively.
These characteristics are typical of the copolymer supplied by the manufacturer and confirm that the pH-dependent solubility of the polymer is maintained for usage as a pH-responsive 3D printing filament. When the Eudragit® E100 solid matrix containing Amlodipine is placed in contact with the simulated acid fluid, the superficial radial layers of the filament are hydrated, and the copolymer chains in this region are relaxed and then dismantled, releasing the drug into the medium via an erosion mechanism of the polymer matrix. This release mechanism is established rapidly from the surface to the core of the filament until all the previously encapsulated drugs are available in the solution. In addition, the rapid-release profile of Amlodipine in the acidic medium was successfully modeled using the Weibull function (with solid lines following the experimental data in Figure 8) for the case of drug diffusion from cylindrical matrices [,]:
In this expression, is the available concentration of the drug at time t; is the asymptotic concentration of the drug; is the time scale factor; and is the parameter representing the degree of kinetics release. The modeling allowed a value of for both /Aml(−) and /Aml(+) samples to be estimated, which is quantitatively in accordance with the exponential kinetics of the drug release pattern found. The combination of these results indicates that the /Aml filaments provide the rapid release of Amlodipine, with reproducibility and a typical diffusive characteristic.
On the other hand, a negligible concentration of the drug available in the basic medium was observed throughout the analyzed time interval, indicating that the matrix acted as a barrier device to minimize the drug’s contact with the external medium.
The application of E100/Aml filaments in FDM 3D printing was exemplified by printing a model part of one typical pharmaceutical form previously designed in CAD software (https://www.autodesk.com/hk/solutions/3d-cad-software, accessed on 20 October 2024) (Figure 11).
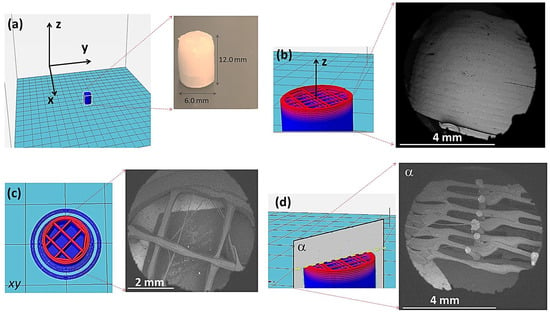
Figure 11.
(a) CAD project of a 3D model part with a cylindrical shape and a photo image of the corresponding pharmaceutical form, produced from E100/Aml filaments; (b) CAD design and SEM image of the lateral surface of the part; (c) design of the part inner circular section and SEM image of the printed material; and (d) interior of the printed part seen from section α.
As mentioned in Section 2.8 (FDM 3D printing), the fabricated filaments were used with a commercial direct drive 3D printer to create a volumic shape as proof of concept. One part, with cylindrical geometry (Figure 11a), was previously subdivided into 24 layers (details in Figure 11b) using the slicer software. A grid type with internal filling was used (layers with parallel filling lines, perpendicular to the parallel filling lines of the subsequent layer—see details of the CAD project of the internal region of the part in Figure 11b,c), with a filling factor of 20%. The extrusion temperature of 145 °C was suitable so that the printing process could take place on the flat working surface of the printer. The total printing time of the part was 5 min and 24 s.
Figure 11b shows an SEM image of the lateral surface of the printed pharmaceutical form. It is possible to observe in detail the overlapping layers of /Aml material in the cylindrical geometry. SEM images in Figure 11c,d detail, in that order, the normal and parallel cross-sections (relative to the z-axis) of the three-dimensional printed part, demonstrating the high execution level of the printing procedure, even considering the small dimensions of the designed geometry. In this sense, given the characteristics observed for the polymer/drug filament, the filling level of the part obtained with them is an important factor to consider when determining the amount of drug available for parts with the same external dimensions.
As previously explained, although the importance of the E100 polymer in the pharmaceutical industry is recognized, a limited number of studies on the development of 3D printing filaments from E100 polymer are found in the literature [,]. The acrylic nature of the polymer, with brittle and sticky aspects, makes it difficult to obtain filaments with adequate flexibility for their workability in FDM 3D printers (given their extruded cylindrical geometric shape with millimeter diameters).
Cardoso and his collaborators [] developed FDM 3D printing filaments of this copolymer containing hesperidin, which is a flavonoid extracted from citrus fruits. The authors highlighted the difficulties in optimizing filament extrusion process parameters. One of the actions involved was adapting the design of a commercial mini-extruder machine so that the mixture between the drug and polymer would be uniform in the heated reservoir of the device and so that the extruded filaments would not display surface defects and possess a constant diameter. The filaments were produced at a temperature of 130 °C and were then used to print cylindrical three-dimensional parts, 10 mm in diameter and 3 mm in height, with a grid-type infill pattern (60% infill), using a printing temperature equal to that of the filament production. The results of microstructural and thermal characterization and in vitro drug release tests demonstrated the feasibility of the materials for the production of customized pharmaceutical forms of the drug.
In the work by Choudhury et al. [] FDM 3D printing filaments containing the drug berberine chloride were fabricated and applied to print customized hollow capsules. The authors cited the difficulty of producing E100 filaments, but they overcame the problem by using an optimized combination of talc (20% w/w) and triethyl citrate (3.5% w/w) as additives. Microstructural and thermal analyses showed that the extrusion of the filaments at 100 °C and the printing process of the prototypes at 160 °C did not cause the degradation of either the polymer or drug, therefore preserving the original chemical characteristics of both materials in the printed parts.
Sadia et al. [] used fused deposition modeling 3D printing to fabricate pH-soluble pharmaceutical tablets capable of the immediate release of various model drugs. They investigated the addition of non-melting fillers to the methacrylic matrix to facilitate FDM 3D printing and explored the impact of the filler nature, the compatibility with the gears of the 3D printer, and the polymer/filler ratio on the 3D printing process. The optimized filaments were based on Eudragit E and TCP (tribasic calcium phosphate). Following the two thermal fabrication processes, hot melt extrusion and fused deposition modeling 3D printing and drug contents of 94.22%, 88.53%, 96.51%, and 93.04% of 5-ASA, captopril, theophylline, and prednisolone, respectively, were added to the filaments. They concluded that the optimal range of the non-melting component of 20–50% within the filament composition enabled the fabrication of well-defined caplets. The level of drug incorporation was above 94% after the thermal fabrication steps, remaining intact in the tablets for the cases of theophylline, 5-ASA, and prednisolone, whilst a significant drop in captopril content was observed.
In comparison with previous results, the present study demonstrated that the production of E100 filaments containing the model drug Amlodipine was viable, significantly contributing to this field of study. The use of the chemical protonation process in the post-extrusion preparation of the filaments improved their flexibility for use in FDM 3D printers, eliminating the need for chemical additives and grafting. Potential challenges for production scalability and/or programmed modification in the release rates of three-dimensional drug parts produced from these filaments involved the adequate control of printing parameters such as extruder nozzle diameter, printing speed, geometry, and the infill rate of these parts.
4. Conclusions
The combination of the mechanical, microstructural, and thermal characterization results proved appropriate as the hot-melt extrusion process parameters were chosen for the production of flexible and uniform E100 filaments containing Amlodipine. The extrusion process was set at 105 °C, and one exemplifying three-dimensional parts was printed at 145 °C using the produced filament. The thermal stability of the polymer and the drug was preserved in the hybrid filaments after two heating and cooling cycles, and a smaller Z-average size of the drug particles released from the amorphous matrix was observed (with a reduction from 691.7 nm, as provided by the manufacturer to 332.4 nm and 284.8 nm 284.8–332.4 nm after their interaction with the polymer and release in the acidic medium). These observations were important to demonstrate the efficiency of the 3D printable Amlodipine delivery systems with improved action and the possibility of reusing the material to produce new filaments.
In vitro release tests showed that the pH-dependent characteristic of the polymer was preserved in the filaments, with rapid release of the drug occurring only in an acidic environment. In addition, the saturation of the accumulated drug concentration in the acidic medium occurred after 30 min of testing. The low variability of the mean drug concentration values in the acidic medium as a function of time, observed for independent filament samples, indicates a homogeneous dispersion of Amlodipine in the cylindrical polymeric matrix in accordance with the results of thermal and microstructural characterizations. The 3D printing of the amorphous solid solution of the drug (which was projected with customized surface and filling geometry) from these filaments, using a conventional FDM 3D printer, proved the potential of the technique for the development of new pharmaceutical forms of Amlodipine, with reproducibility, individualized therapy, controlled release, and improved drug dissolution.
After the satisfactory results of this initial study, future research will be continued by the authors, with the objective of studying variations in the drug release profile from copolymer matrices with different three-dimensional geometries of the same specific surface area (evaluating simultaneously the reproducibility of the printing process); establishing a correlation between the fill rate of a polymer/drug part and the concentration of the available drug; developing E100 filaments with other poorly water-soluble drugs and/or with broad-spectrum antimicrobial drugs for innovative applications in biotechnology.
Author Contributions
Conceptualization, E.S.A., G.M.N., P.H.N.C., G.F.T., F.S.S. and P.M.F.; methodology, E.S.A., G.M.N., P.H.N.C., G.F.T., N.C.O., F.S.S. and P.M.F.; writing—review and editing, E.S.A., G.M.N., P.H.N.C., G.F.T., P.M.F., F.S.S. and N.C.O.; data curation, E.S.A., G.M.N., P.H.N.C., G.F.T., P.M.F., N.C.O., F.S.S. and E.M.E.d.S.; writing—original draft preparation, E.S.A., G.M.N., P.H.N.C., P.M.F. and F.S.S.; visualization, E.S.A., G.M.N., F.S.S., P.H.N.C., G.F.T., P.M.F., N.C.O. and E.M.E.d.S.; project administration, E.S.A. and F.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco—FACEPE, Brazil, APQ 1387-3.03/22 and IBPG-0849-3.03/20 projects; Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Brazil; and Portuguese national funds through FCT—Fundação para a Ciência e a Tecnologia, Portugal, UIDB/00285/2020 and LA/P/0112/2020 projects.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Elhadad, A.A.; Rosa-Sainz, A.; Canete, R.; Peralta, E.; Begines, B.; Balbuena, M.; Alcudia, A.; Torres, Y. Applications and multidisciplinary perspective on 3D printing techniques: Recent developments and future trends. Mater. Sci. Eng. R Rep. 2023, 156, 100760. [Google Scholar] [CrossRef]
- Gao, G.; Ahn, M.; Cho, W.-W.; Kim, B.-S.; Cho, D.-W. 3D Printing of Pharmaceutical Application: Drug Screening and Drug Delivery. Pharmaceutics 2021, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- DePalma, K.; Walluk, M.R.; Murtaugh, A.; Hilton, J.; McConky, S.; Hilton, B. Assessment of 3D printing using fused deposition modeling and selective laser sintering for a circular economy. J. Clean. Prod. 2020, 264, 121567. [Google Scholar] [CrossRef]
- Padmakumar, M. Additive manufacturing of tungsten carbide hardmetal parts by selective laser melting (SLM), selective laser sintering (SLS) and binder jet 3D printing (BJ3DP) techniques. Lasers Manuf. Mater. Process 2020, 7, 338–371. [Google Scholar]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Cardoso, P.H.N.; Araújo, E.S. An Approach to 3D Printing Techniques, Polymer Materials, and Their Applications in the Production of Drug Delivery Systems. Compounds 2024, 4, 71–105. [Google Scholar] [CrossRef]
- Reddy, N.; Ananthaprasad, M.G. Polymeric materials for three-dimensional printing. In Additive Manufacturing; Woodhead Publishing: Sawston, UK, 2021; pp. 233–274. [Google Scholar] [CrossRef]
- Pistone, M.; Racaniello, G.F.; Arduino, I.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Rizzi, R.; Franco, M.; Lopedota, A.; Denora, N. Direct cyclodextrin-based powder extrusion 3D printing for one-step production of the BCS class II model drug niclosamide. Drug Deliv. Transl. Res. 2022, 12, 1895–1910. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, S.; Almotairy, A.; Bandari, S.; Repka, M.A. Development of multifunctional drug delivery system via hot-melt extrusion paired with fused deposition modeling 3D printing techniques. Eur. J. Pharm. Biopharm. 2023, 183, 102–111. [Google Scholar] [CrossRef]
- Lu, M. Novel Excipients and Materials Used in FDM 3D Printing of Pharmaceutical Dosage Forms. In 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing; Mohammed Maniruzzaman, M., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 211–237. [Google Scholar] [CrossRef]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T. Development of 3D printed tablets by fused deposition modeling using polyvinyl alcohol as polymeric matrix for rapid drug release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and fabrication of drug-delivery systems toward adjustable release profiles for personalized treatment. View 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Shojaie, F.; Ferrero, C.; Caraballo, I. Development of 3D-Printed Bicompartmental Devices by Dual-Nozzle Fused Deposition Modeling (FDM) for Colon-Specific Drug Delivery. Pharmaceutics 2023, 15, 2362. [Google Scholar] [CrossRef]
- Mahmood, M.A. 3D Printing in Drug Delivery and Biomedical Applications: A State-of-the-Art Review. Compounds 2021, 1, 94–115. [Google Scholar] [CrossRef]
- Sester, C.; Ofridam, F.; Lebaz, N.; Gagnière, E.; Mangin, D.; Elaissari, A. pH-Sensitive methacrylic acid–methyl methacrylate copolymer Eudragit L100 and dimethylaminoethyl methacrylate, butyl methacrylate, and methyl methacrylate tri-copolymer Eudragit E100. Polym. Adv. Technol. 2020, 31, 440–450. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S.; Jena, G.K.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef]
- Cardoso, P.H.N.; Oliveira, C.Y.B.; Nunes, M.; Tavares, G.F.; Faia, P.M.; Araújo, E.S. Eudragit E100/Hesperidin 3D Printing Filaments: Preparation, Characterization, and In Vitro Release Studies. Appl. Sci. 2023, 13, 11558. [Google Scholar] [CrossRef]
- Elizondo-Luevano, J.H.; Castro-Ríos, R.; Parra-Saldívar, R.; Larqué-García, H.; Garza-Tapia, M.; Melchor-Martínez, E.M.; Chávez-Montes, A. Influence of the polymer and solvent variables on the nanoencapsulation of the flavonoid quercetin: Preliminary study based on Eudragit® polymers. Appl. Sci. 2023, 13, 7816. [Google Scholar] [CrossRef]
- Llera-Rojas, V.G.; Hernández-Salgado, M.; Quintanar-Guerrero, D.; Leyva-Gómez, G.; Mendoza-Elvira, S.; Villalobos-García, R. Comparative study of the release profiles of ibuprofen from polymeric nanocapsules and nanospheres. J. Mex. Chem. Soc. 2019, 63, 61–70. [Google Scholar] [CrossRef]
- Choudhury, D.; Murty, U.S.; Banerjee, S. 3D printing and enteric coating of a hollow capsular device with controlled drug release characteristics prepared using extruded Eudragit® filaments. Pharm. Dev. Technol. 2021, 26, 1010–1020. [Google Scholar] [CrossRef]
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Material. ASTM International: West Conshohocken, PA, USA, 2001.
- ISO13321; Particle Size Analysis—Photon Correlation Spectroscopy. ISO: Geneva, Switzerland, 1996.
- United States Pharmacopeia. Reagents, Simulated Gastric Fluid TS; USP-NF: Rockville, MD, USA, 2023. [Google Scholar] [CrossRef]
- United States Pharmacopeia. Reagents, Simulated Intestinal Fluid TS; USP-NF: Rockville, MD, USA, 2023. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.; Tzounis, L. Fused Filament Fabrication Three-Dimensional Printing Multi-Functional of Polylactic Acid/Carbon Black Nanocomposites. C 2021, 7, 52. [Google Scholar] [CrossRef]
- Alhnan, M.A. Solid Dosage Form Production. Patent EP3191084B1, 28 November 2018. [Google Scholar]
- Ai, J.R.; Peng, F.; Joo, P.; Vogt, B.D. Enhanced dimensional accuracy of material extrusion 3D-printed plastics through filament architecture. ACS Appl. Polym. Mater. 2021, 3, 2518–2528. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Pohar, A.; Likozar, B. Dissolution, nucleation, crystal growth, crystal aggregation, and particle breakage of amlodipine salts: Modeling crystallization kinetics and thermodynamic equilibrium, scale-up, and optimization. Ind. Eng. Chem. Res. 2014, 53, 10762–10774. [Google Scholar] [CrossRef]
- Hadžidedić, Š.; Uzunović, A.; Šehić Jazić, S.; Kocova El-Arini, S. The impact of chirality on the development of robust and stable tablet formulation of (S-) amlodipine besylate. Pharm. Dev. Technol. 2014, 19, 930–941. [Google Scholar] [CrossRef]
- Jalilov, A.K.; Khaidarov, V.R. Microscopic studies of the shape and size of particles of samples of amlodipine besylate substances from selected pharmaceutical manufacturers. Am. J. Med. Sci. Pharm. Res. 2023, 5, 16–24. [Google Scholar] [CrossRef]
- Saleh, M.; Anwar, S.; AlFaify, A.Y.; Al-Ahmari, A.M.; Abd Elaty, E. Development of PLA/recycled-desized carbon fiber composites for 3D printing: Thermal, mechanical, and morphological analyses. J. Mater. Res. Technol. 2024, 29, 2768–2780. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A.; Evtuguin, D. Polyamide 6/modified pine bark particle composites for additive manufacturing. J. Mater. Sci. 2021, 56, 19093–19105. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wu, J.; Shi, Z.; Zhao, P.; Su, H.; Wang, Q.; Jin, L. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: New delivery system for amlodipine besylate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128096. [Google Scholar] [CrossRef]
- Pezik, E.; Gulsun, T.; Sahin, S.; Vural, I. Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate. Eur. J. Pharm. Sci. 2021, 156, 105597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Zhao, X.; Zhang, S.; Cao, M.; Wang, X.; Li, W. Development and characterization of an amorphous curcumin-Eudragit® E100 solid dispersions with improved solubility, stability, and pharmacokinetic properties. Pharm. Dev. Technol. 2022, 27, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Cengage: Belmont, CA, USA, 2009; pp. 1–104. [Google Scholar]
- Ahmed, Z.A.G.; Hussein-Al-Ali, S.H.; Ibrahim, I.A.A.; Haddad, M.K.; Ali, D.K.; Hussein, A.M.; Abu Sharar, A.A. Development and Evaluation of Amlodipine-Polymer Nanocomposites Using Response Surface Methodology. Int. J. Polym. Sci. 2022, 2022, 3427400. [Google Scholar] [CrossRef]
- Kapor, A.; Nikolić, V.; Nikolić, L.; Stanković, M.; Cakić, M.; Stanojević, L.; Ilić, D. Inclusion complexes of amlodipine besylate and cyclodextrins. Open Chem. 2010, 8, 834–841. [Google Scholar] [CrossRef]
- Nanda, A.; Sahoo, R.N.; Pramanik, A.; Mohapatra, R.; Pradhan, S.K.; Thirumurugan, A.; Das, D.; Mallick, S. Drug-in-mucoadhesive type film for ocular anti-inflammatory potential of amlodipine: Effect of sulphobutyl-ether-beta-cyclodextrin on permeation and molecular docking characterization. Colloids Surf. B Biointerfaces 2018, 172, 555–564. [Google Scholar] [CrossRef]
- Hrichi, H.; Monser, L.; Adhoum, N. A novel electrochemical sensor based on electropolymerized molecularly imprinted poly (aniline-co-anthranilic acid) for sensitive detection of amlodipine. J. Electroanal. Chem. 2017, 805, 133–145. [Google Scholar] [CrossRef]
- Beaman, C.W.; Lees, R.M.; Xu, L.H.; Billinghurst, B.E. FTIR synchrotron spectroscopy of lower modes of methyl-D3 mercaptan (CD3SH). J. Mol. Spectrosc. 2023, 392, 111739. [Google Scholar] [CrossRef]
- Quinteros, D.A.; Manzo, R.H.; Allemandi, D.A. Interaction between Eudragit® E100 and anionic drugs: Addition of anionic polyelectrolytes and their influence on drug release performance. J. Pharm. Sci. 2011, 100, 4664–4673. [Google Scholar] [CrossRef]
- Hanif, M.; Ameer, N.; Akram, H.; Mahmood, K.; Bano, S.; Qaiser, M.; Rahman, H.M.A. Raft-forming gastroretentive tablets incorporating solidly dispersed Curcumin-Eudragit E100; in vitro and in vivo approaches for treatment of gastric ulcer. Polym. Bull. 2023, 80, 9833–9851. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Ahmed, N.; Manzoor, S.; Elaissari, A. Encapsulation of doxorubicin in magnetic-polymer hybrid colloidal particles of Eudragit E100 and their hyperthermia and drug release studies. Polym. Adv. Technol. 2020, 31, 1732–1743. [Google Scholar] [CrossRef]
- Carrascal, J.J.; Pinal, R.; Carvajal, T.; Pérez, L.D.; Baena, Y. Benzoic acid complexes with Eudragit E100®: New alternative antimicrobial preservatives. Int. J. Pharm. 2021, 607, 120991. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.M.; Gálico, D.A.; Guerra, R.B.; Perpétuo, G.L.; Legendre, A.O.; Rinaldo, D.; Bannach, G. Thermal stability and thermal decomposition of the antihypertensive drug amlodipine besylate. J. Therm. Anal. Calorim. 2015, 120, 889–892. [Google Scholar] [CrossRef]
- Linares, V.; Yarce, C.J.; Echeverri, J.D.; Galeano, E.; Salamanca, C.H. Relationship between Degree of Polymeric Ionisation and Hydrolytic Degradation of Eudragitfi E Polymers under Extreme Acid Conditions. Polym. J. 2019, 11, 1010. [Google Scholar] [CrossRef]
- Chourasiya, V.; Bohrey, S.; Pandey, A. Formulation, optimization, and characterization of amlodipine besylate loaded polymeric nanoparticles. Polym. Polym. Compos. 2021, 29, S1555–S1568. [Google Scholar] [CrossRef]
- Ramirez-Tagle, R.; Salgado-Moran, G.; Mendez-Luna, D.; Correa-Basurto, J.; Villada, W.C.; Candia, L.G.; Mendoza-Huizar, L.H. Synthesis, chemical identification, drug release and docking studies of the amlodipine–chitosan nanobiopolymer composite. J. Chil. Chem. Soc. 2021, 66, 5063–5066. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Alshangiti, D.M.; Alkhursani, S.A.; Al-Gahtany, S.A.; Shokr, F.S.; Madani, M. Improvement of In Vitro Dissolution of the Poor Water-Soluble Amlodipine Drug by Solid Dispersion with Irradiated Polyvinylpyrrolidone. ACS Omega 2020, 5, 21476–21487. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Shaikh, S.S.; Shahi, S.R.; Shookur, M.A.; Reddy, L.K.; Padalkar, A.N.; Thube, M. Formulation and evaluation of s-(-)-amlodipine besylate and nebivolol hydrochloride tablets. J. Adv. Pharm. Technol. Res. 2010, 1, 199–206. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q₁₀ as naked nanocrystals. Int J Nanomed. 2012, 7, 5733–5744. [Google Scholar] [CrossRef]
- Lin, X.; Su, L.; Li, N.; Hu, Y.; Tang, G.; Liu, L.; Li, H.; Yang, Z. Understanding the mechanism of dissolution enhancement for poorly water-soluble drugs by solid dispersions containing Eudragit® E PO. J. Drug Deliv. Sci. Technol. 2018, 48, 328–337. [Google Scholar] [CrossRef]
- Vlachou, M.; Kikionis, S.; Siamidi, A.; Kyriakou, S.; Tsotinis, A.; Ioannou, E.; Roussis, V. Development and Characterization of Eudragit®-Based Electrospun Nanofibrous Mats and Their Formulation into Nanofiber Tablets for the Modified Release of Furosemide. Pharmaceutics 2019, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Vedha Hari, B.N.; Narayanan, N.; Dhevedaran, K. Efavirenz–eudragit E-100 nanoparticle-loaded aerosol foam for sus-tained release: In-vitro and ex-vivo evaluation. Chem. Pap. 2015, 69, 358–367. [Google Scholar] [CrossRef]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. A reappraisal of drug release laws using Monte Carlo simulations: The preva-lence of the Weibull function. Pharm. Res. 2003, 20, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).