Rheological, Thermal, and Textural Characteristics of White, Milk, Dark, and Ruby Chocolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Particle Size Distribution (PSD)
2.2.2. Density of Chocolate Samples
2.2.3. Melting Properties of Chocolates
2.2.4. Chocolate Hardness
2.2.5. Rheological Properties of Chocolate Mass
2.2.6. Statistical Analysis
3. Results and Discussion
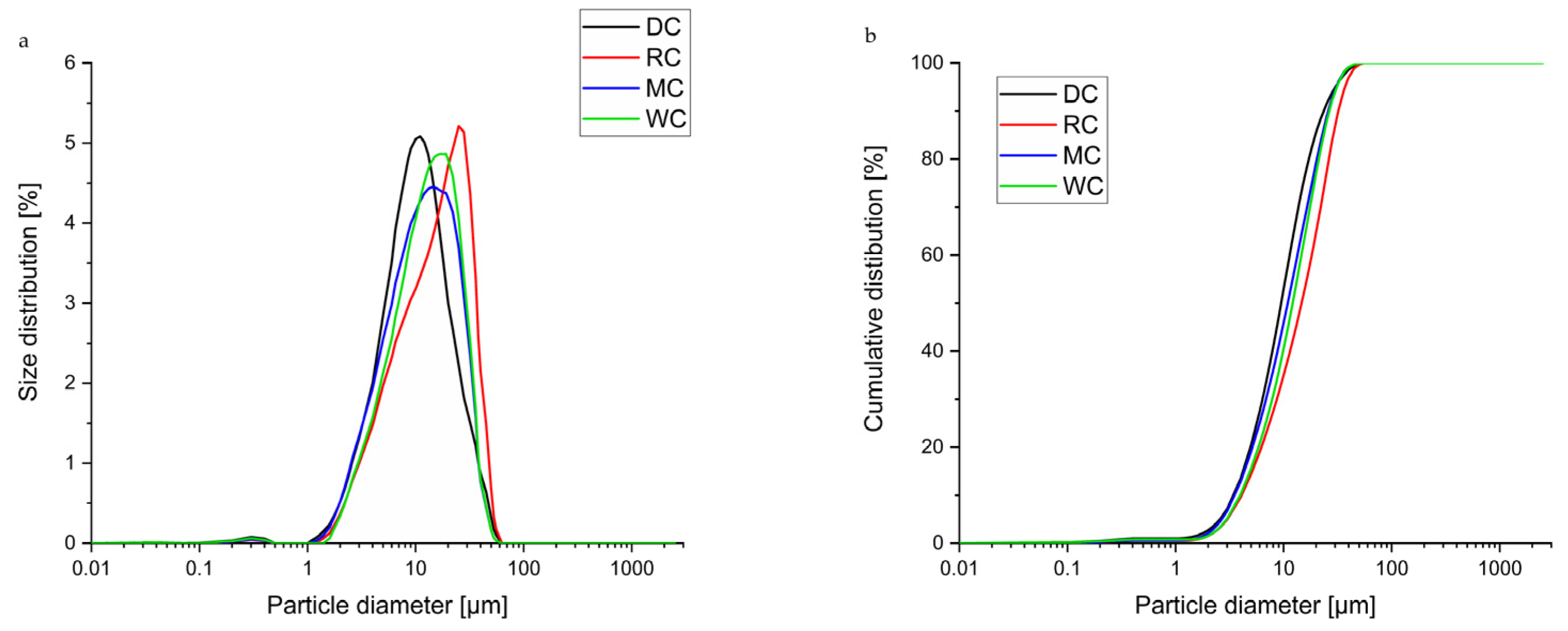
3.1. Comparative Overview of Particle Size Distribution in Tested Chocolate Samples
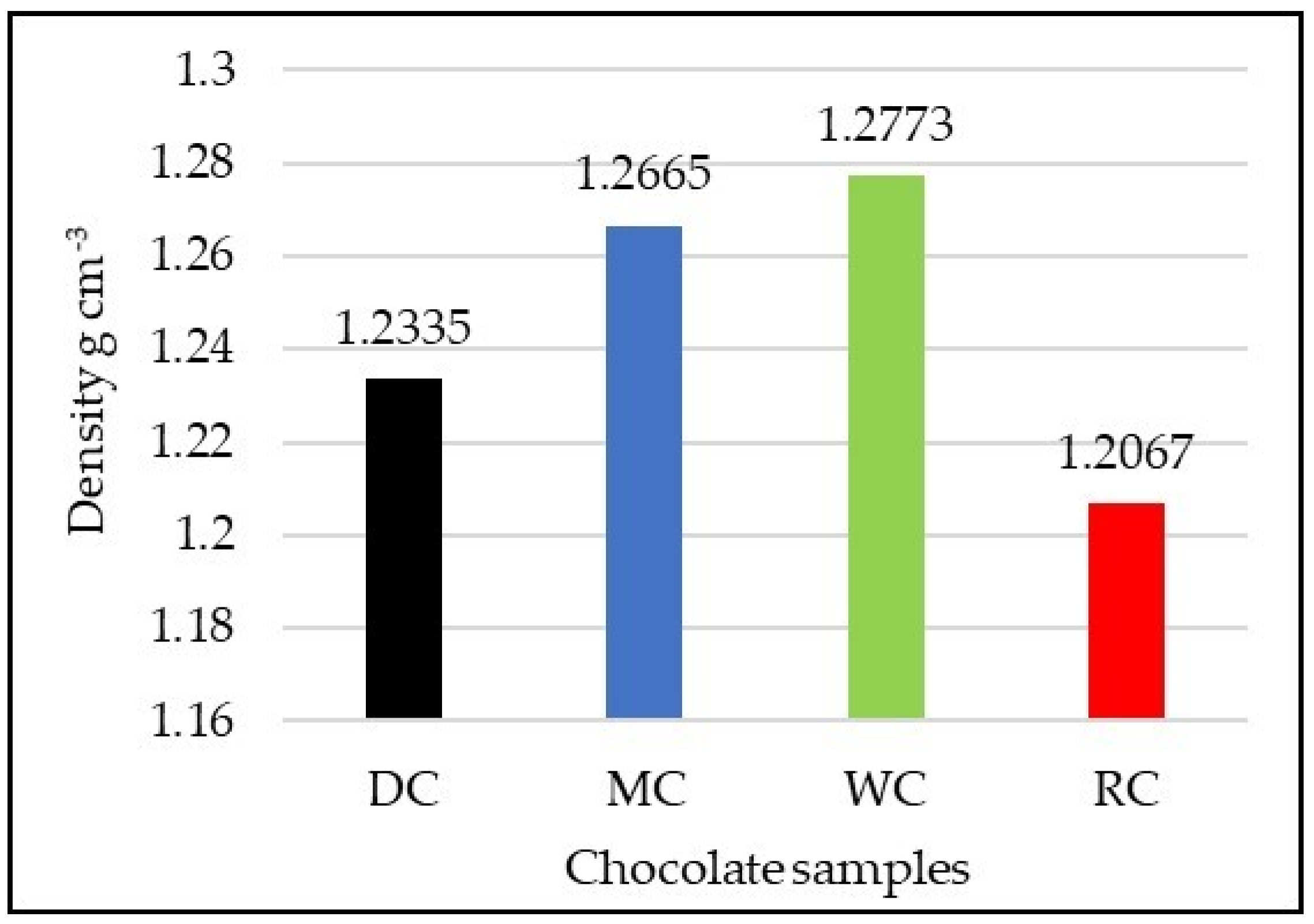
3.2. Comparative Overview of Density in Tested Chocolate Samples
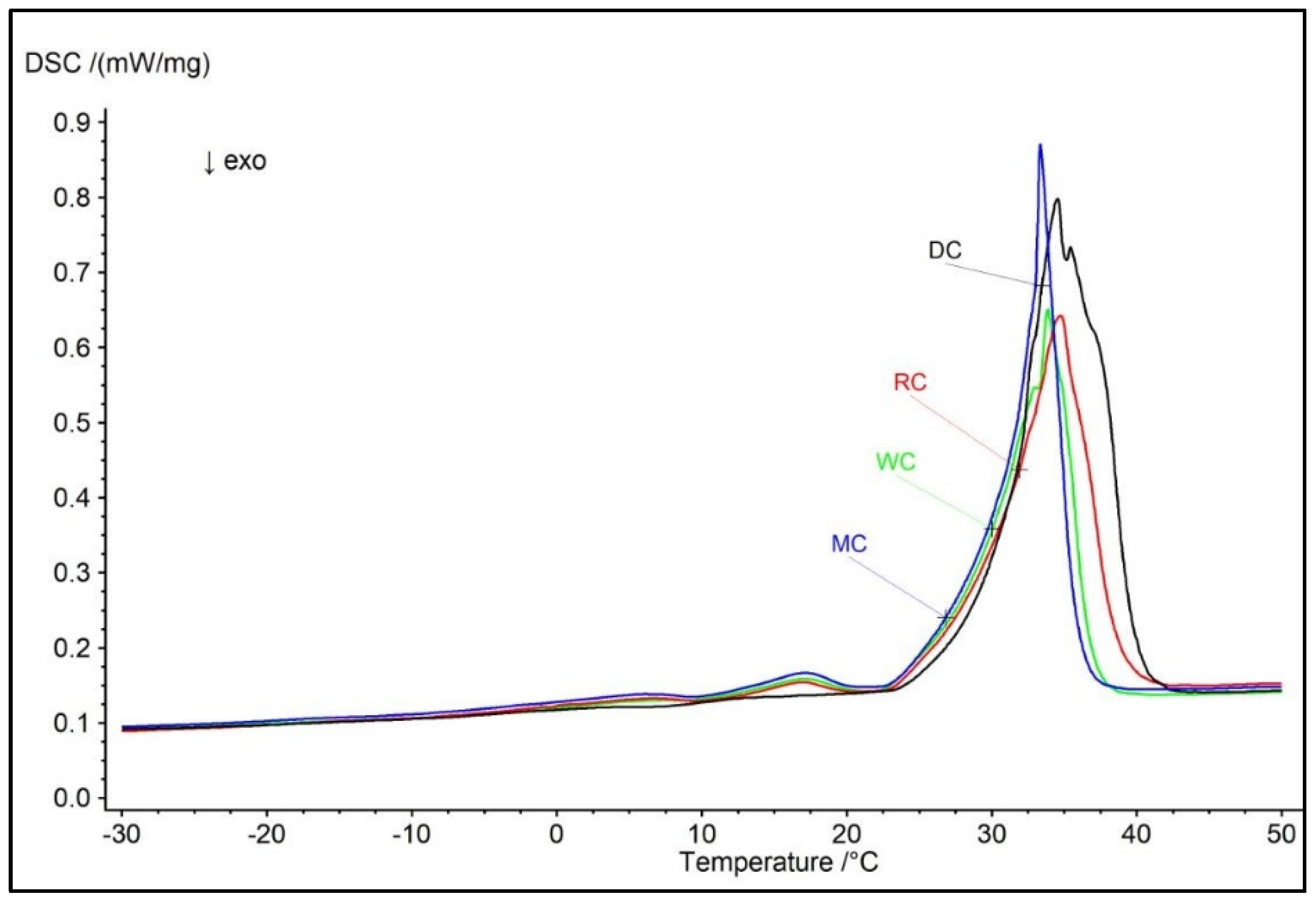
3.3. Comparative Overview of Melting Properties and Hardness in Tested Chocolate Samples
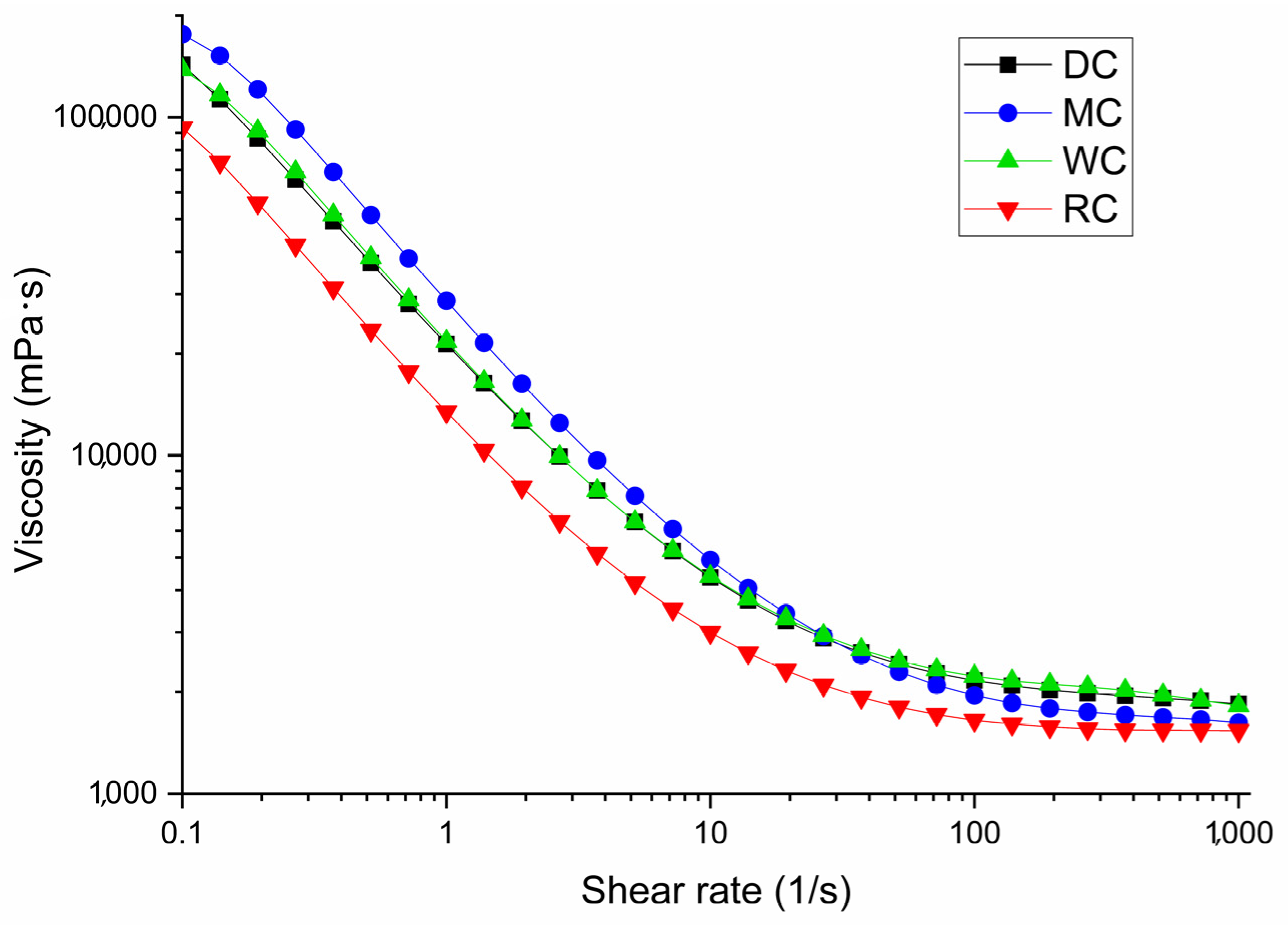
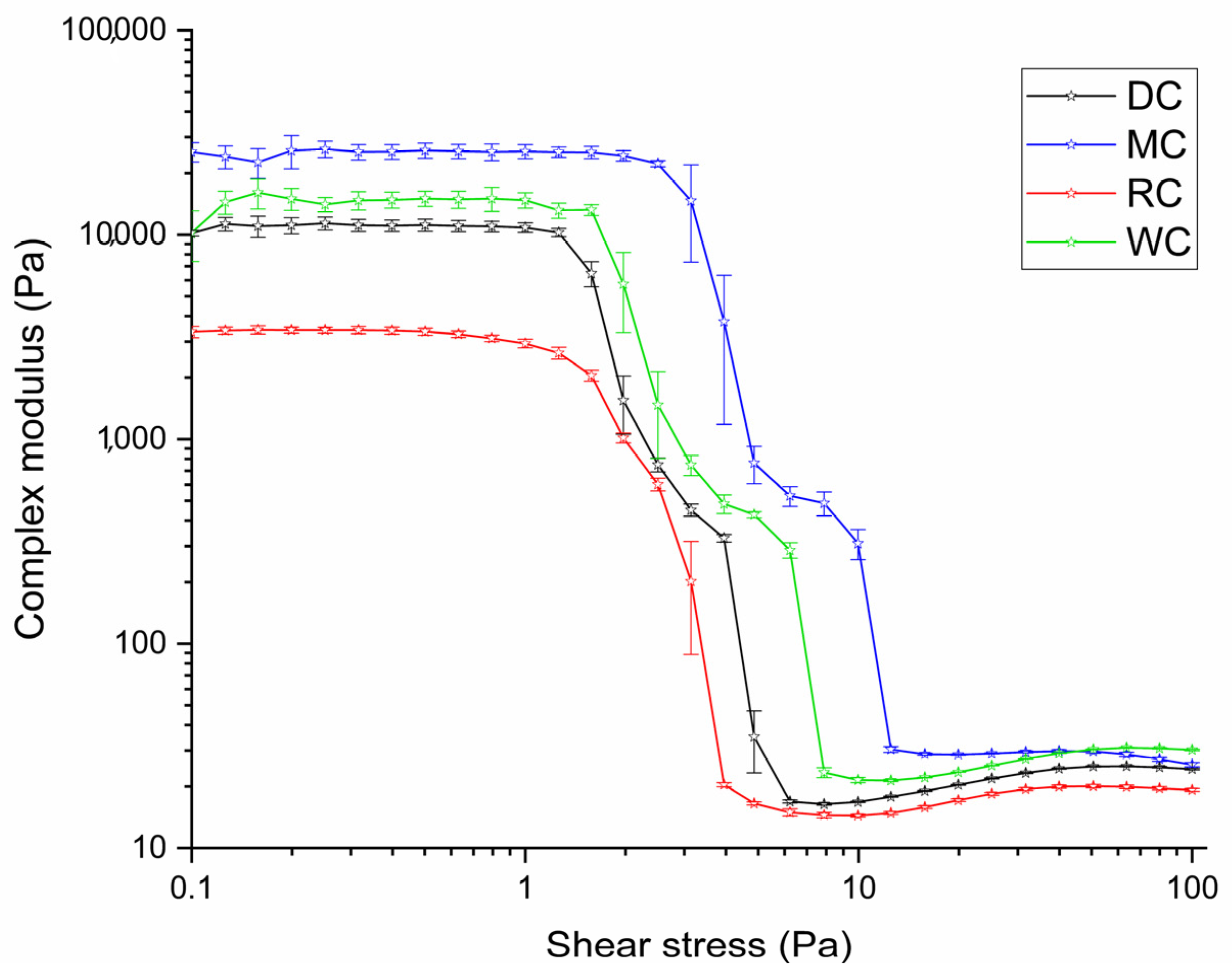
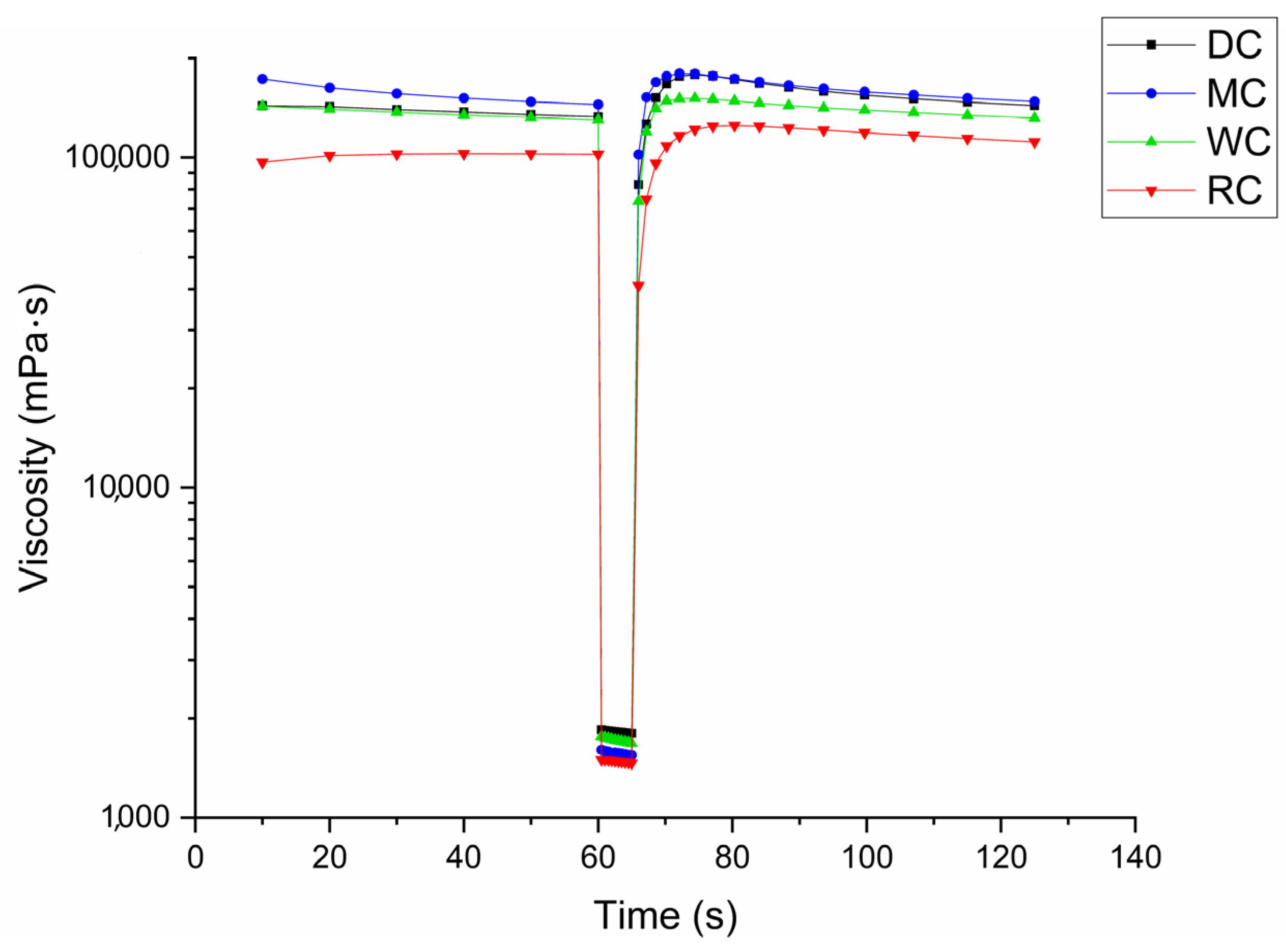
3.4. Comparative Overview of Rheology Properties in Tested Chocolate Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, V.A.; Müller, A.J.; Sandoval, A.J. Thermal, structural and rheological characteristics of dark chocolate with different compositions. J. Food Eng. 2013, 116, 97–108. [Google Scholar] [CrossRef]
- Directive 2000/36/EC of the European Parliament and of the Council; Official Journal of the European Communities, 23 June 2000. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32000L0036 (accessed on 30 September 2024).
- Regulation on Cocoa and Chocolate Products Intended for Human Consumption, Official Gazette Serbia 24/2019 and 18/2024. Available online: https://www.paragraf.rs/propisi/pravilnik-o-kvalitetu-i-drugim-zahtevima-kakao-cokoladne-proizvode.html (accessed on 30 September 2024).
- Dumarche, A.; Troplin, P.; Bernaert, H.; Lechevalier, P.; Beerens, H.; Landuyt, A. Process for Producing Cocoa-Derived Material. European Patent EP2237677B1, 13 June 2012. [Google Scholar]
- Tuenter, E.; Sakavitsi, M.E.; Rivera-Mondragon, A.; Hermans, N.; Foubert, K.; Halabalaki, M.; Pieters, L. Ruby chocolate: A study of its phytochemical composition and quantitative comparison with dark, milk and white chocolate. Food Chem. 2021, 343, 128446. [Google Scholar] [CrossRef] [PubMed]
- Šeremet, D.; Mandura, A.; Vojvodić Cebin, A.; Oskomić, M.; Champion, E.; Martinić, A.; Komes, D. Ruby chocolate—Bioactive potential and sensory quality characteristics compared with dark, milk and white chocolate. Food Health Dis. 2019, 8, 89–96. Available online: https://api.semanticscholar.org/CorpusID:209754360 (accessed on 30 September 2024).
- Kumbár, V.; Kouřilová, V.; Dufková, R.; Votava, J.; Hřivna, L. Rheological and Pipe Flow Properties of Chocolate Masses at Different Temperatures. Foods 2021, 10, 2519. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Dolatowska-Żebrowska, K.; Brzezińska, R.; Wirkowska-Wojdyła, M.; Bryś, J.; Piasecka, I.; Górska, A. Characterization of Thermal Properties of Ruby Chocolate Using DSC, PDSC and TGA Methods. Appl. Sci. 2023, 13, 5221. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Marzec, A.; Górska, A.; Wirkowska-Wojdyła, M.; Bryś, J.; Rejch, A.; Czarkowska, K. A Comparative Study of Thermal and Textural Properties of Milk, White, and Dark Chocolates. Thermochim. Acta 2019, 671, 60–69. [Google Scholar] [CrossRef]
- Dolatowska-Żebrowska, K.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Brys, J. Characterization of thermal properties of goat milk fat and goat milk chocolate by using DSC, PDSC and TGA methods. J. Therm. Anal. Calorim. 2019, 138, 2769–2779. [Google Scholar] [CrossRef]
- Rousseau, D.; Sonwai, S. Influence of the Dispersed Particulate in Chocolate on Cocoa Butter Microstructure and Fat Crystal Growth during Storage. Food Biophys. 2008, 3, 273–278. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M. Effects of particle size distribution and composition on rheological properties of dark chocolate. Eur. Food Res. Technol. 2008, 226, 1259–1268. [Google Scholar] [CrossRef]
- Beckett, S.T. The Science of Chocolate, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2008; pp. 104–110. [Google Scholar]
- Bolenz, S.; Holm, M.; Langkrär, C. Improving particle size distribution and flow properties of milk chocolate produced by ball mill and blending. Eur. Food Res. Technol. 2014, 238, 139–147. [Google Scholar] [CrossRef]
- Taylor, J.E.; Van Damme, I.; Johns, M.L.; Routh, A.F.; Wilson, D.I. Shear rheology of molten crumb chocolate. J. Food Sci. 2009, 74, E55–E61. [Google Scholar] [CrossRef] [PubMed]
- De Graef, V.; Depypere, F.; Minnaert, M.; Dewettinck, K. Chocolate Yield Stress as Measured by Oscillatory Rheology. Food Res. Int. 2011, 44, 2660–2665. [Google Scholar] [CrossRef]
- Naining, W.; Hongjian, Z. A study of the accuracy of optical fraunhofer diffraction size analyzer. Part. Sci. and Technol. 1986, 4, 403–408. [Google Scholar] [CrossRef]
- Rakin, M.; Bulatović, M.; Zarić, D.; Krunić, T.; Lončarević, I.; Petrović, J.; Pajin, B. Influence of added whey proteins and hydrolysates on rheological and textural characteristics of milk chocolate. Food Sci. Technol. Int. 2023, 29, 610–618. [Google Scholar] [CrossRef]
- Jovanović, P.; Pajin, B.; Lončarić, A.; Jozinović, A.; Petrović, J.; Fišteš, A.; Zarić, D.; Tumbas Šaponjac, V.; Ačkar, Đ.; Lončarević, I. Whey as a Carrier Material for Blueberry Bioactive Components: Incorporation in White Chocolate. Sustainability 2022, 14, 14172. [Google Scholar] [CrossRef]
- IA004EN-A Application Report: Density and Percent Solids of a Slurry. Anton Paar. 2020. Available online: https://www.anton-paar.com (accessed on 1 October 2024).
- IOCCC. Viscosity of Cocoa and Chocolate Products. Anal. Method 2000, 46, 1–7. [Google Scholar]
- Lončarević, I.; Pajin, B.; Petrović, J.; Nikolić, I.; Maravić, N.; Ačkar, Đ.; Šubarić, D.; Zarić, D.; Miličević, B. White chocolate with resistant starch: Impact on physical properties, dietary fiber content and sensory characteristics. Molecules 2021, 26, 5908. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Particle size distribution and compositional effects on textural properties and appearance of dark chocolates. J. Food Eng. 2008, 87, 181–190. [Google Scholar] [CrossRef]
- Ziegler, G.R.; Mongia, G.; Hollender, R. Role of particle size distribution of suspended solids in defining the sensory properties of milk chocolate. Int. J. Food Prop. 2001, 4, 175–192. [Google Scholar] [CrossRef]
- Bolenz, S.; Manske, A. Impact of Fat Content during Grinding on Particle Size Distribution and Flow Properties of Milk Chocolate. Eur. Food Res. Technol. 2013, 236, 863–872. [Google Scholar] [CrossRef]
- Glicerina, V.; Balestra, F.; Dalla Rosa, M.; Romani, S. Rheological, textural and calorimetric modifications of dark chocolate during process. J. Food Eng. 2013, 119, 173–179. [Google Scholar] [CrossRef]
- Bettersize Instruments Ltd. The Role of True Density Analysis in Chocolate Manufacturing Quality. AZoM. 2024. Available online: https://www.azom.com/article.aspx?ArticleID=23293 (accessed on 17 October 2024).
- Zaric, D.; Rakin, M.; Bulatovic, M.; Krunić, T.; Lončarević, I.; Pajin, B.; Blaževska, Z. Influence of added extracts of herbs (Salvia lavandulifolia, Salvia officinalis) and fruits (Malpighia glabra) on rheological, textural, and functional (AChE-inhibitory and antioxidant activity) characteristics of dark chocolate. J. Food Meas. Charact. 2022, 18, 772–782. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Fišteš, A.; Tumbas Šaponjac, V.; Petrović, J.; Jovanović, P.; Vulić, J.; Zarić, D. Enrichment of white chocolate with blackberry juice encapsulate: Impact on physical properties, sensory characteristics and polyphenol content. LWT 2018, 92, 458–464. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Molecular origins of polymorphism in cocoa butter. Food Sci. Technol. 2021, 12, 567–590. [Google Scholar] [CrossRef]
- Merken, G.; Vaeck, S. Étude du polymorphisme du beurre de cacao par calorimetrie DSC, Lebensm. Wiss. Technol. 1980, 13, 314–317. [Google Scholar]
- Wang, F.; Liu, Y.; Jin, Q.; Meng, Z.; Wang, X. Characterization of cocoa butter substitutes, milk fat and cocoa butter mixtures. Eur. J. Lipid Sci. Technol. 2011, 113, 1145–1151. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Saputro, A.D.; Rottiers, H.; Van de Walle, D.; Dewettinck, K. Physicochemical Properties and Antioxidant Activities of Chocolates Enriched with Engineered Cinnamon Nanoparticles. Eur. Food Res. Technol. 2018, 244, 1185–1202. [Google Scholar] [CrossRef]
- Konar, N.; Özhan, B.; Artık, N.; Dalabasmaz, S.; Poyrazoglu, E.S. Rheological and physical properties of inulin-containing milk chocolate prepared at different process conditions. CYTA J. Food 2013, 12, 55–64. [Google Scholar] [CrossRef]
- Feichtinger, A.; Scholten, E.; Sala, G. Effect of particle size distribution on rheological properties of chocolate. Food Funct. 2020, 11, 9547–9559. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Microstruc-ture and mechanical properties related to particle size distribu-tion and composition in dark chocolate. Int. J. Food Sci. Technol. 2009, 44, 111–119. [Google Scholar] [CrossRef]
- Pajin, B. Tehnologija Čokolade i Kakao Proizvoda, 1st ed.; Tehnološki Fakultet: Novi Sad, Serbia, 2014; pp. 100–105. [Google Scholar]
- Do, T.-A.L.; Hargreaves, J.M.; Wolf, B.; Mitchell, J.R. Impact of particle Size Distribution on Rheological and Textural Properties of Chocolate Models with Reduced Fat Content. J. Food Sci. 2007, 72, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Walls, H.J.; Caines, S.B.; Sanchez, A.M.; Khan, S.A. Yield stress and wall slip phenomena in colloidal silica gels. J. Rheol. 2003, 47, 847–868. [Google Scholar] [CrossRef]
- Gao, X.; Guo, T.; Han, F.; Tian, Y.; Zhang, Z. Rheological and Sensory Properties of Four Kinds of Dark Chocolates. AJAC 2015, 6, 1010–1018. [Google Scholar] [CrossRef]
- Kalić, M.D. Physico-Chemical and Rheological Characterization of Fish Oil Microcapsules Incorporated in a Chocolate Matrix. Ph.D. Thesis, Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia, 2019. [Google Scholar]


| RC | DC | MC | WC | |
|---|---|---|---|---|
| Energy value (kJ) | 2340 | 2255 | 2357 | 2379 |
| Fats (g) | 36 | 39 | 36 | 36 |
| Of which are saturated fatty acids | 21 | 23 | 22 | 21.6 |
| Carbohydrates (g) | 50 | 31 | 51.9 | 55.5 |
| Of which are sugars | 49 | 26 | 50 | 55 |
| Proteins (g) | 9.3 | 8.5 | 7 | 6 |
| Salt (g) | 0.27 | 0.02 | 0.2 | 0.2 |
| Ingredients | sugar, cocoa butter, skimmed milk powder, whole milk powder, cocoa mass, emulsifier soy lecithin, citric acid, natural vanilla flavor. | cocoa mass, sugar, cocoa powder with reduced cocoa butter content, emulsifier soy lecithin, natural vanilla flavor. | sugar, cocoa butter, whole milk powder, cocoa mass, emulsifier soy lecithin, natural vanilla flavor. | sugar, cocoa butter, whole milk powder, emulsifier soy lecithin, natural vanilla flavor. |
| Cocoa parts: min. (%) | 47 | 70 | 33 | 28 |
| Parameters | Sample of Chocolate | |||
|---|---|---|---|---|
| DC | RC | MC | WC | |
| d(0.1) µm | 3.392 ± 0.039 a | 4.078 ± 0.018 b | 3.525 ± 0.052 c | 3.917 ± 0.046 d |
| d(0.5) µm | 9.441 ± 0.034 a | 14.569 ± 0.133 b | 10.790 ± 0.062 c | 11.831 ± 0.225 d |
| d(0.9) µm | 23.487 ± 0.112 a | 31.965 ± 0.211 b | 25.181 ± 0.375 c | 25.575 ± 0.505 c |
| D[4.3] µm | 12.301 ± 0.036 a | 17.427 ± 0.118 b | 13.445 ± 0.144 c | 14.155 ± 0.259 d |
| SPAN | 2.128 ± 0.020 a | 1.914 ± 0.011 b | 2.007 ± 0.260 ab | 1.831 ± 0.018 c |
| Chocolate | Melting Parameters | Textural Properties | |||
|---|---|---|---|---|---|
| Tons (°C) | Tend (°C) | Tpeak (°C) | ΔH (J/g) | Hardness (g) | |
| DC | 24.4 ± 0.08 a | 40.2 ± 0.16 a | 34.5± 0.21 a | 55.04 ± 0.54 a | 4994.0 ± 28.50 a |
| MC | 24.3 ± 0.14 a | 35.9 ± 0.19 b | 33.3± 0.15 ab | 36.76 ± 0.32 b | 4782.0 ± 26.70 b |
| RC | 19.9 ± 0.11 b | 38.9 ± 0.12 c | 34.7± 0.17 ac | 39.91 ± 0.34 c | 4648.0 ± 38.20 c |
| WC | 23.6 ± 0.09 c | 36.8 ± 0.13 b | 33.9± 0.20 acd | 35.30 ± 0.30 b | 4082.0 ± 44.30 d |
| Parameters | DC | MC | WC | RC |
|---|---|---|---|---|
| Casson | ||||
| Yield stress τ0, Pa | 11.45 ± 0.08 a | 17.61 ± 0.10 b | 11.41 ± 0.07 a | 6.47 ± 0.05 c |
| Infinite shear viscosity η∞, Pa s | 1.21 ± 0.05 a | 0.89 ± 0.01 b | 1.27 ± 0.07 a | 1.01 ± 0.05 b |
| Correlation coefficient R2 | 0.99256 | 0.99145 | 0.99098 | 0.99196 |
| Windhab | ||||
| Yield stress τ0, Pa | 19.38 ± 0.09 a | 26.52 ± 0.12 b | 20.08 ± 0.09 c | 11.91 ± 0.06 d |
| Infinite shear viscosity η∞, Pa s | 1.92 ± 0.06 a | 1.61 ± 0.04 b | 2.01 ± 0.13 a | 1.52 ± 0.08 b |
| Linear yield stress τ1, Pa | 27.06 ± 0.18 a | 35.81 ± 0.15 b | 26.80 ± 0.11 a | 16.48 ± 0.09 c |
| Characteristic shear rate D*, s−1 | 5.825 | 6.744 | 6.499 | 5.295 |
| Correlation coefficient R2 | 0.99999 | 0.99999 | 0.99996 | 0.99998 |
| Other parameters | ||||
| Tixotropic curve area, Pas−1 | 277.37 ± 5.18 a | 692.26 ± 3.62 b | 1447.5 ± 9.88 c | 639.74 ± 3.98 b |
| Viscosity from the viscosity curve at 1000 s−1, Pa s | 1.84 ± 0.05 a | 1.62 ± 0.03 b | 1.82 ± 0.06 a | 1.54 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarić, D.B.; Rakin, M.B.; Bulatović, M.L.; Dimitrijević, I.D.; Ostojin, V.D.; Lončarević, I.S.; Stožinić, M.V. Rheological, Thermal, and Textural Characteristics of White, Milk, Dark, and Ruby Chocolate. Processes 2024, 12, 2810. https://doi.org/10.3390/pr12122810
Zarić DB, Rakin MB, Bulatović ML, Dimitrijević ID, Ostojin VD, Lončarević IS, Stožinić MV. Rheological, Thermal, and Textural Characteristics of White, Milk, Dark, and Ruby Chocolate. Processes. 2024; 12(12):2810. https://doi.org/10.3390/pr12122810
Chicago/Turabian StyleZarić, Danica B., Marica B. Rakin, Maja Lj. Bulatović, Ivan D. Dimitrijević, Vanja D. Ostojin, Ivana S. Lončarević, and Milica V. Stožinić. 2024. "Rheological, Thermal, and Textural Characteristics of White, Milk, Dark, and Ruby Chocolate" Processes 12, no. 12: 2810. https://doi.org/10.3390/pr12122810
APA StyleZarić, D. B., Rakin, M. B., Bulatović, M. L., Dimitrijević, I. D., Ostojin, V. D., Lončarević, I. S., & Stožinić, M. V. (2024). Rheological, Thermal, and Textural Characteristics of White, Milk, Dark, and Ruby Chocolate. Processes, 12(12), 2810. https://doi.org/10.3390/pr12122810






