Performance of CaO-Promoted Ni Catalysts over Nanostructured CeO2 in Dry Reforming of Methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Characterization
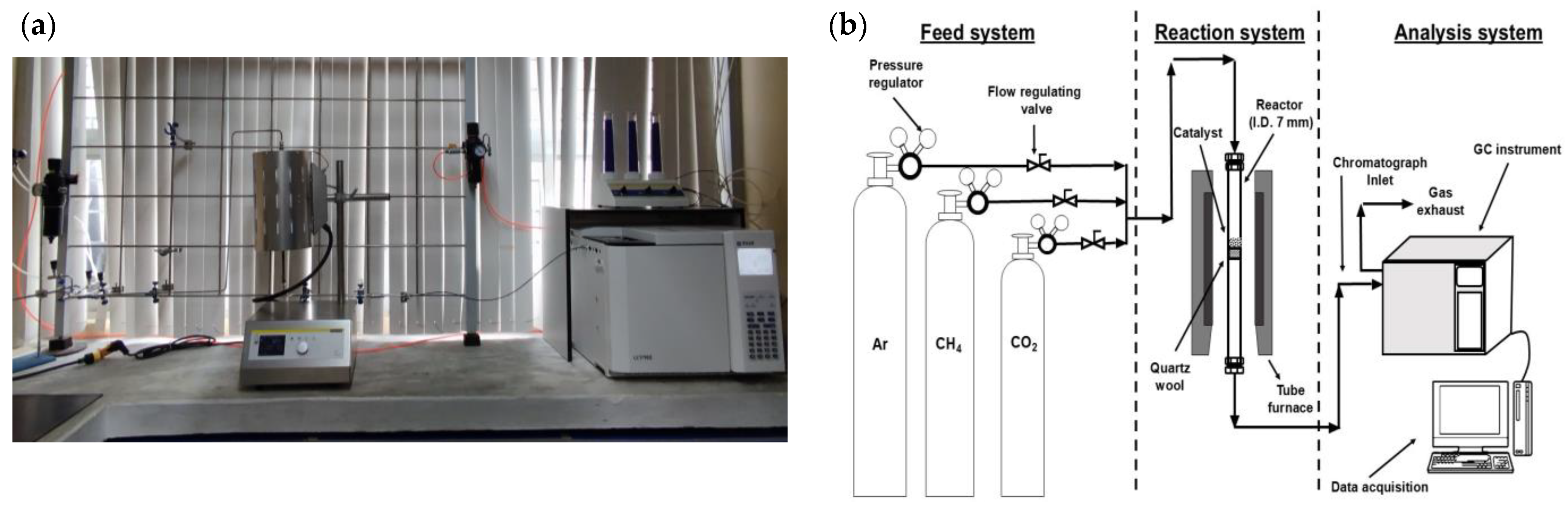
2.3. Catalytic Evaluation
3. Results and Discussion
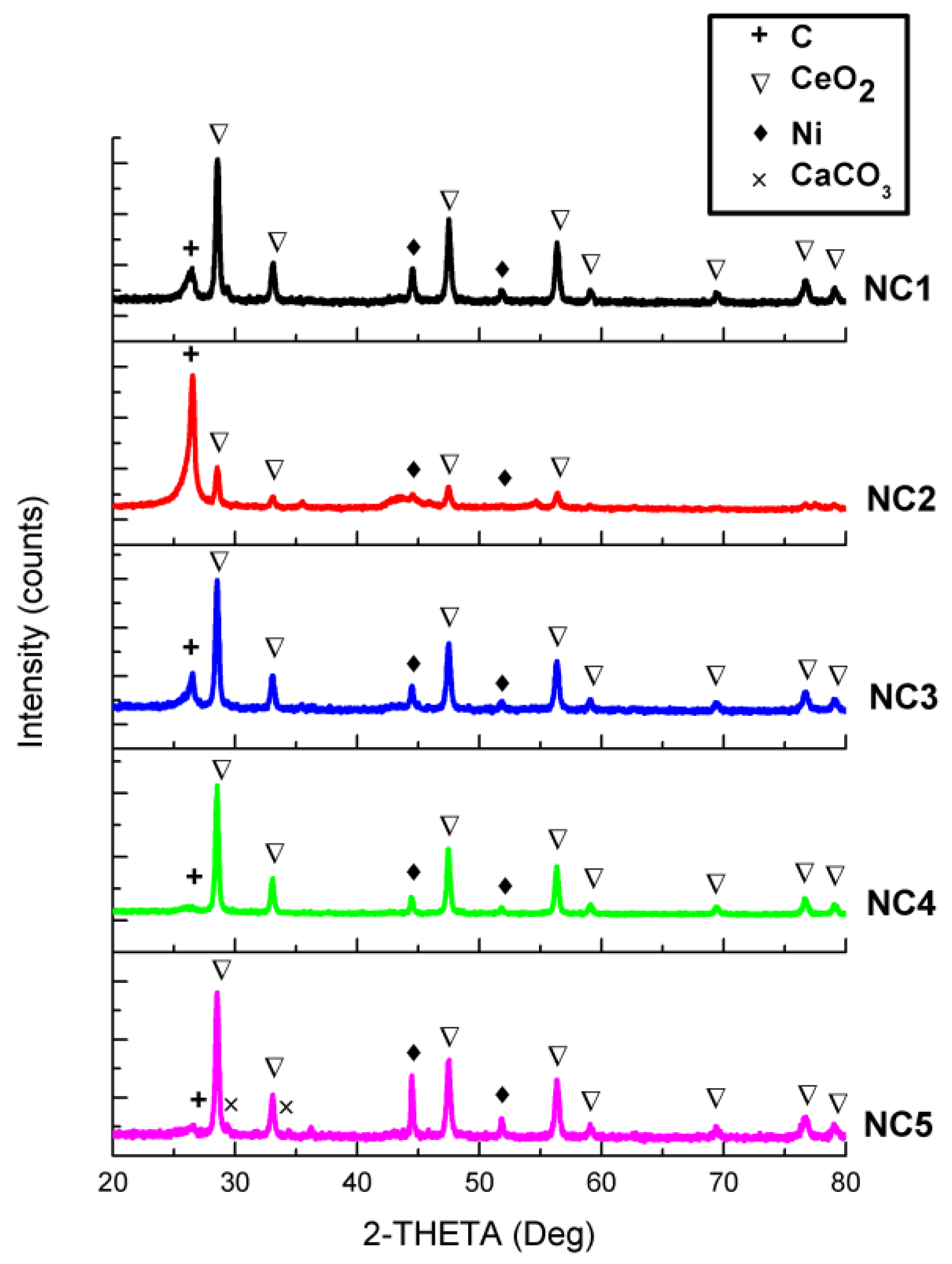
3.1. X-Ray Diffraction
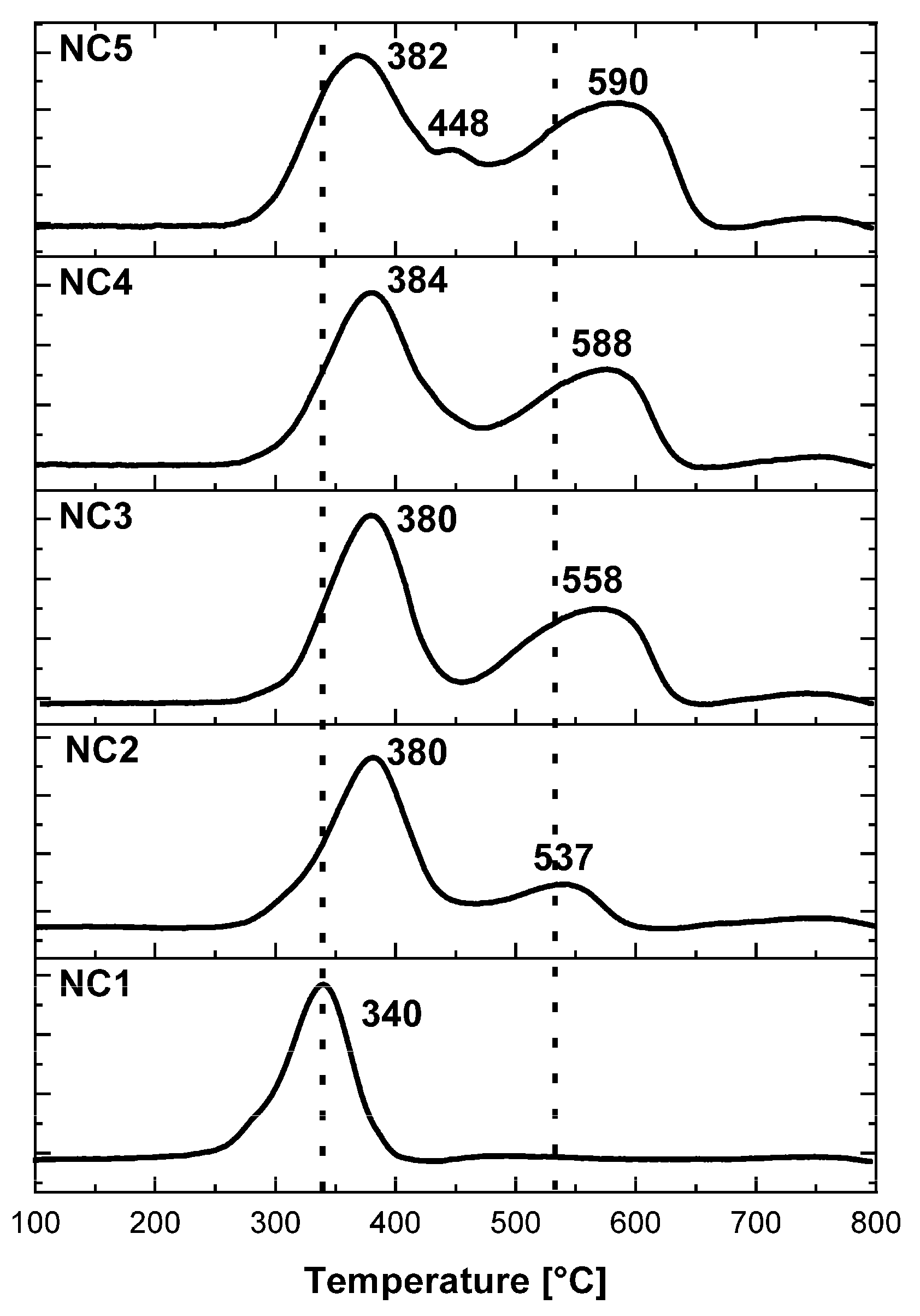
3.2. H2-TPR
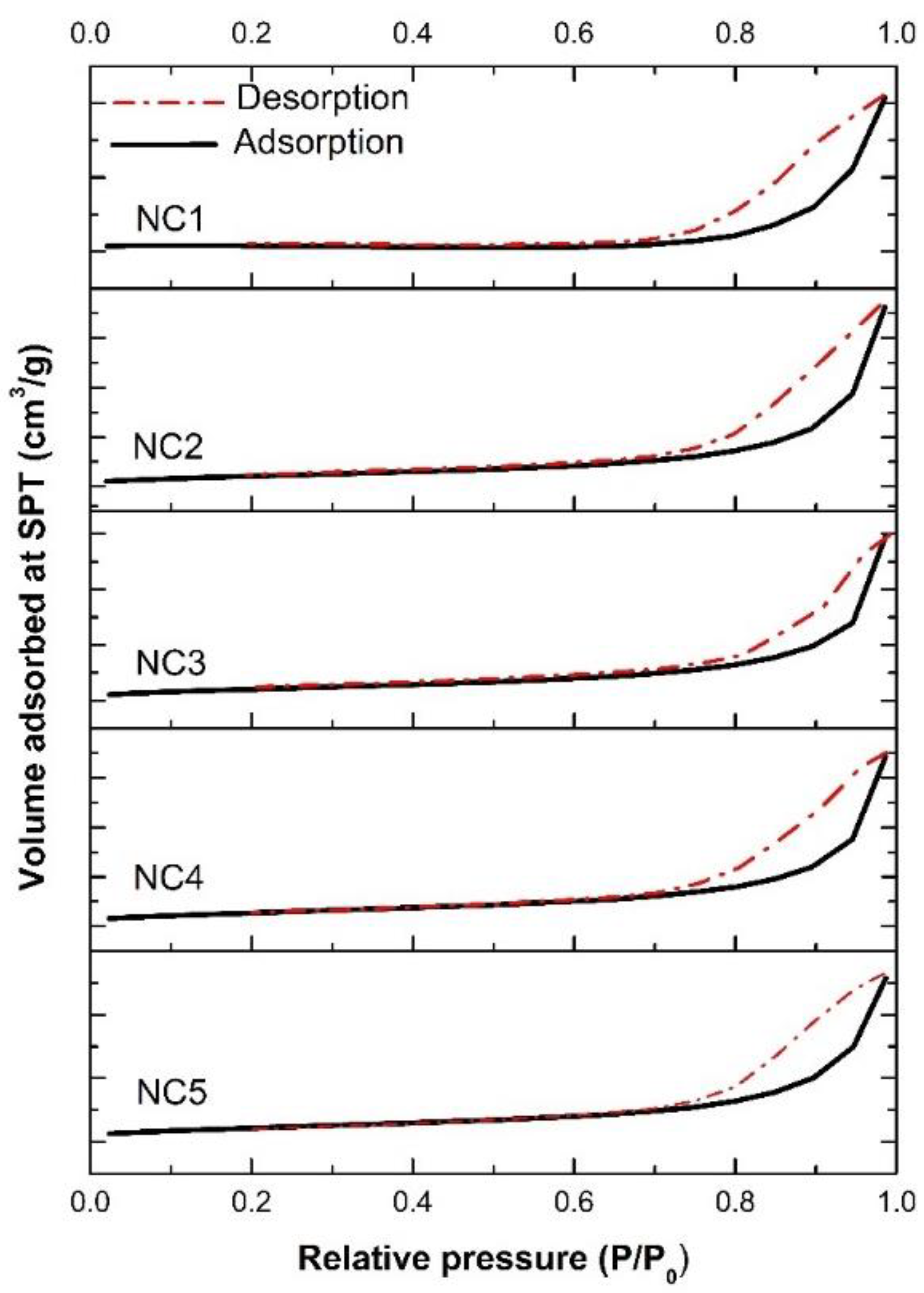
3.3. Particle Size and Textural Properties
3.4. Scanning Electron Micrographs (SEMs)
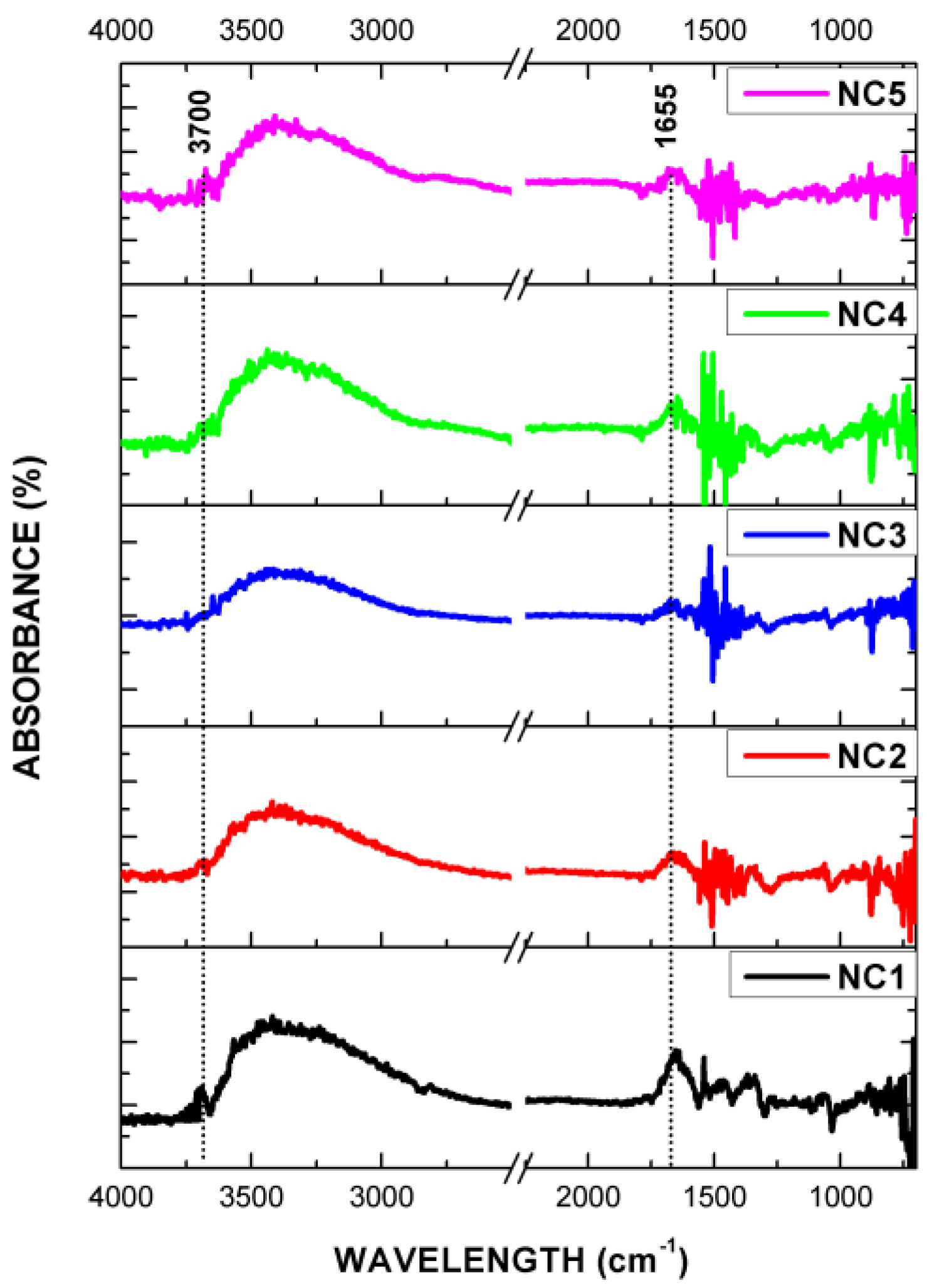
3.5. FT-IR (ATR and DRIFTS)
3.6. Catalytic Performance
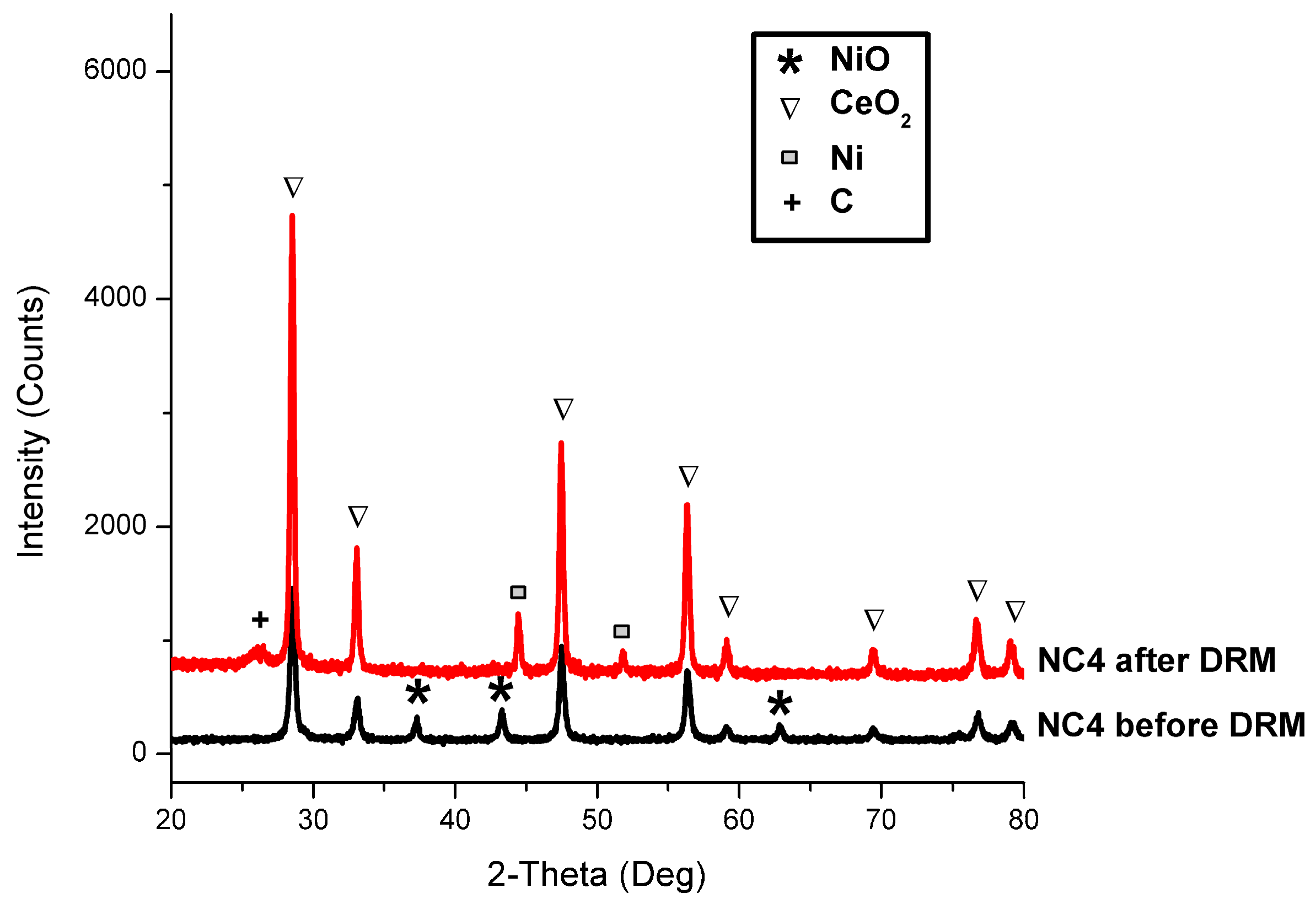
3.7. Spent Catalysts
4. Conclusions
- (a)
- CaO plays a crucial role in enhancing catalytic performance by influencing basicity, CO2 adsorption, and catalytic activity. The NC4 catalyst (15 wt.% CaO) demonstrated the highest activity and stability, making it the optimal formulation;
- (b)
- Raman analysis (ID/IG ratio) suggests that the addition of Ca promotes the formation of more disordered or amorphous carbon, giving rise to higher stability compared to Ni/CeO2 catalysts;
- (c)
- The presence of CaCO3, likely formed due to environmental CO2 adsorption and reaction with CaO, reduced the number of basic sites on the catalyst surface, as evidenced by DRIFTS results. Higher Ca2+ content correlated with reduced conversion of MBOH to acetylene groups, highlighting the effect of Ca on catalyst efficiency;
- (d)
- While no direct evidence of CaO–CeO2 interactions was observed, TPR results indicate that increased calcium content enhances CeO2 reduction, suggesting a potential interaction that improves reducibility and oxygen mobility.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pompeo, F.; Nichio, N.N.; González, M.G.; Montes, M. Characterization of Ni/SiO2 and Ni/Li-SiO2 catalysts for methane dry reforming. Catal. Today 2005, 107, 856–862. [Google Scholar] [CrossRef]
- Roblero, J.G.; Pola-Albores, F.; Valenzuela, M.A.; Rojas-García, E.; Ríos-Valdovinos, E.; Valverde-Aguilar, G. Ni and Ni3C catalysts supported on mesoporous silica for dry reforming of methane. Int. J. Hydrogen Energy 2019, 44, 10473–10483. [Google Scholar] [CrossRef]
- Naeem, M.A.; Al-Fatesh, A.S.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Syngas Production from Dry Reforming of Methane over Nano Ni Polyol Catalysts. Int. J. Chem. Eng. Appl. 2013, 4, 315–320. [Google Scholar] [CrossRef]
- de la Cruz-Flores, V.G.; Martinez-Hernandez, A.; Gracia-Pinilla, M.A. Deactivation of Ni-SiO2 catalysts that are synthetized via a modified direct synthesis method during the dry reforming of methane. Appl. Catal. A Gen. 2020, 594, 117455. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.; Li, J.; Wang, P.; Zhu, J.; Ge, F.; Zhou, J.; Song, M.; Zhu, W. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2019, 47, 199–208. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Ren, H.; Gao, X. A short review on nickel-based catalysts in dry reforming of methane: Influences of oxygen defects on anti-coking property. Mater. Today Proc. 2019, 42, 153–160. [Google Scholar] [CrossRef]
- Meric, G.G.; Arbag, H.; Degirmenci, L. Coke minimization via SiC formation in dry reforming of methane conducted in the presence of Ni-based core–shell microsphere catalysts. Int. J. Hydrogen Energy 2017, 42, 16579–16588. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Q.; Wang, J.; Gui, K.; Thomas, H.R. Effect of CaCO3 on catalytic activity of Fe-Ce/Ti catalysts for NH3-SCR reaction. RSC Adv. 2020, 10, 44876–44883. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Li, B.; Yuan, X.; Li, B.; Wang, X. Ceria-modified nickel supported on porous silica as highly active and stable catalyst for dry reforming of methane. Fuel 2021, 301, 121027. [Google Scholar] [CrossRef]
- Pappacena, A.; Razzaq, R.; de Leitenburg, C.; Boaro, M.; Trovarelli, A. The role of neodymium in the optimization of a Ni/CeO2 and Ni/CeZrO2 Methane dry reforming catalyst. Inorganics 2018, 6, 39. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Ayub, A.; Bahruji, H.; Mahadi, A.H. Ni nanoparticles on reducible metal oxides (Sm2O3, CeO2, ZnO) as catalysts for CO2 methanation. Bull. Chem. React. Eng. Catal. 2021, 16, 641–650. [Google Scholar] [CrossRef]
- Nichele, V.; Signoretto, M.; Pinna, F.; Menegazzo, F.; Rossetti, I.; Cruciani, G.; Cerrato, G.; Di Michele, A. Ni/ZrO2 catalysts in ethanol steam reforming: Inhibition of coke formation by CaO-doping. Appl. Catal. B 2014, 150–151, 12–20. [Google Scholar] [CrossRef]
- Pérez-Madrigal, V.; Santiago-Salazar, D.; Ríos-Valdovinos, E.; Albiter, E.; Valenzuela, M.A.; Pola-Albores, F. Calcium-based promoter in Ni catalysts supported over nanostructured ZrO2. MRS Adv. 2024, 9, 172–176. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Fakeeha, A.H.; Alotibi, M.F.; Alreshaidan, S.B.; Kumar, R. Reforming of methane: Effects of active metals, supports, and promoters. Catal. Rev. 2023, 66, 2209–2307. [Google Scholar] [CrossRef]
- Pérez-Madrigal, V.; Ríos-Valdovinos, E.; Rojas-García, E.; Valenzuela, M.A.; Pola-Albores, F. Dry Reforming of Methane over Li-Doped Ni/TiO2 Catalysts: Effect of Support Basicity. Methane 2023, 2, 452–469. [Google Scholar] [CrossRef]
- Lauren-Pernot, H.; Luck, F.; Popa, J.M. Methylbutynol: A new and simple diagnostic tool for acidic and basic sites of solids. Appl. Catal. 1991, 78, 213–225. [Google Scholar] [CrossRef]
- Bailly, M.L.; Chizallet, C.; Costentin, G.; Krafft, J.M.; Lauron-Pernot, H.; Che, M. A spectroscopy and catalysis study of the nature of active sites of MgO catalysts: Thermodynamic Brønsted basicity versus reactivity of basic sites. J. Catal. 2005, 235, 413–422. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Tian, S.; Yan, F.; Zhang, Z.; Jiang, J. Calcium-looping reforming of methane realizes in situ CO2 utilization with improved energy efficiency. Sci. Adv. 2019, 5, eaav5077. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, J.; Chu, M.; Yue, J.; Cui, Y.; Xu, G. Cooperation between Active Metal and Basic Support in Ni-Based Catalyst for Low-Temperature CO2 Methanation. Catal. Lett. 2020, 150, 1418–1426. [Google Scholar] [CrossRef]
- Atzori, L.; Cutrufello, M.; Meloni, D.; Cannas, C.; Gazzoli, D.; Monaci, R.; Sini, M.; Rombi, E. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation. Catal. Today 2018, 299, 183–192. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, T.W.; Lee, S.H.; Park, E.D. Effects of Na content in Na/Ni/SiO2 and Na/Ni/CeO2 catalysts for CO and CO2 methanation. Catal. Today 2018, 303, 159–167. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Cui, S.; Wang, X.; Wang, L.; Zheng, X. Enhanced selectivity of the CO2 reverse water-gas reaction over a Ni2P/CeO2 catalyst. Dalton Trans. 2021, 50, 5978–5987. [Google Scholar] [CrossRef]
- Aberkane, A.B.; Yeste, M.P.; Fayçal, D.; Goma, D.; Cauqui, M.Á. Catalytic soot oxidation activity of NiO-CeO2 catalysts prepared by a coprecipitation method: Influence of the preparation pH on the catalytic performance. Materials 2019, 12, 3436. [Google Scholar] [CrossRef]
- Martos, C.; Coto, B.; Peña, J.L.; Rodríguez, R.; Merino-Garcia, D.; Pastor, G. Effect of precipitation procedure and detection technique on particle size distribution of CaCO3. J. Cryst. Growth 2010, 312, 2756–2763. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Mosinska, M.; Maniukiewicz, W.; Szynkowska-jozwik, M.I.; Mierczynski, P. The effect of modifiers on the performance of Ni/CeO2 and Ni/La2O3 catalysts in the oxy–steam reforming of lng. Int. J. Mol. Sci. 2021, 22, 9076. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund, P.C. Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]






| Sample | XRD | WD-XRF | ||||||
|---|---|---|---|---|---|---|---|---|
| NiO Crystallite Size [nm] | NiO | CeO2 | CaCO3 | CaO | NiO | CeO2 | Ca Content * | |
| NC1 | 39.64 | 25.6% | 74.4% | --- | --- | 26.9% | 72.1% | --- |
| NC2 | 38.988 | 28.98% | 71.02% | --- | --- | 28% | 66.7% | 4.2% |
| NC3 | 43.948 | 27.03 | 72.97% | <1% | --- | 24.7% | 68% | 7.0% |
| NC4 | 36.77 | 33.59% | 65.45% | <1% | --- | 29.5% | 60.8% | 8.6% |
| NC5 | 28.832 | 38.89% | 51.38% | 5.44% | 4.28% | 32.9% | 53.3% | 13.5% |
| Sample | Calcium Content [%] | Particle Size [nm] | Bet Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|---|
| NC1 | --- | 464.1 | 4.89 | 12.24 | 0.088 |
| NC2 | 5 | 633.8 | 9.30 | 9.58 | 0.117 |
| NC3 | 10 | 720.2 | 16.39 | 6.50 | 0.093 |
| NC4 | 15 | 814.5 | 17.46 | 7.78 | 0.090 |
| NC5 | 20 | 534.7 | 15.29 | 9.50 | 0.075 |
| Sample | Area in 1655 cm−1 |
|---|---|
| NC1 | 1491.4 |
| NC2 | 672.75 |
| NC3 | 567.9 |
| NC4 | 527.1 |
| NC5 | 889.1 |
| Samples | CeO2 | Metallic Ni | C (Graphite) | CaCO3 | Carbon Formed [mg] |
|---|---|---|---|---|---|
| NC1 | 17.1% | 3.1% | 79.8% | --- | 13.9 |
| NC2 | 4% | 3% | 92.9% | --- | 59.9 |
| NC3 | 12.8% | 2.7% | 83.9% | <1% | 43.2 |
| NC4 | 63.4% | 10.5% | 25.8% | <1% | 54.6 |
| NC5 | 58.6% | 6.4% | 58.6% | 20.1% | 15.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Madrigal, V.; Santiago-Salazar, D.; Ortega-Avilés, M.; Ríos-Valdovinos, E.; Albiter, E.; Valenzuela, M.A.; Pola-Albores, F. Performance of CaO-Promoted Ni Catalysts over Nanostructured CeO2 in Dry Reforming of Methane. Processes 2024, 12, 2815. https://doi.org/10.3390/pr12122815
Pérez-Madrigal V, Santiago-Salazar D, Ortega-Avilés M, Ríos-Valdovinos E, Albiter E, Valenzuela MA, Pola-Albores F. Performance of CaO-Promoted Ni Catalysts over Nanostructured CeO2 in Dry Reforming of Methane. Processes. 2024; 12(12):2815. https://doi.org/10.3390/pr12122815
Chicago/Turabian StylePérez-Madrigal, Vicente, Didier Santiago-Salazar, Mayahuel Ortega-Avilés, Edna Ríos-Valdovinos, Elim Albiter, Miguel A. Valenzuela, and Francisco Pola-Albores. 2024. "Performance of CaO-Promoted Ni Catalysts over Nanostructured CeO2 in Dry Reforming of Methane" Processes 12, no. 12: 2815. https://doi.org/10.3390/pr12122815
APA StylePérez-Madrigal, V., Santiago-Salazar, D., Ortega-Avilés, M., Ríos-Valdovinos, E., Albiter, E., Valenzuela, M. A., & Pola-Albores, F. (2024). Performance of CaO-Promoted Ni Catalysts over Nanostructured CeO2 in Dry Reforming of Methane. Processes, 12(12), 2815. https://doi.org/10.3390/pr12122815







