Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
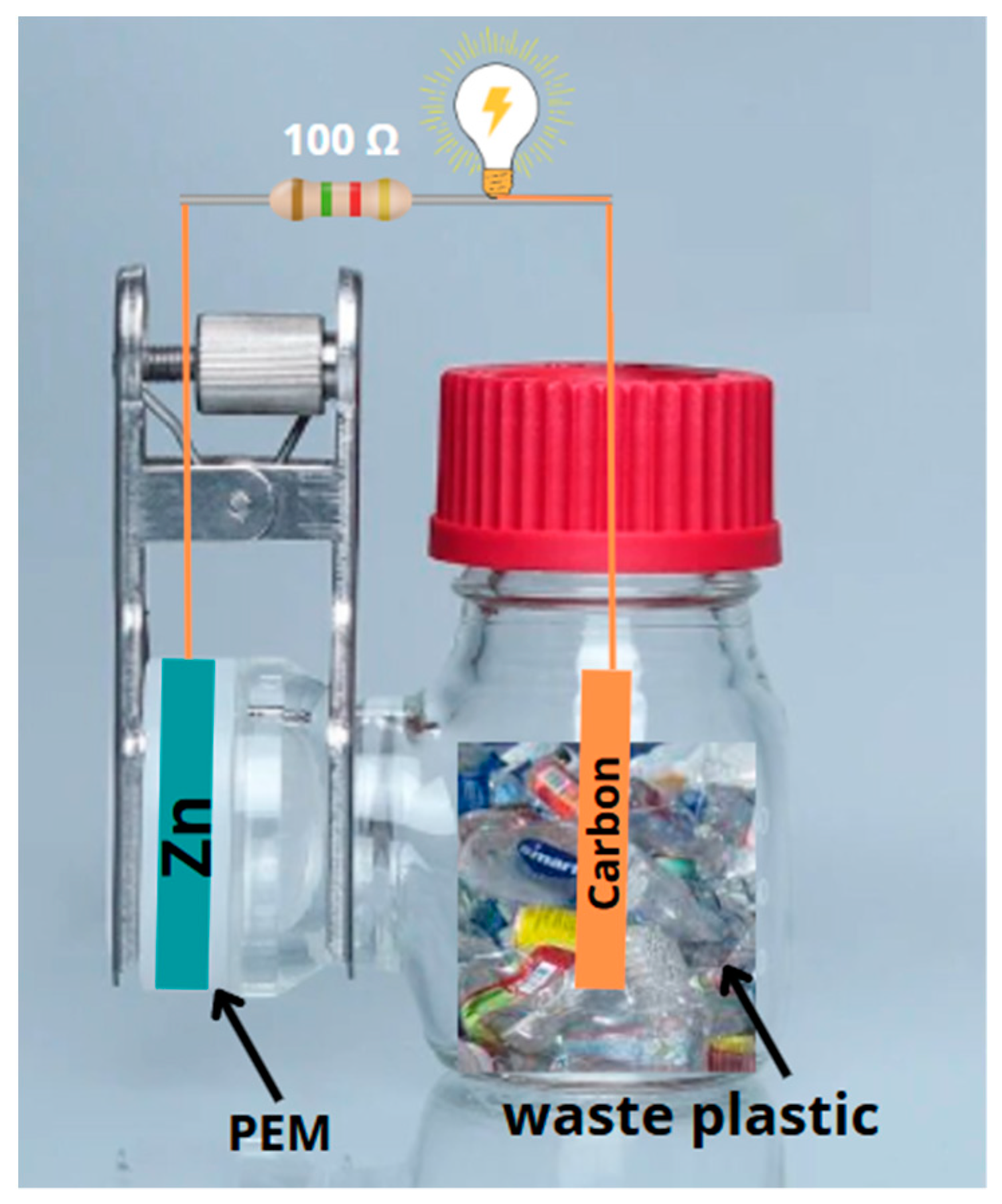
2.1. Construction and Operation of Single-Chamber MFCs
2.2. Characterization of Electrochemical and Morphological Parameters
2.3. Obtaining the Plastic and Straw Waste Sample
2.4. Isolation and Selection of Trichoderma sp.
2.5. Obtaining the Spore Inoculum
2.6. Installation of the Microbial Fuel Cell
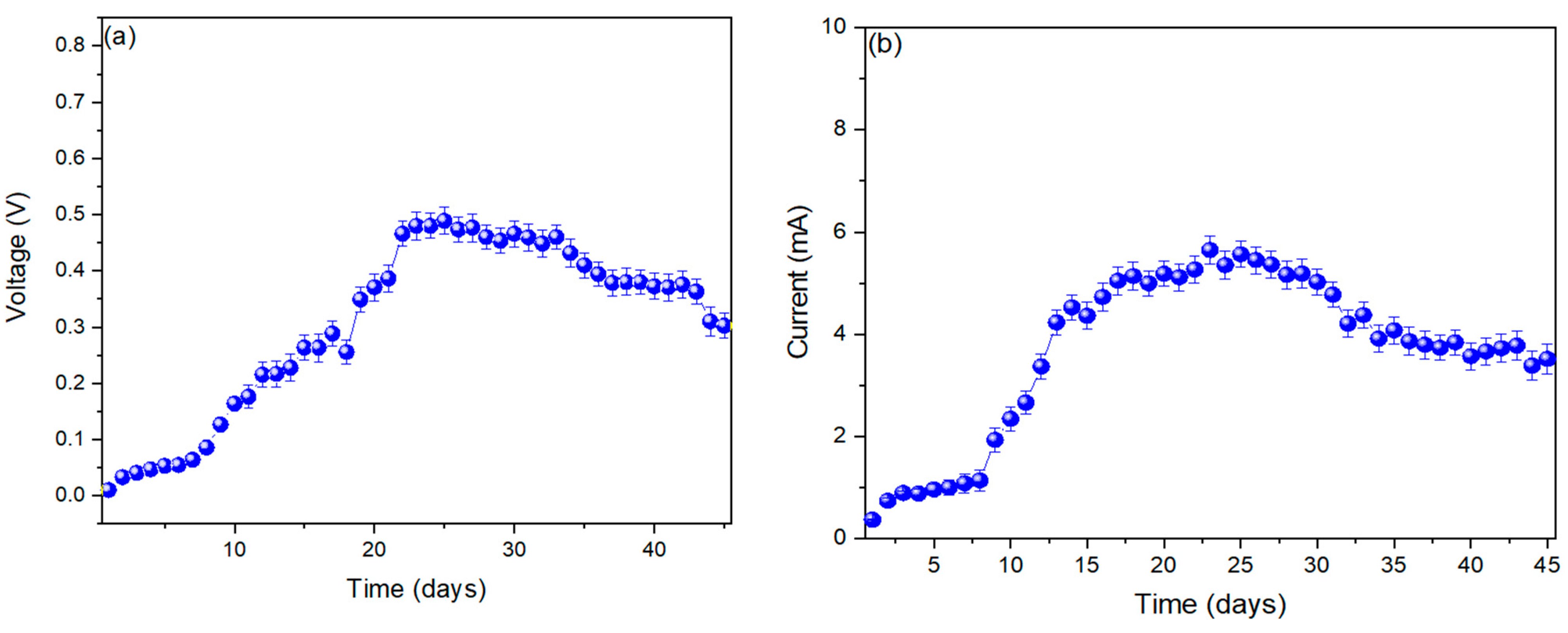
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Sustainable energy from waste organic matters via efficient microbial processes. Sci. Total Environ. 2020, 722, 137927. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Yuwono, N.W.; Mategeko, B.; Smetana, S.; Saki, M.; Nagdalian, A. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of sustainable approaches for converting the organic waste to bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: An integrated system design approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira LF, R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry—Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Cortina, J.L. Use of membrane technologies in dairy industry: An overview. Foods 2021, 10, 2768. [Google Scholar] [CrossRef]
- Lalwani, J.; Gupta, A.; Thatikonda, S.; Subrahmanyam, C. An industrial insight on treatment strategies of the pharmaceutical industry effluent with varying qualitative characteristics. J. Environ. Chem. Eng. 2020, 8, 104190. [Google Scholar] [CrossRef]
- Šimatović, A.; Udiković-Kolić, N. Antibiotic resistance in pharmaceutical industry effluents and effluent-impacted environments. Antibiot. Resist. Environ. A Worldw. Overv. 2020, 101–122. [Google Scholar]
- Heidarpanah, M.; Hooshyaripor, F.; Fazeli, M. Daily electricity price forecasting using artificial intelligence models in the Iranian electricity market. Energy 2023, 263, 126011. [Google Scholar] [CrossRef]
- Lin, B.; Huang, C. How will promoting the digital economy affect electricity intensity? Energy Policy 2023, 173, 113341. [Google Scholar] [CrossRef]
- Peters, R.; Berlekamp, J.; Kabiri, C.; Kaplin, B.A.; Tockner, K.; Zarfl, C. Sustainable pathways towards universal renewable electricity access in Africa. Nat. Rev. Earth Environ. 2024, 5, 137–151. [Google Scholar] [CrossRef]
- Nti, I.K.; Teimeh, M.; Nyarko-Boateng, O.; Adekoya, A.F. Electricity load forecasting: A systematic review. J. Electr. Syst. Inf. Technol. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Cardinal, J. Evolution and reform of UK electricity market. Renew. Sustain. Energy Rev. 2022, 161, 112317. [Google Scholar] [CrossRef]
- Ida, T.K.; Mandal, B. Microbial fuel cell design, application and performance: A review. Mater. Today Proc. 2023, 76, 88–94. [Google Scholar]
- Meylani, V.; Surahman, E.; Fudholi, A.; Almalki, W.H.; Ilyas, N.; Sayyed, R.Z. Biodiversity in microbial fuel cells: Review of a promising technology for wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109503. [Google Scholar] [CrossRef]
- Gupta, S.; Patro, A.; Mittal, Y.; Dwivedi, S.; Saket, P.; Panja, R.; Saeed, T.; Martínez, F.; Yadav, A.K. The race between classical microbial fuel cells, sediment-microbial fuel cells, plant-microbial fuel cells, and constructed wetlands-microbial fuel cells: Applications and technology readiness level. Sci. Total Environ. 2023, 879, 162757. [Google Scholar] [CrossRef]
- Sun, F.; Chen, J.; Tang, M.; Yang, Y. Recent research progress, challenges and future directions of sediment microbial fuel cell: A comprehensive review. Int. J. Hydrogen Energy 2023, 50, 870–886. [Google Scholar] [CrossRef]
- Sharma, A.; Chhabra, M. The versatility of microbial fuel cells as tools for organic matter monitoring. Bioresour. Technol. 2023, 377, 128949. [Google Scholar] [CrossRef]
- Verma, J.; Kumar, D.; Singh, N.; Katti, S.S.; Shah, Y.T. Electricigens and microbial fuel cells for bioremediation and bioenergy production: A review. Environ. Chem. Lett. 2021, 19, 2091–2126. [Google Scholar] [CrossRef]
- Garbini, G.L.; Barra Caracciolo, A.; Grenni, P. Electroactive bacteria in natural ecosystems and their applications in microbial fuel cells for bioremediation: A review. Microorganisms 2023, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, C.; do Valle Trotta, C.; Martins, G.; Matos, J.; Maiorano, A.E.; Brito, A.G.; Peixoto, L. In Situ Trametes versicolor Laccase Biocathode Performance Assessment in Dual-Chamber Microbial Fuel Cells. BioEnergy Res. 2023, 16, 2616–2624. [Google Scholar] [CrossRef]
- Dusabe, S.; Juliastuti, S.R.; Darmawan, R. HYDROLYSIS OF FOOD WASTE AND PRODUCTION OF BIOELECTRICITY USING SINGLE CHAMBER MICROBIAL FUEL CELL FOR THE SUSTAINABLE FUTURE. J. Sustain. Sci. Manag. 2023, 18, 151–166. [Google Scholar] [CrossRef]
- Segundo, R.F.; Benites, S.M.; De La Cruz-Noriega, M.; Vives-Garnique, J.; Otiniano, N.M.; Rojas-Villacorta, W.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Impact of dragon fruit waste in microbial fuel cells to generate friendly electric energy. Sustainability 2023, 15, 7316. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Druzhinina, I.S.; Xie, X.; Wang, E.; Martin, F.; Yuan, Z. Phosphorus/nitrogen sensing and signaling in diverse root–fungus symbioses. Trends Microbiol. 2024, 32, 200–215. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Lai, C.; Chen, J.; Liu, J.; Tian, D.; Lan, D.; Liu, T.; Hongkai, B.; Tang, J. New polyketides from a hydrothermal vent sediment fungus Trichoderma sp. JWM29-10-1 and their antimicrobial effects. Mar. Drugs 2022, 20, 720. [Google Scholar] [CrossRef]
- Rusetskaya, V.; Różalska, S.; Słaba, M.; Bernat, P. Degradation ability of Trichoderma spp. in the presence of poly (butylene adipate-co-terephthalate) microparticles. Int. Biodeterior. Biodegrad. 2024, 193, 105829. [Google Scholar] [CrossRef]
- Zaman, K.A.U.; Wu, X.; Sarotti, A.M.; Cao, S. New and bioactive polyketides from Hawaiian marine-derived fungus Trichoderma sp. FM652. Nat. Prod. Res. 2022, 36, 5984–5990. [Google Scholar] [CrossRef]
- Simões, M.F.; Maiorano, A.E.; dos Santos, J.G.; Peixoto, L.; de Souza, R.F.B.; Neto, A.O.; Ottoni, C.A.; Brito, A.G. Microbial fuel cell-induced production of fungal laccase to degrade the anthraquinone dye Remazol Brilliant Blue R. Environ. Chem. Lett. 2019, 17, 1413–1420. [Google Scholar] [CrossRef]
- Votat, S.; Pontié, M.; Jaspard, E.; Lebrun, L. Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies 2024, 17, 247. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Wang, J.H.; Shen, Y.; Guo, Z.W.; Yang, Y.; Zhu, D.C.; Mahmoud, A.S. Performance of the Dual-Chamber Fungal Fuel Cell in Treating Tannery Wastewater. Appl. Sci. 2023, 13, 10710. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Chen, H.L.; Nath, T.K.; Chong, S.; Foo, V.; Gibbins, C.; Lechner, A.M. The plastic waste problem in Malaysia: Management, recycling and disposal of local and global plastic waste. SN Appl. Sci. 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Zamparas, M.G.; Kapsalis, V.C. Investigating the human impacts and the environmental consequences of microplastics disposal into water resources. Sustainability 2022, 14, 828. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Peters, R.W.; Mostafa, M.K.; Rochman, C.M. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Castañeta, G.; Gutiérrez, A.F.; Nacaratte, F.; Manzano, C.A. Microplásticos: Un contaminante que crece en todas las esferas ambientales, sus características y posibles riesgos para la salud pública por exposición. Rev. Boliv. De Química 2020, 37, 142–157. [Google Scholar] [CrossRef]
- Agüero Quiñones, R.A.; Avila Sanchez, Z.M. Biomasa de Chlorella sp. en la remoción de cadmio y DQO de aguas residuales municipales usando celdas de combustible microbianas. 2023. Available online: https://hdl.handle.net/20.500.12692/125198 (accessed on 12 November 2024).
- Jeraldine, D.V.M.; Wim, L.; Ellen, V.E. A comparative study for optimization of MALDI-TOF MS identification of filamentous fungi. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1153–1161. [Google Scholar] [CrossRef]
- Troconis, G.P.; Taylhardat, L. La educación superior agrícola en Venezuela (Génesis y primeros tiempos). Areté Rev. Digit. Del Dr. En Educ. De La Univ. Cent. De Venez. 2015, 1, 7–25. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi. 1972. Available online: https://archive.org/details/cftri.9574illustratedgener0000hlba (accessed on 12 November 2024).
- Bustillo, A. Método para Cuantificar Suspensiones de Esporas de Hongos y Otros Organismos; Universidad Nacional de Colombia: Palmira, Colombia, 2010; Volume 10. [Google Scholar]
- Sandt, C.; Waeytens, J.; Deniset-Besseau, A.; Nielsen-Leroux, C.; Réjasse, A. Use and misuse of FTIR spectroscopy for studying the bio-oxidation of plastics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119841. [Google Scholar] [CrossRef]
- Doğan, M. Ultraviolet light accelerates the degradation of polyethylene plastics. Microsc. Res. Tech. 2021, 84, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Mulyono, T.; Misto, M.; Cahyono, B.E.; Fahmidia, N.H. The impact of adding vegetable waste on the functioning of microbial fuel cell. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2022; Volume 2663. [Google Scholar]
- Mulyono, T.; Nasifatul, S.M. The effect of addition of vegetable waste on microbial fuel cell performance. J. Phys. Conf. Ser. 2021, 1825, 012073. [Google Scholar] [CrossRef]
- Ahmad, A. Conventional vegetable waste: A potential source for the high performance of benthic microbial fuel cells. Biomass Convers. Biorefinery 2023, 14, 24641–24653. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Al-Zaqri, N.; Yaakop, A.S.; Umar, K. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Singh, S.P.; Atkinson, J.D.; Lam, S.S.; Iqbal, H.M.; Tong, Y.W. Biotransformation of food waste into biogas and hydrogen fuel–a review. Int. J. Hydrogen Energy 2024, 52, 46–60. [Google Scholar] [CrossRef]
- Rojas-Villacorta, W.; Rojas-Flores, S.; Benites, S.M.; Nazario-Naveda, R.; Romero, C.V.; Gallozzo-Cardenas, M.; Murga-Torres, E. Preliminary Study of Bioelectricity Generation Using Lettuce Waste as Substrate by Microbial Fuel Cells. Sustainability 2023, 15, 10339. [Google Scholar] [CrossRef]
- Basu, A.; Manna, S.; Sil, A.K. A new electro-active bacterium, Paraclostridium sp. AKS46, converts waste efficiently into electricity in microbial fuel cell. Chem. Eng. J. 2023, 475, 145626. [Google Scholar] [CrossRef]
- Sukri, A.; Othman, R.; Abd-Wahab, F.; Noor, N.M. Self-sustaining bioelectrochemical cell from fungal degradation of lignin-rich agrowaste. Energies 2021, 14, 2098. [Google Scholar] [CrossRef]
- Laily, F.N.; Juliastuti, S.R.; Darmawan, R. MFC Performance with Additional Micronutrients in Food Waste Substrate. J. Adv. Res. Fluid Mech. Therm. Sci. 2024, 115, 103–112. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Jayabalan, T.; Sethupathi, M.; Kim, W.; Muniyasamy, S.; Sengottuvelan, N.; Alagarsamy, A. Bioelectricity generation by natural microflora of septic tank wastewater (STWW) and biodegradation of persistent petrogenic pollutants by basidiomycetes fungi: An integrated microbial fuel cell system. J. Hazard. Mater. 2021, 412, 125228. [Google Scholar] [CrossRef]
- Zafar, Z.; Naz, S.; Malik, N.H.; Ahmed, F.; Ali, N. Bioelectrogeneic performance of air-cathode microbial fuel cells with diesel contaminants. Fuel 2024, 355, 129407. [Google Scholar] [CrossRef]
- Sarma, R.; Tamuly, A.; Kakati, B.K. Recent developments in electricity generation by Microbial Fuel Cell using different substrates. Mater. Today Proc. 2022, 49, 457–463. [Google Scholar] [CrossRef]
- Ullah, Z.; Zeshan, S. Effect of substrate type and concentration on the performance of a double chamber microbial fuel cell. Water Sci. Technol. 2020, 81, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Luo, H.; Shi, Z.; Wu, Y.; Liu, H. Substrate salinity: A critical factor regulating the performance of microbial fuel cells, a review. Sci. Total Environ. 2021, 763, 143021. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Das, S. Effect of pH, COD, and HRT on the performance of microbial fuel cell using synthetic dairy wastewater. Water 2023, 15, 3472. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghasemi, M.; Sedighi, M. Comparative study of energy production and treatment of municipal and dairy wastewater via microbial fuel cell technology: Process evaluation towards optimization. Biomass Convers. Biorefinery 2024, 14, 6285–6298. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Zhang, J. Enhanced biogas production from anaerobic digestion of solid organic wastes: Current status and prospects. Bioresour. Technol. Rep. 2019, 5, 280–296. [Google Scholar] [CrossRef]
- Zafar, H. Microbial Fuel Cells: A Comparative Analysis of Operational Factors, Response Metrics, and Degradation Response to Fruit Waste Degradation. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2023. [Google Scholar]
- Simeon, M.I.; Asoiro, F.U.; Aliyu, M.; Raji, O.A.; Freitag, R. Polarization and power density trends of a soil-based microbial fuel cell treated with human urine. Int. J. Energy Res. 2020, 44, 5968–5976. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, P.S. A review on recent advancements in bioenergy production using microbial fuel cells. Chemosphere 2022, 288, 132512. [Google Scholar] [CrossRef]
- Rossi, R.; Logan, B.E. Using an anion exchange membrane for effective hydroxide ion transport enables high power densities in microbial fuel cells. Chem. Eng. J. 2021, 422, 130150. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Bakar, M.A.B.A.; Kim, H.C.; Ahmad, A.; Alshammari, M.B.; Yaakop, A.S. Oxidation of food waste as an organic substrate in a single chamber microbial fuel cell to remove the pollutant with energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102282. [Google Scholar] [CrossRef]
- Choudhury, P.; Ray, R.N.; Bandyopadhyay, T.K.; Basak, B.; Muthuraj, M.; Bhunia, B. Process engineering for stable power recovery from dairy wastewater using microbial fuel cell. Int. J. Hydrogen Energy 2021, 46, 3171–3182. [Google Scholar] [CrossRef]
- Lawson, K.; Rossi, R.; Regan, J.M.; Logan, B.E. Impact of cathodic electron acceptor on microbial fuel cell internal resistance. Bioresour. Technol. 2020, 316, 123919. [Google Scholar] [CrossRef]
- Naveenkumar, M.; Senthilkumar, K. Microbial fuel cell for harvesting bio-energy from tannery effluent using metal mixed biochar electrodes. Biomass Bioenergy 2021, 149, 106082. [Google Scholar] [CrossRef]
- Wang, H.; Long, X.; Sun, Y.; Wang, D.; Wang, Z.; Meng, H.; Lu, N. Electrochemical impedance spectroscopy applied to microbial fuel cells: A review. Front. Microbiol. 2022, 13, 973501. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, S.; Chhabra, V.A.; Marwaha, A.; Kim, K.H.; Tripathi, S.K. A sustainable approach towards utilization of plastic waste for an efficient electrode in microbial fuel cell applications. J. Hazard. Mater. 2021, 417, 125992. [Google Scholar] [CrossRef]
- Malachová, K.; Novotný, Č.; Adamus, G.; Lotti, N.; Rybková, Z.; Soccio, M.; Fava, F. Ability of Trichoderma hamatum isolated from plastics-polluted environments to attack petroleum-based, synthetic polymer films. Processes 2020, 8, 467. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Trichoderma harzianum—SEM, FTIR, and NMR analyses. Environ. Monit. Assess. 2014, 186, 6577–6586. [Google Scholar] [CrossRef]
- Parit, A.A.; Jadhav, A.S.; Raut, P.D. Screening and Isolation of Polypropylene Degrading Fungi from Waste Dumping Site, Kolhapur, India. Nat. Environ. Pollut. Technol. 2023, 22, 1599–1605. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ionescu, D.; Grossart, H.P. Tapping into fungal potential: Biodegradation of plastic and rubber by potent Fungi. Sci. Total Environ. 2024, 934, 173188. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segundo, R.-F.; Rocío, P.-C.; Luis, C.-C.; Angelats Silva, L.M. Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells. Processes 2024, 12, 2904. https://doi.org/10.3390/pr12122904
Segundo R-F, Rocío P-C, Luis C-C, Angelats Silva LM. Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells. Processes. 2024; 12(12):2904. https://doi.org/10.3390/pr12122904
Chicago/Turabian StyleSegundo, Rojas-Flores, Pimentel-Castillo Rocío, Cabanillas-Chirinos Luis, and Luis M. Angelats Silva. 2024. "Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells" Processes 12, no. 12: 2904. https://doi.org/10.3390/pr12122904
APA StyleSegundo, R.-F., Rocío, P.-C., Luis, C.-C., & Angelats Silva, L. M. (2024). Potential Use of the Fungus Trichoderma sp. as a Plastic-Reducing Agent and Electricity Generator in Microbial Fuel Cells. Processes, 12(12), 2904. https://doi.org/10.3390/pr12122904






