Study on the Repair of Irregular and Deep Cracks Induced by Thermal Shock Using Al-Cu-O Reactions in Al2O3 Ceramics
Abstract
:1. Introduction
| Materials | Crack Generation | Crack Length (μm) | Reference No. |
|---|---|---|---|
| Al2O3/15vol%SiC | Vickers indentation | ~100 | [9] |
| Al2O3/SiCw/TiSi2 | Vickers indentation | 150~500 | [10] |
| Si3N4/SiCw | Vickers indentation | 200~1200 | [11] |
| Al2O3/Ti | Vickers indentation | ~80 | [13] |
| Al2O3/Ti2AlC | Vickers indentation | ~20 | [14] |
| Al2O3 | Thermal shock | - | [15] |
| Al2O3/Silica glass | Vickers indentation and bridge loading | 2500 | [16] |
2. Experimental Procedure
2.1. Preparation of Specimen
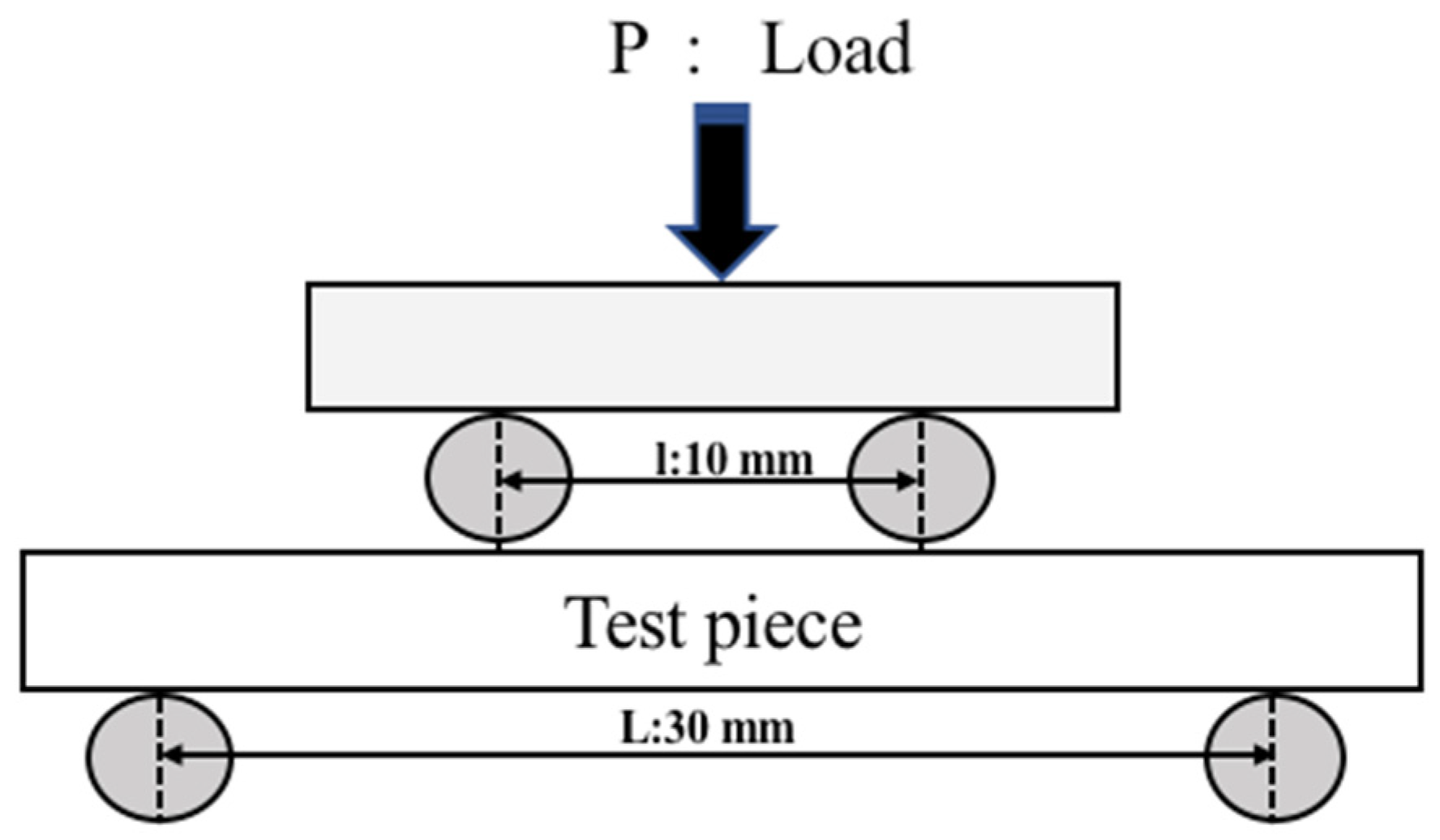
2.2. Evaluation Method
3. Results and Discussion
3.1. Crack Formation After Thermal Shock
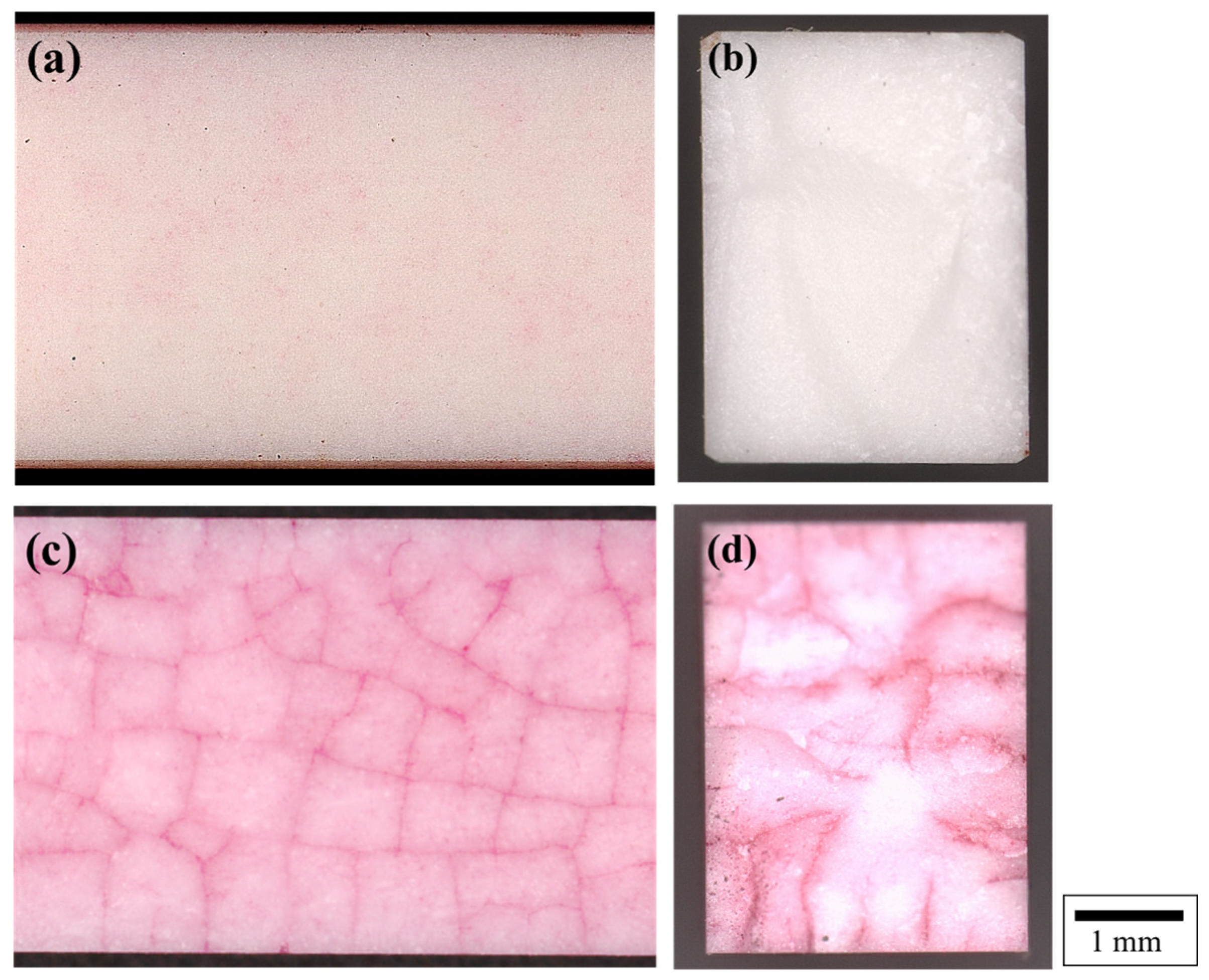
3.1.1. Cracks Pattern Appeared on the Surface of the Quenched Al2O3
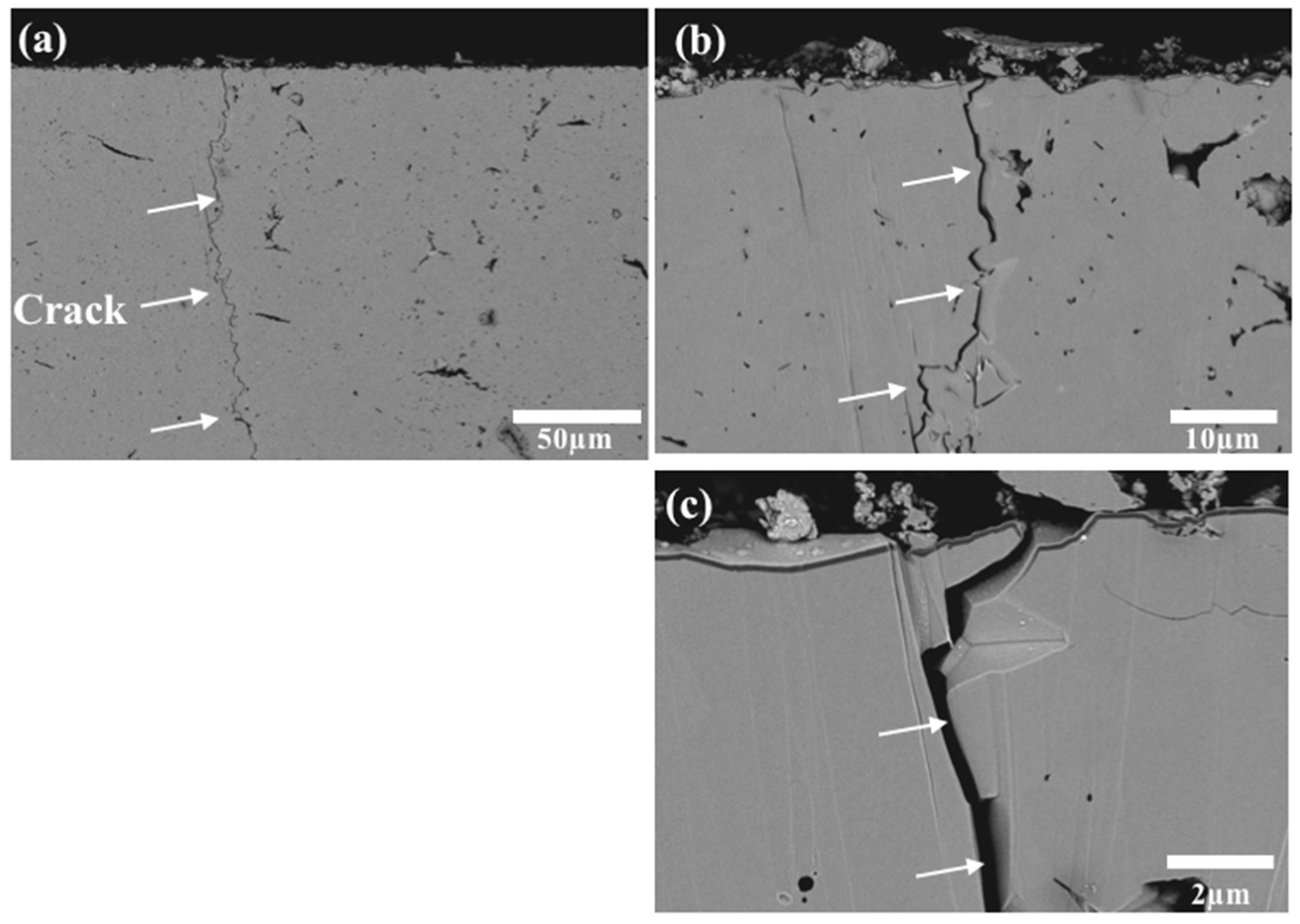
3.1.2. Microstructure and Cracks Extending in Al2O3 After Thermal Shock
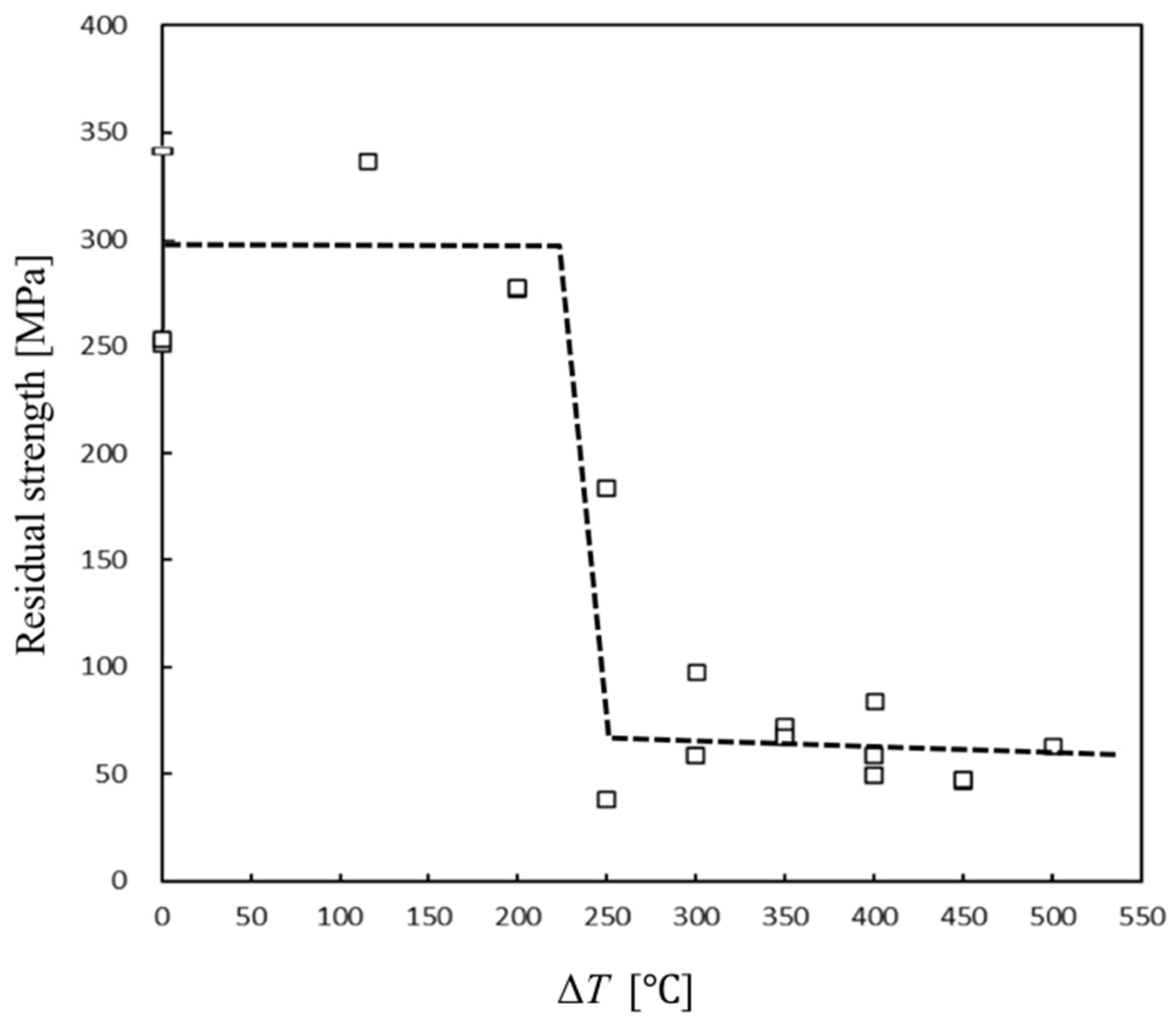
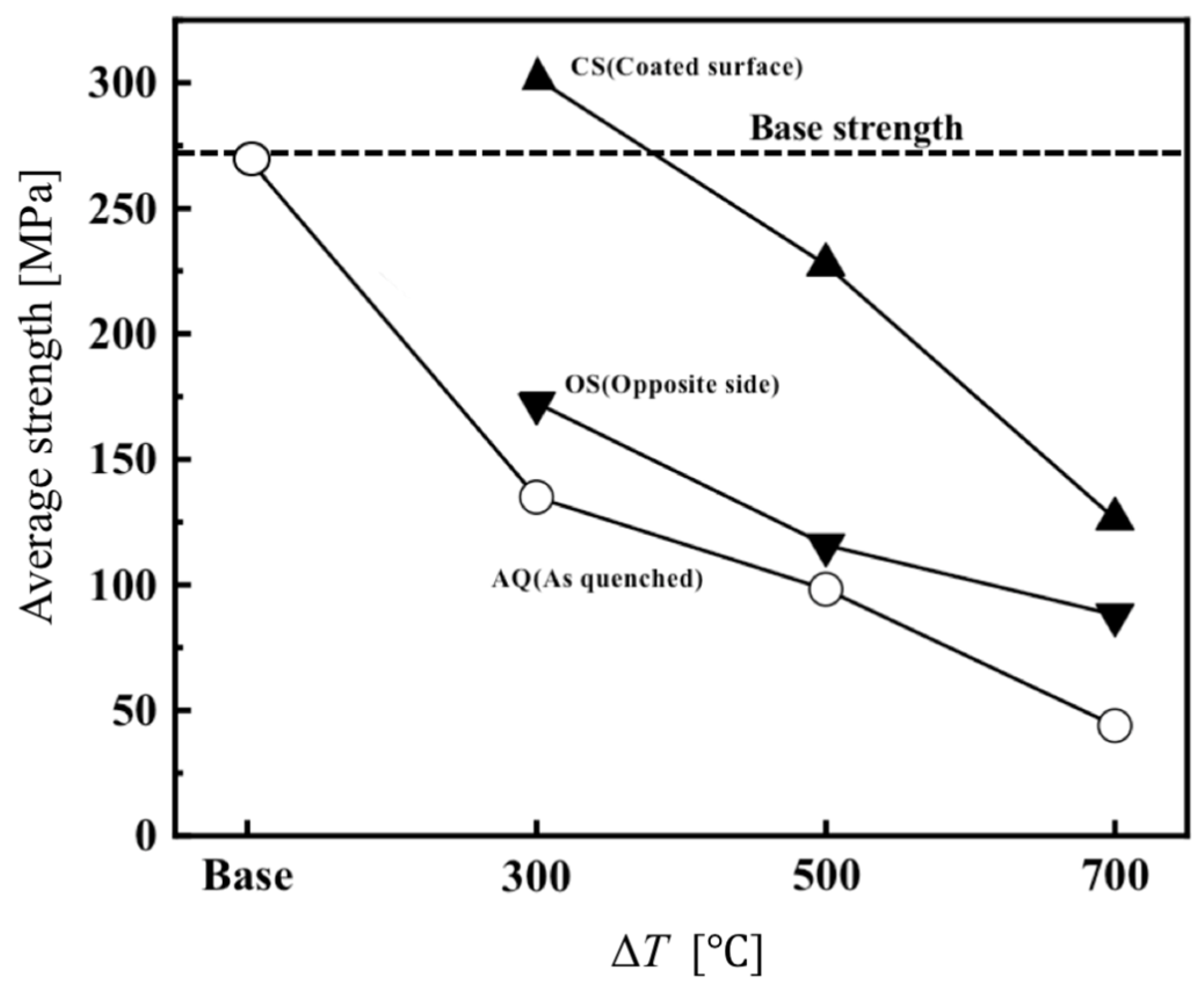
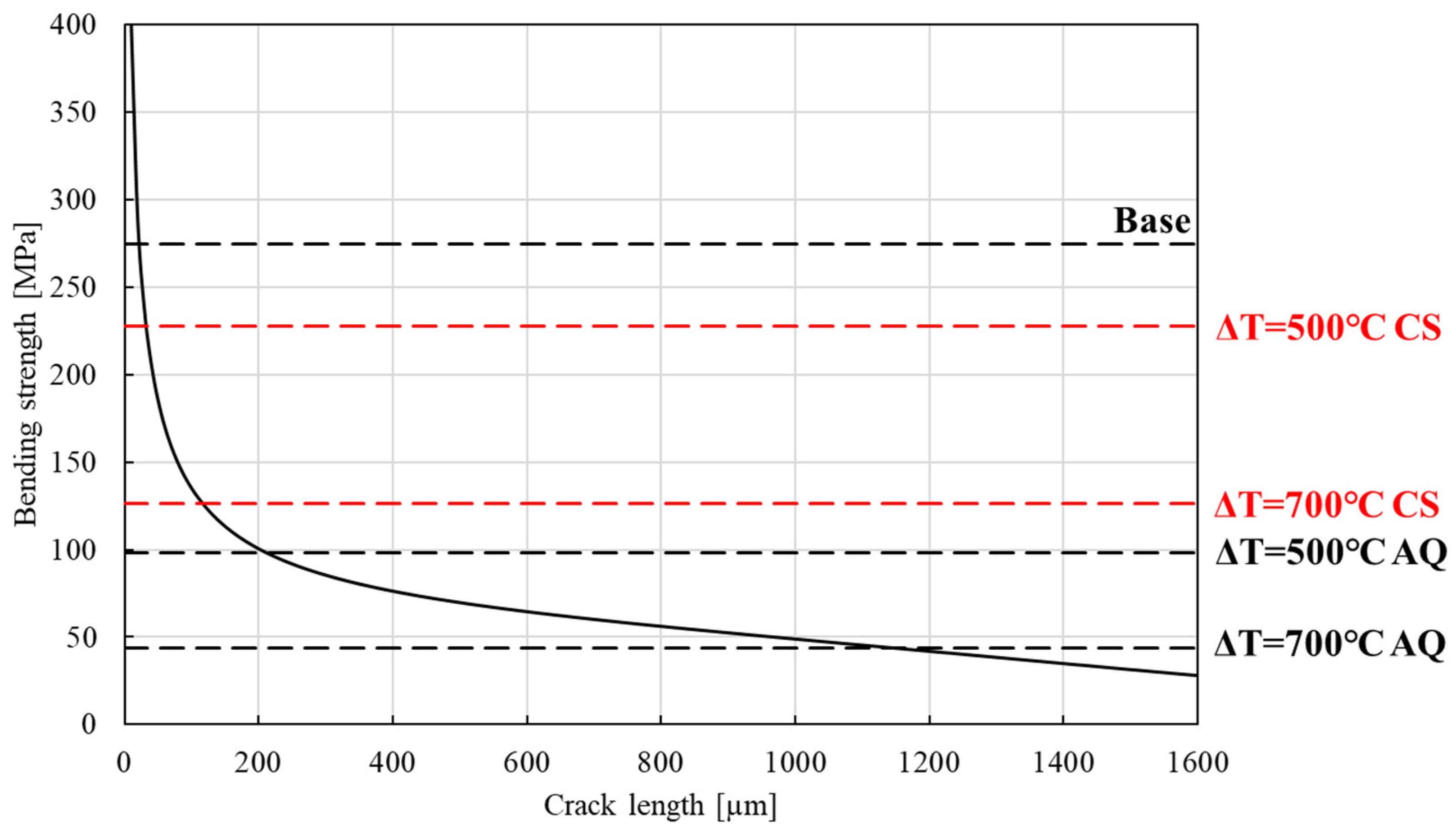
3.1.3. Determination of Thermal Shock Temperature for Crack Introduction: Residual Strength Test Results After Quenching at Different ΔT
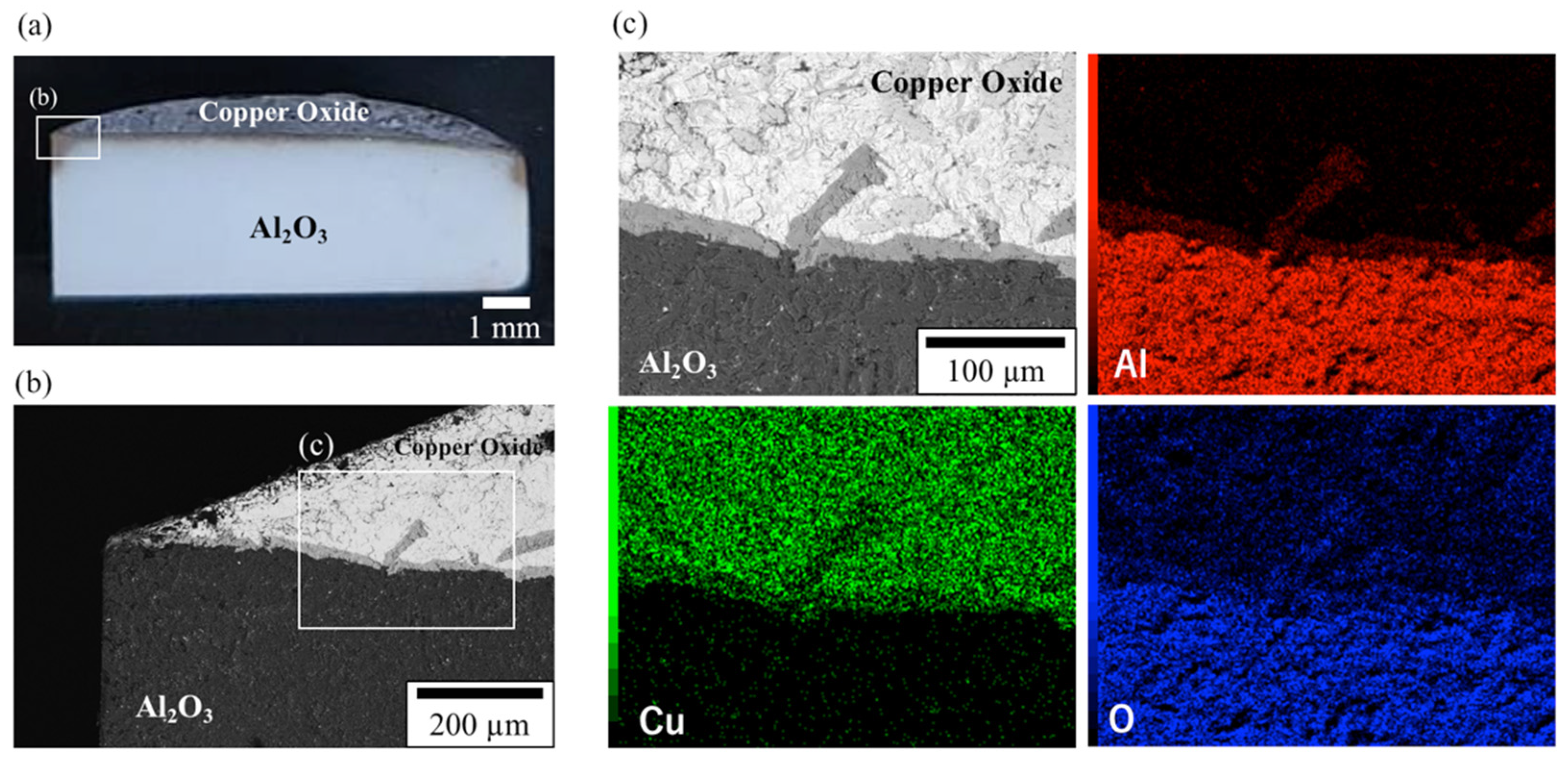
3.2. Microstructure of Sintered Al2O3 Samples Not Subjected to Thermal Shock After Heat Treatment at 1200 °C for 1 h Following Cu Pasting
3.3. Strength After Thermal Shock Followed by Cu Application and Heat Treatment
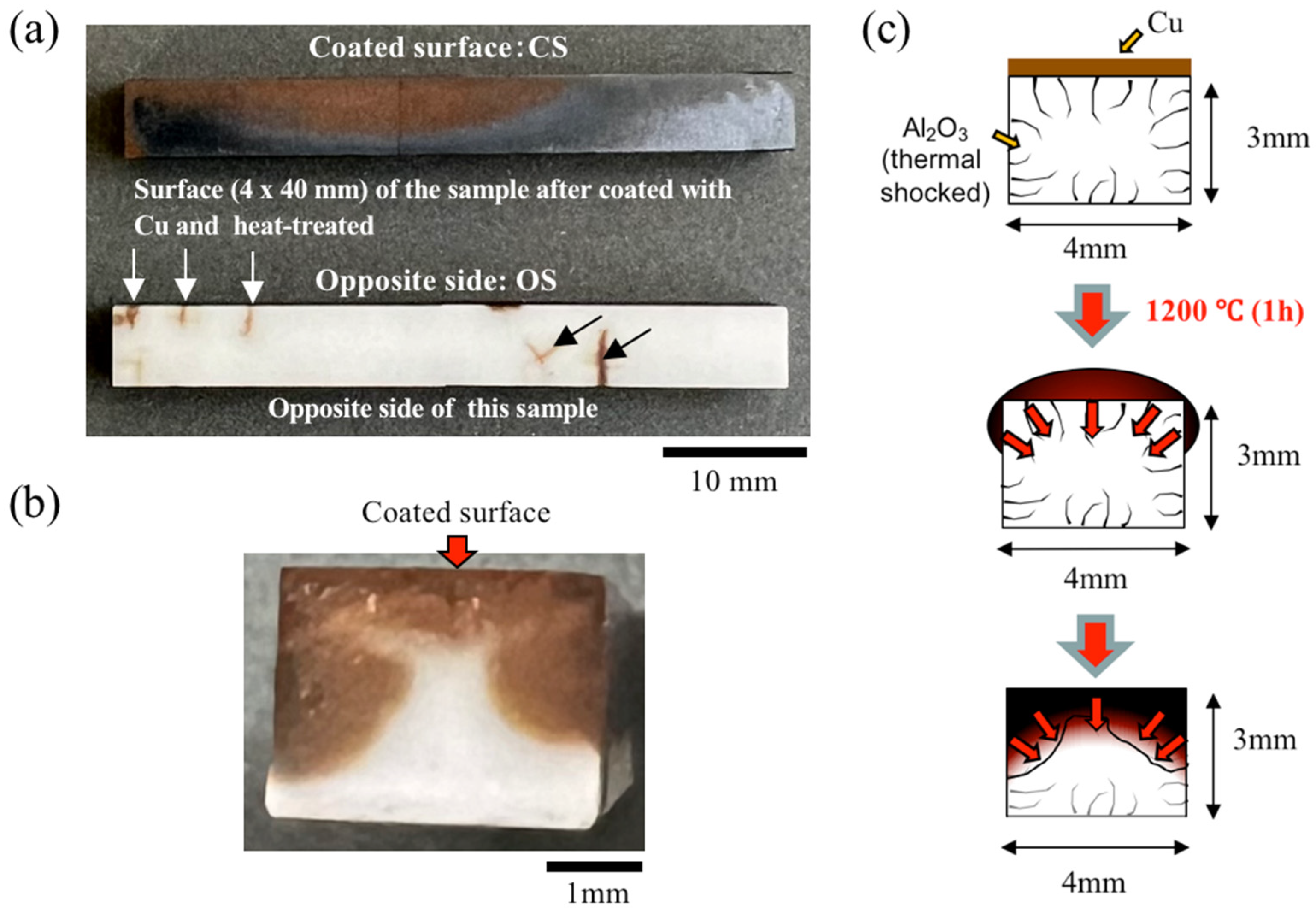
3.3.1. Low-Magnification Images of the Surface and Cross-Sectional Structure of a Sample Coated with Cu Paste Exclusively on the Upper Surface
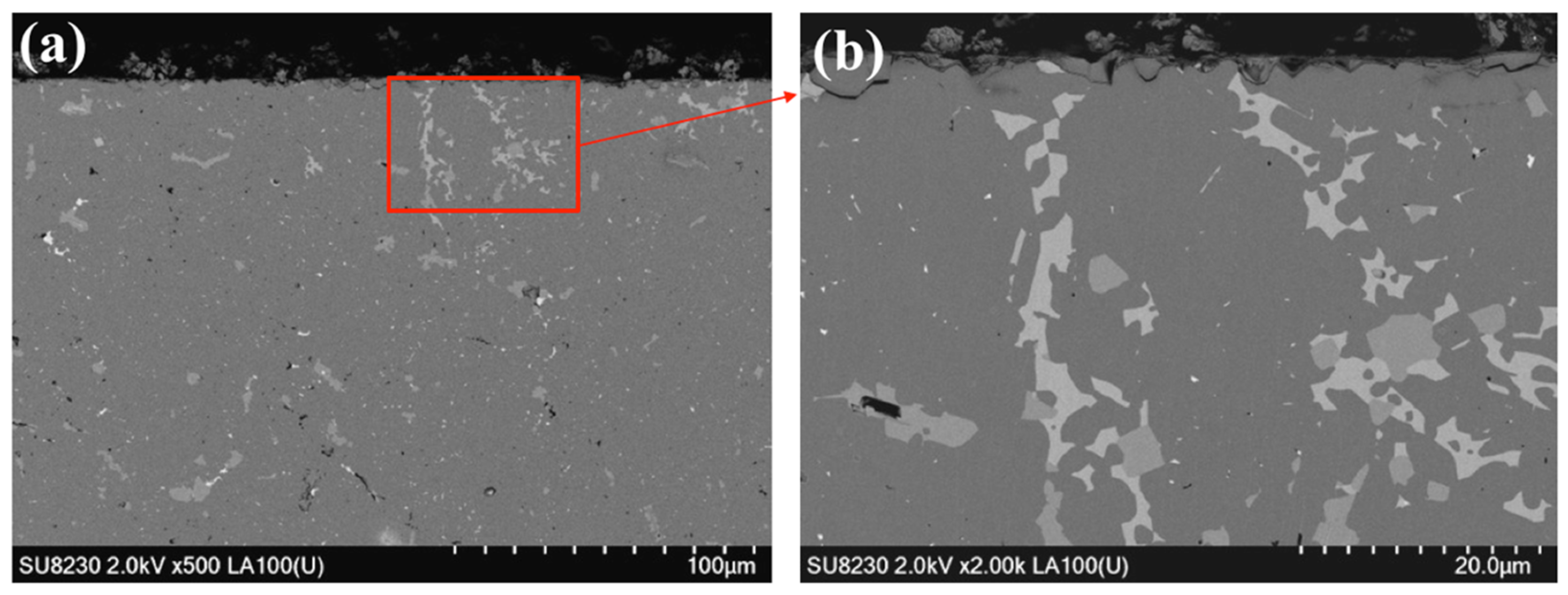
3.3.2. SEM Results of the Cross-Sectional Microstructure of a Sample Heat-Treated with Cu at 1200 °C for 1 h in Air
3.3.3. Strength of Samples Subjected to Various Treatment Conditions
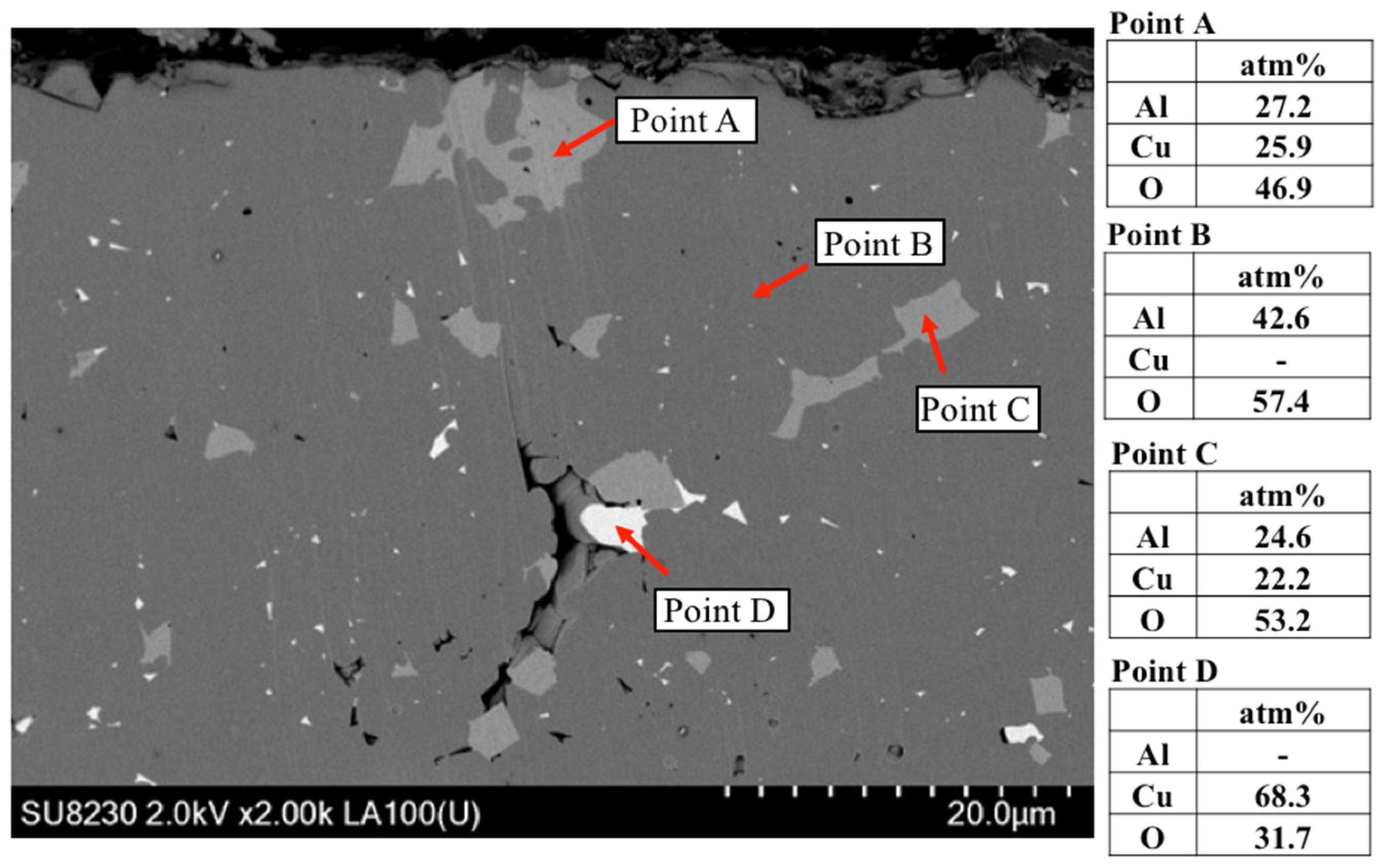
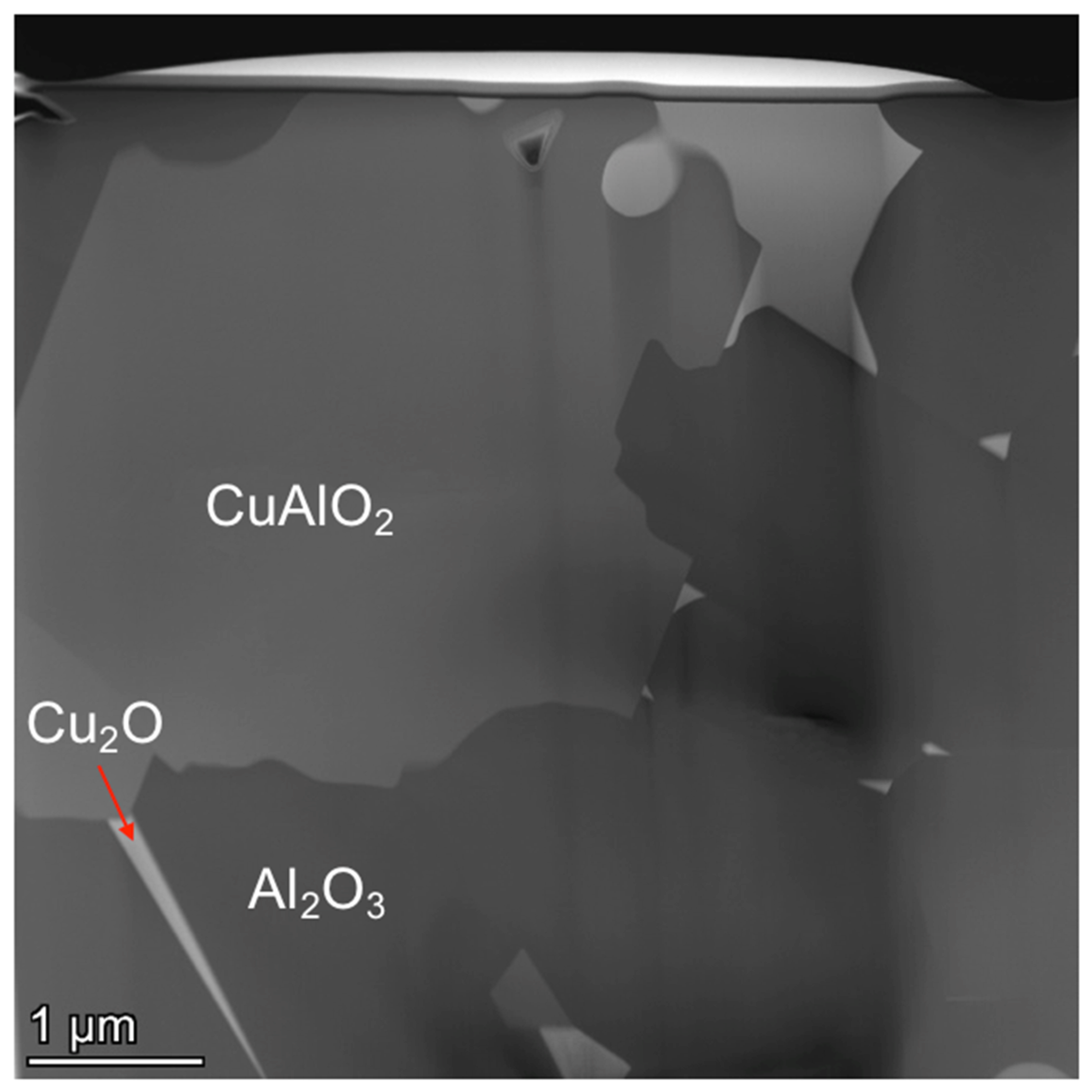
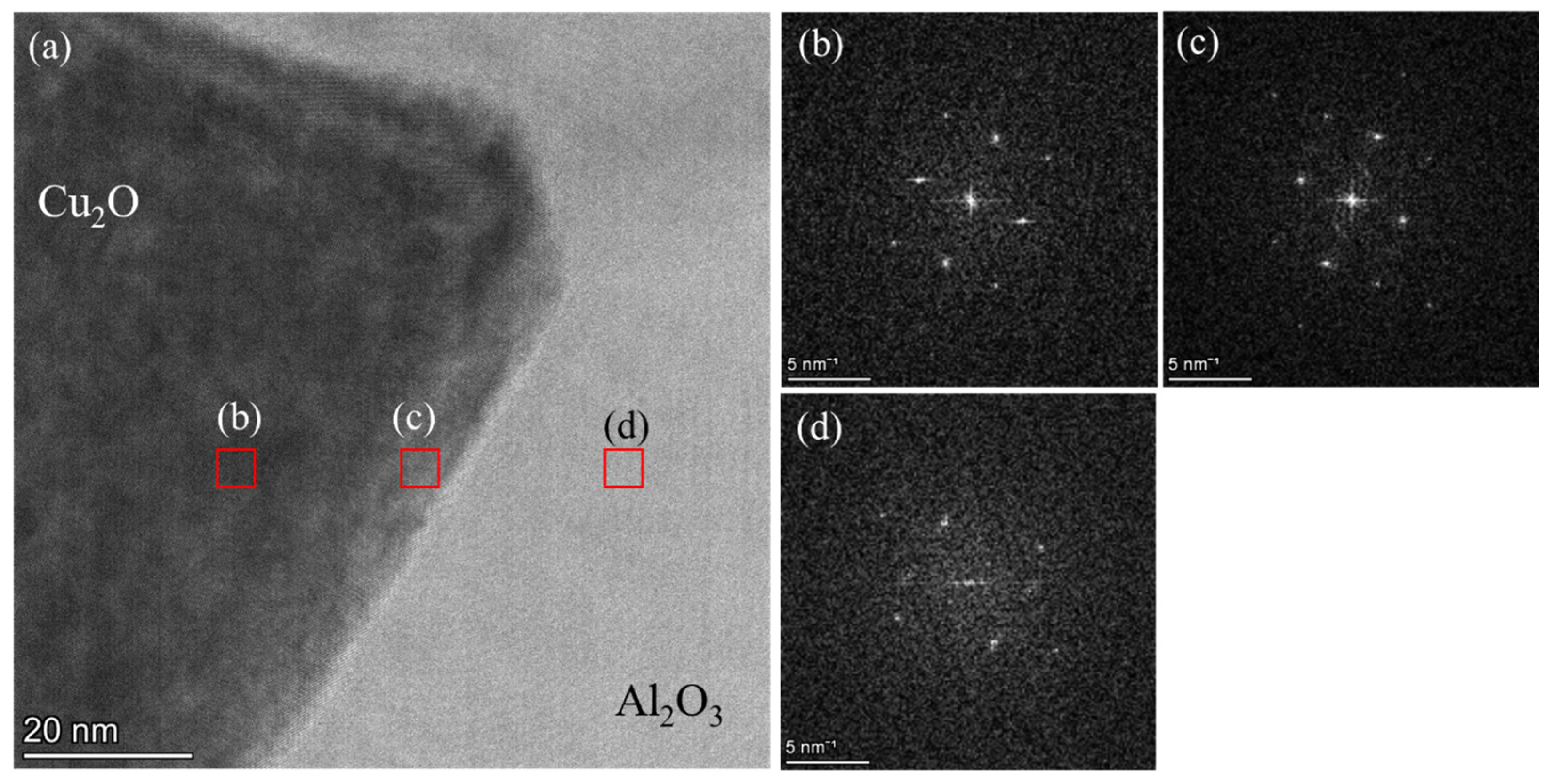
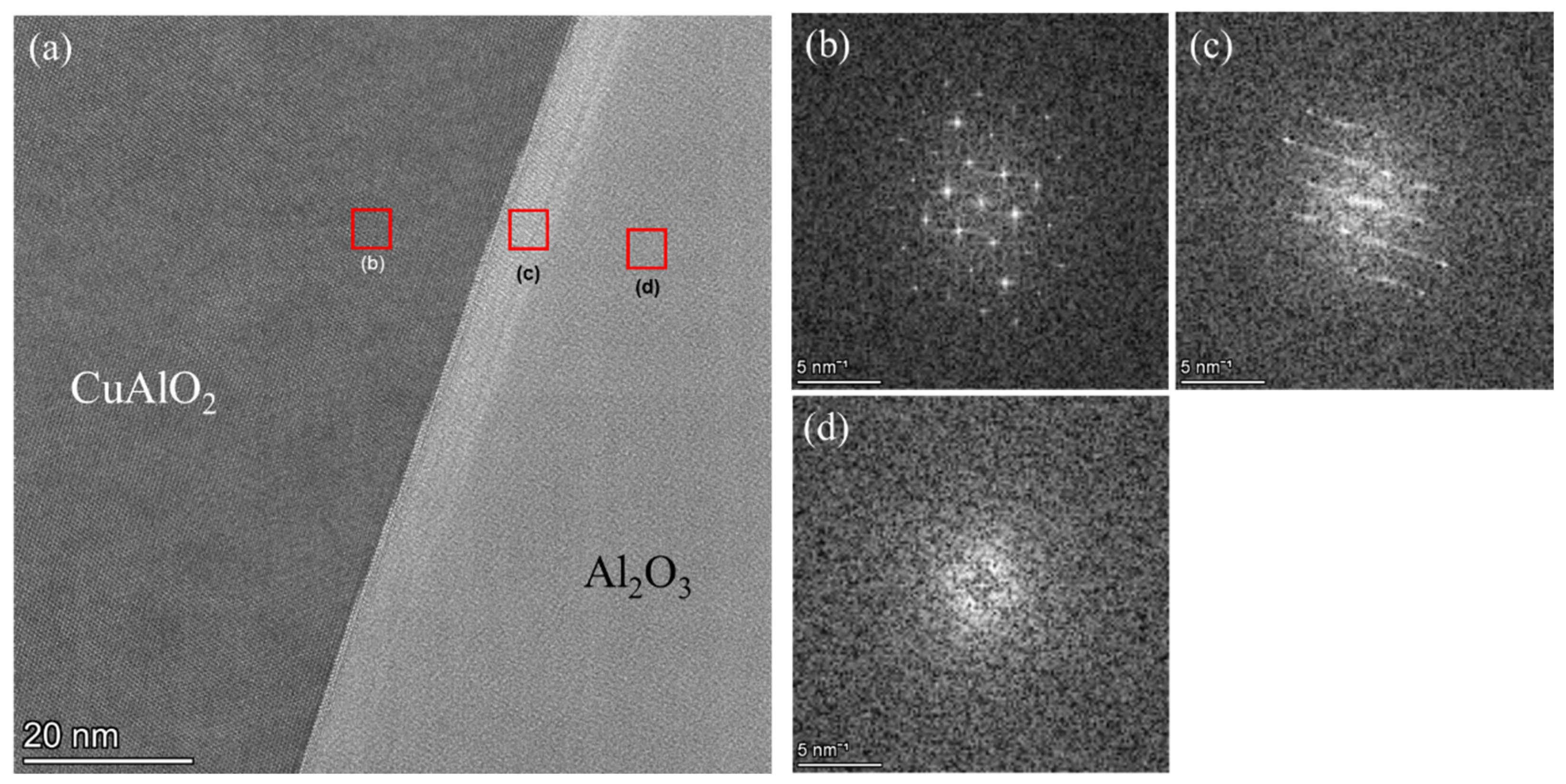
3.3.4. TEM Results of the Al2O3 and CuAlO2 Interface
3.4. Relationship Between Different Crack Lengths and Bending Strengths
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajak, P.; Kalia, R.K.; Nakano, A.; Vashishta, P. Faceting, Grain Growth, and Crack Healing in Alumina. ACS Nano 2018, 12, 9005–9010. [Google Scholar] [CrossRef] [PubMed]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive manufacturing of ceramics issues, potentialities, and opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Sekine, N.; Nakao, W. Advanced Self-Healing Ceramics with Controlled Degradation and Repair by Chemical Reaction. Materials 2023, 16, 6368. [Google Scholar] [CrossRef] [PubMed]
- Greil, P. Generic principles of crack-healing ceramics. J. Adv. Ceram. 2012, 1, 249–267. [Google Scholar] [CrossRef]
- Greil, P. Self-healing engineering ceramics with oxidation-induced crack repair. Adv. Eng. Mater. 2020, 22, 1901121. [Google Scholar] [CrossRef]
- Tseng, W.J.; Kita, H. As-fired strength of sintered silicon nitride ceramics. Ceram. Int. 2000, 26, 197–202. [Google Scholar] [CrossRef]
- Nakao, W.; Osada, T.; Nishiwaki, T.; Otsuka, H. Focus on self-healing materials: Recent challenges and innovations. Sci. Technol. Adv. Mater. 2021, 22, 234. [Google Scholar] [CrossRef]
- Pham, H.V.; Nanko, M.; Nakao, W. High-temperature Bending Strength of Self-Healing Ni/Al2O3 Nanocomposites. Int. J. Appl. Ceram. Technol. 2016, 13, 973–983. [Google Scholar] [CrossRef]
- Osada, T.; Nakao, W.; Takahashi, K.; Ando, K. Kinetics of self-crack-healing of alumina/silicon carbide composite including oxygen partial pressure effect. J. Am. Ceram. Soc. 2009, 92, 864–869. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Huang, C.; Ji, L.; Wang, L.; Yuan, Y.; Liu, Q.; Han, Q. Study on crack healing performance of Al2O3/SiCw/TiSi2 new ceramic tool material. Ceram. Int. 2023, 49, 13790–13798. [Google Scholar] [CrossRef]
- Hu, J.F.; Deng, X.; Xu, T.Z.; Chen, Z. Experimental and theoretical investigation on the effect of crack dimension on the crack-healing performance of Si3N4/SiCw composite ceramic. Results Phys. 2019, 14, 102411. [Google Scholar] [CrossRef]
- Boatemaa, L.; Brouwer, J.C.; van der Zwaag, S.; Sloof, W.G. The effect of the TiC particle size on the preferred oxidation temperature for self-healing of oxide ceramic matrix materials. J. Mater. Sci. 2018, 53, 5973–5986. [Google Scholar] [CrossRef]
- Boatemaa, L.; van der Zwaag, S.; Sloof, W.G. Self-healing of Al2O3 containing Ti microparticles. Ceram. Int. 2018, 44, 11116–11126. [Google Scholar] [CrossRef]
- Boatemaa, L.; Bosch, M.; Farle, A.S.; van der Zwaag, S.; Sloof, W.G. Autonomous high-temperature healing of surface cracks in Al2O3 containing Ti2AlC particles. J Am Ceram Soc. 2018, 101, 5684–5693. [Google Scholar] [CrossRef]
- Gupta, T.K. Crack healing and strengthening of thermally shocked alumina. J. Am. Ceram. Soc. 1976, 59, 259–262. [Google Scholar] [CrossRef]
- Chu, M.C.; Cho, S.J.; Yoon, K.J.; Park, H.M. Crack Repairing in Alumina by Penetrating Glass. J. Am. Ceram. Soc. 2005, 88, 491–493. [Google Scholar] [CrossRef]
- Yoshino, Y. Role of Oxygen in Bonding Copper to Alumina. J. Am. Ceram. Soc. 1989, 72, 1322–1327. [Google Scholar] [CrossRef]
- Yoshino, Y.; Ohtsu, H. Interface Structure and Bond Strength of Copper-Bonded Alumina Substrates. J. Am. Ceram. Soc. 1991, 74, 2184–2188. [Google Scholar] [CrossRef]
- Yoshino, Y.; Shibata, T. Structure and Bond Strength of a Copper–Alumina Interface. J. Am. Ceram. Soc. 1992, 75, 2756–2760. [Google Scholar] [CrossRef]
- Moulla, F.; Chekirou, W.; Karaali, A.; Karaali, N.; Mirouh, K. Solid state synthesis and spectroscopic analysis of CuAlO2 and spinel CuAl2O4. Phase Transit. 2020, 93, 813–825. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Khan, U.; Jangid, R.; Khan, S. Hardness and wear analysis of Cu/Al2O3 composite for application in EDM electrode. Mater. Sci. Eng. 2018, 310, 012044. [Google Scholar] [CrossRef]
- Sang, K.; Weng, Y.; Huang, Z.; Hui, X.; Li, H. Preparation of interpenetrating alumina–copper composites. Ceram. Int. 2016, 42, 6129–6135. [Google Scholar] [CrossRef]
- Hu, W.; Donat, F.; Scott, S.A.; Dennis, J.S. The interaction between CuO and Al2O3 and the reactivity of copper aluminates below 1000 °C and their implication on the use of the Cu–Al–O system for oxygen storage and production. RSC Adv. 2016, 6, 113016–113024. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Feng, L.; Zang, D. Effect of oxygen partial pressure on the structure and properties of Cu–Al–O thin films. Appl. Surf. Sci. 2012, 258, 5354–5359. [Google Scholar] [CrossRef]
- Trinidad, C.S.; Angel, G.D.; Torres, G.T.; Uribe, A.C.; Pavón, A.A.S.; Que, Z.G.; Perez, J.C.A.; Morales, F.J.T. Effect of the CuAl2O4 and CuAlO2 Phases in Catalytic Wet Air Oxidation of ETBE and TAME using CuO/γ-Al2O3 catalysts. ChemistryOpen 2019, 8, 1143–1150. [Google Scholar] [CrossRef]
- Hernandez, G.C.; Hernandeza, S.M.; Tostado, E.C.; Flores, F.D.; Gonzalez, E.C.; Alonso, C.M.; Cruz, J.S. CuAlO2 and CuAl2O4 thin films obtained by stacking Cu and Al films using physical vapor deposition. Results Phys. 2018, 9, 745–752. [Google Scholar] [CrossRef]
- Zhou, X.; Yamashita, S.; Kubota, M.; Kita, H. Encapsulated copper-based phase-change material for high-temperature heat storage. ACS Omega 2022, 7, 5442–5452. [Google Scholar] [CrossRef]
- Zhou, X.; Yamashita, S.; Kubota, M.; Zhang, C.; Hong, F.; Kita, H. Macro encapsulated Cu-based phase change material for high temperature heat storage with characteristic of self-sealing and high durability. Appl. Therm. Eng. 2023, 229, 120491. [Google Scholar] [CrossRef]
- Bao, F.; Yamashita, S.; Daki, H.; Nakagawa, K.; Kita, H. Microstructure modification of alumina prepared by water-stabilized plasma spraying method using Al-Cu-O reaction. J. Eur. Ceram. Soc. 2024, 44, 6113–6123. [Google Scholar] [CrossRef]
- Japanese Industrial Standards, Testing Method for Flexural Strength (Modulus of Rupture) of Fine Ceramics at Room Temperature. JIS R 1601. 2008. Available online: https://standards.globalspec.com/std/1150428/jis-r-1601 (accessed on 20 March 2008).
- Misra, S.K.; Chaklader, A.C.D. The System Copper Oxide—Alumina. J. Am. Ceram. Soc. 1963, 46, 509. [Google Scholar] [CrossRef]
- Zmak, I.; Coric, D.; Mandic, V.; Curkovic, L. Hardness and Indentation Fracture Toughness of Slip Cast Alumina and Alumina-Zirconia Ceramics. Materials 2019, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Paris, P.C.; Irwin, G.R. The Stress Analysis of Cracks Handbook; Elias, A.C., Ed.; ASME Press: New York, NY, USA, 2000. Available online: https://books.google.co.jp/books/about/The_Stress_Analysis_of_Cracks_Handbook.html?id=9tfAQgAACAAJ&redir_esc=y (accessed on 26 July 2000).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, F.; Yamashita, S.; Kita, H. Study on the Repair of Irregular and Deep Cracks Induced by Thermal Shock Using Al-Cu-O Reactions in Al2O3 Ceramics. Processes 2024, 12, 2606. https://doi.org/10.3390/pr12112606
Bao F, Yamashita S, Kita H. Study on the Repair of Irregular and Deep Cracks Induced by Thermal Shock Using Al-Cu-O Reactions in Al2O3 Ceramics. Processes. 2024; 12(11):2606. https://doi.org/10.3390/pr12112606
Chicago/Turabian StyleBao, Fuhai, Seiji Yamashita, and Hideki Kita. 2024. "Study on the Repair of Irregular and Deep Cracks Induced by Thermal Shock Using Al-Cu-O Reactions in Al2O3 Ceramics" Processes 12, no. 11: 2606. https://doi.org/10.3390/pr12112606
APA StyleBao, F., Yamashita, S., & Kita, H. (2024). Study on the Repair of Irregular and Deep Cracks Induced by Thermal Shock Using Al-Cu-O Reactions in Al2O3 Ceramics. Processes, 12(11), 2606. https://doi.org/10.3390/pr12112606







